Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud mental
versión impresa ISSN 0185-3325
Salud Ment vol.36 no.4 México jul./ago. 2013
Artículo original
An alarm pheromone increases the responsivity of amygdaline-hippocampal neurons
Una feromona de alarma incrementa la responsividad de neuronas amigdalino-hipocampales
Tania Molina-Jiménez,1 Ana G. Gutiérrez-García,1,2 Carlos M. Contreras1,3
1 Laboratorio de Neurofarmacología, Instituto de Neuroetología, Universidad Veracruzana, Xalapa, Veracruz, México.
2 Facultad de Psicología, Universidad Veracruzana, Xalapa Veracruz, México.
3 Unidad Periférica del Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México. Xalapa, Veracruz, México.
Correspondence:
Carlos M. Contreras, Laboratorio de Neurofarmacología.
Av. Dr. Luis Castelazo s/n, col. Industrial Las Ánimas, 91190, Xalapa, Ver., México.
Tel: +52 (228) 841-8900, ext. 13613.
Fax: +52 (228) 841-8918.
E-mail: ccontreras@uv.mx; contreras@biomedicas.unam.mx.
Recibido: 22 de junio de 2012.
Aceptado: 11 de febrero de 2013.
ABSTRACT
The capability to perceive and emit alarm substances, such as 2-heptanone, makes animals aware of the presence of danger, leading to some strategies directed towards survival. Strategies of survival involve emotional memory which is processed by deep temporal lobe structures, such as amygdaloid complex and hippocampus. In the Wistar rat, 2-heptanone produces anxiety-like behavior and an increased firing rate of basal amygdaline neurons. However, it is unknown whether 2-heptanone modifies the responsivity of medial amygdaline-hippocampal connection. Therefore, we placed a group (n=10) of Wistar rats in a plexiglass cage impregnated with 2-heptanone. Rats from control group (n=10) were introduced into a similar clean cage. Twenty four hours later we obtained single-unit extracellular recordings from the hippocampus (CA1-CA3) neurons identified by their connection to medial amygdala. Although the basal neuronal firing rate was similar between groups, first order interval distribution histogram analysis showed that 2-heptanone produced shorter intervals of firing rate. Peristimulus histograms indicated that: i) the amygdaline stimulation produces an increased firing rate in hippocampal neurons; and ii) this response is increased and enlarged on the 2-heptanone group. Since a single exposure to an alarm pheromone seems to facilitate the amygdala-hippocampal connection, results suggest the initial formation of contextual memories related with fear.
Key words: 2-heptanone, medial amygdala, hippocampus, alarm pheromone, anxiety, emotional memory.
RESUMEN
La percepción olfatoria de feromonas de alarma, como la 2-heptanona, promueve ciertas estrategias de supervivencia con la participación de la memoria emocional, integrada en estructuras del lóbulo temporal, como la amígdala y el hipocampo. En la rata Wistar, la olfacción de 2-heptanona genera conductas sugerentes de ansiedad y un incremento de la tasa de disparo neuronal del núcleo basal de la amígdala. Sin embargo, no se conoce si la 2-heptanona modifica la responsividad de la conexión amígdala medial-hipocampo. Un grupo de ratas Wistar (n=10) fue colocado dentro de una caja de acrílico impregnada con 2-heptanona; el grupo control (n=10) fue introducido en una caja limpia. Veinticuatro horas después se obtuvo el registro unitario extracelular de neuronas del hipocampo (CA1-CA3) identificadas por su conexión con la amígdala medial. Aunque la tasa de disparo basal fue similar entre los grupos experimentales, el histograma de distribución de intervalos de primer orden indicó un predominio de intervalos de breve duración en el grupo 2-heptanona. Los histogramas periestímulo indicaron que: i) las neuronas hipocampales responden con un incremento en la tasa de disparo neuronal ante la estimulación amigdalina; ii) la respuesta es de mayor magnitud y duración en el grupo previamente expuesto a 2-heptanona. Dado que una sola exposición a una feromona de alarma facilita la conexión amígdala medial-hipocampo, los resultados sugieren la formación inicial de una memoria contextual relacionada con el miedo.
Palabras clave: 2-heptanona, amígdala medial, hipocampo, feromona de alarma, ansiedad, memoria emocional.
INTRODUCTION
Emotional memories allow emotional arousing1 and are namely processed by the interaction of two temporal lobe deep structures, the amygdala nuclei and hippocampus.2,3 From amygdaline nuclei, medial amygdala integrates olfactory information coming from the main and accessory olfactory system.4,5 The chemical cues perceived by olfactory system seems to modulate maternal behavior,6 aggression,7 and reproductive behavior,8 and, inclusively, they allow the identification of individuals of the same species.9 Also, the interaction of olfactory structures with medial amygdala facilitates retrieval olfactory information leading to fear expression,10 including alarm messages, such as the presence of a predator identified by its odor.11,12 The anatomical circuit beginning in olfactory pathways, and relying in medial amygdale, is completed by connections with the hippocampus. The hippocampus receives information from medial amygdala through the entorhinal cortex13 and in the presence of an alarm substance modulates the discrimination of significant events by contextual memories.1,14
Alarm substances are delivered on dangerous situations and elicit defensive behavior and anxiety-like behavior.15,16 Among others, 2-heptanone is the main volatile component produced in mandibular gland of the honeybee and ants, as well as in the anal glands of some ants, and it has been described as alarm pheromone.17,18 In rodents, 2-heptanone elicited activity in both the main and accessory olfactory bulbs.19 This substance is delivered by urine, and its concentration increases in animals physically stressed and a single exposure to 2-heptanone causes signs of anxiety in conspecifics in the short term and despair in the long-term.20,21
Some odors can elicit unconditioned and conditioned fear behavior,22,23 depending on context. But any response involving fear must affect the neuronal activity of amygdaline and hippocampal structures. Certainly, the acute inhalation of 2-heptanone produces an increased firing rate in basal amygdaline neurons, which is enhanced by epithelial olfactory structures removal,24 but it is unknown if a single exposure to an alarm pheromone modifies, in the long-term, the responsivity of hippocampal neurons to the medial amygdala nucleus stimulation; in such a case the meaning may be the initial formation of contextual memories related with fear.
MATERIALS AND METHODS
Animals and housing conditions
All the experiments were performed in strict adherence to National Institutes of Health guidelines and international and institutional standards for the care and use of animals in research25 and with the authorization of the Ethical Committee of Biomedical Research Institute of the Universidad Nacional Autónoma de México. We included a total of 20 male Wistar rats, aged three months, weighing 350-400 g. Animals were housed five per cage in transparent plexiglas boxes (45 x 30 x 30 cm) in local housing facilities with a 12 h/12 h light/dark cycle (lights on at 7:00AM), and had ad libitum access to food and water. They were handled daily during 5min 1 week before testing. All procedures were performed during the light period between 10:00 AM and 3:00 PM.
Apparatus
We used a plexiglas box (base: 30cm x 25cm, height 30cm, Modular test cage Instruments Coulbourn, Lehigh Valley, PA, USA). The box contained a stainless steel grid floor (diameter 0.5cm) and the separation between bars was 1.3cm (Model E10-10R, Coulbourn Instruments, Lehigh Valley, PA, USA). The box was placed in a sound-isolated box (56 x 46 x 40 cm; Coulbourn Instruments).
Experimental groups
A 2-heptanone group (n=10) was placed during 16min in an acrylic box previously impregnated with 2-heptanone (0.4mL) sprinkled on the floor below the grids. The control group (n=10) surpassed a similar session, also during 16 min, but in a clean cage. After each session, we carefully cleaned and deodorized the box with a cleaning solution (ammonia 0.5%, ethanol 15%, extran 10%, isopropyl alcohol 5%, Pinol ® 10% and water 59.5%). Since the cleaning solution contains some odors, and that an stressed animal is able to deliver some alarm substance, a minimal lapse of 20 min after cleaning was considered before introducing a new animal into the cage. On the day after, single unit extracellular recordings were obtained.
Stereotaxic surgery
Rats were anesthetized with ethyl carbamate (urethane 1gr/kg i.p. Sigma Chemicals Co., MO). Once rats showed no alert signs, the head was fixed to a stereotaxic frame. Then, an incision was made along the midline of the scalp to expose the skull. We drilled two small holes at the appropriated coordinates.26 The recording electrode was a glass micropipette filled with 3 M KCl (4-5MΩ) containing pontamine blue (Chicago Sky Blue, Sigma Chemicals Co., MO) as a dye having a final concentration of 4%.27 Through a small trephination the recording electrode was lowered by means of a motorized micromanipulator (Trent Wells, South Gate, CA, USA) toward the hippocampus (CA1-CA3) (anterior/posterior =-6 mm, medial/lateral =-3.9mm, dorsal/ventral =-2 to -3 mm). A stainless steel bipolar electrode (diameter: 50 um, resistance: 100kΩ) was placed in the medial amygdala nucleus (anterior/posterior =-3 mm, medial/lateral =-3.3 mm, dorsal/ventral =-9 mm from the brain surface).
Single-unit extracellular recordings
The micropipette signal was connected in series to a 7P511L Grass amplifier (Quincy, MA, USA; bandwidth pass filters: 300 Hz-3 KHz) and oscilloscope (model 5111A, Tektronix, Beaverton, OR, USA) that received a filtered signal free from background noise through a window discriminator and in parallel to an audio amplifier. The absence of sudden changes in the amplitude of the firing rate over 300s verified a stable recording. Afterward, each spike detected by the amplifier was fed to a Grass stimulator S88 (Quincy MA, USA) that delivered a spike-corresponding square pulse of constant amplitude and duration (4 V, 0.6 ms). Then, the signal was sent to an interface (CED MICRO 1401; Cambridge Electronic Design, Cambridge, England) that transformed the analog signal into digital. The Spike2 program delivered digital data for its statistical analysis. The firing-rate was analyzed using frequency histograms, interval histograms and peristimulus histograms (base 50 ms; bin width 0.1 ms).
After one min of basal spontaneous activity recording of hipocampal neurons, amygdaline stimulation began (1 ms, 0.3 Hz, 1 min). For amygdaline stimulation the bipolar electrode was connected to a stimulator (GRASS S88, Quincy, MA, USA) coupled to an isolation unit (GRASS SIU 5a, Quincy, MA, USA). Once finished amygdaline stimulation, the hippocampal recording lasted one more min.
Histological analysis
To mark the last recorded point, we passed a direct current (1mA) during 5min through the recording micropipette so the colorant left a blue mark on the last recorded site. We also passed current (10 mA DC, 30s) through the stimulation electrode to mark the placement of stimulating electrode. Afterwards, each rat was intracardially perfused with 0.9% saline (10mL), followed by 30% formaldehyde (50mL). We removed the brains and placed in 30mL of formaldehyde for 48 hrs. Each brain was placed in a 10% sucrose solution.
After 24h the brain was frozen at -20°C, cut into 40 |im thick sections with a cryocut microtome (Leica-Jung, Nussloch, Germany), and dyed using the Nissl technique to check the stimulation and recording sites. After sectioning, only those recordings we recognized a clear mark left by the electrodes in the hippocampus (CA1-CA3) and medial amygdala were included in the data analysis.
Statistical analysis
First, we used a t-Student test (Sigma-Stat 3.5) to compare the spontaneous firing rate (1min) between both experimental groups (control group vs 2-heptanone group).
Afterwards, we constructed data bases including all data from peristimulus histograms, and first order interval distribution histograms, i.e., we obtained graphs including all recordings for each experimental group. Data from first order interval histograms analysis was first normalized and then subjected to statistical analysis using two-way analysis of variance (two-way ANOVA). First considered factor was group (control and 2-heptanone groups) and an arbitrarily fixed range of intervals (10, 20, 30, 50, 60, 70, 80, 90 and 100 ms) as a second factor. For analysis of peristimulus data we used two-way ANOVA with factors group (control and 2-heptanone groups) and recording period (50ms before and 50ms after electrical stimulation). In any case, when at least one of the factors reached the criterion of significance (p<0.05), the interaction between factors was included, followed by Student-Newman-Keuls (SNK, p≤0.05) as post hoc. The data are expressed as mean and standard error of the mean.
RESULTS
Histological control
We recorded a total amount of 85 neurons from the hippocampus CA1 region, 36 from the control group (n=10 rats) and 48 from the 2-heptanone group (n=10 rats). Histological analysis allowed the identification of hippocampus (CA1: 2.0-2.9 mm beneath the cortical surface) as the recording place and medial amygdala (9.0 mm beneath the cortical surface) as the stimulation place (figure 1).
Single-unit extracellular recording
Spontaneous activity. Before amygdaline stimulation, hippocampal neurons from both groups fired at similar rates 2-heptanone group: 5.0±0.72 spikes; control group 4.7±0.85 spikes; t82=-0.25, p=0.80).
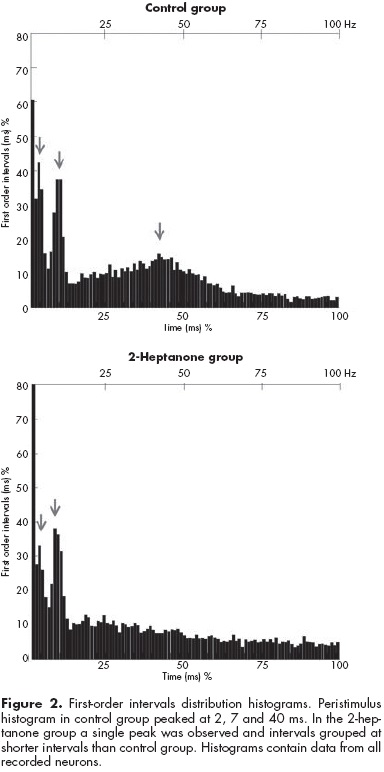
First-order interval histograms. The two-way ANOVA indicated that the factor group analysis did not reach statistical significance (F1,1=0.003, p=1.0), but the interval range factor did so (F1,9=19.40, p<0.001). Independently of experimental group, intervals of firing peaked at the 10 ms range (p<0.05, SNK). The interaction between group X interval range was significant (F9,81=3.64, p<0.001). In control group, first order intervals peaked twice, at the 10 ms range and at 40-50 ms (p<0.05, SNK). However, in the 2-heptanone group the first peak (10 ms) was significantly higher (p<0.05, SNK) than control group, and the second peak (40-50 ms) disappeared (p<0.05, SNK). In other words, a bimodal histogram of first order intervals in control was observed, but such conformation of histogram disappeared in 2-heptanone group, at the expense of a higher (p<0.05) amount of short intervals of firing (figure 2).
Peristimulus histograms analysis (50 ms)
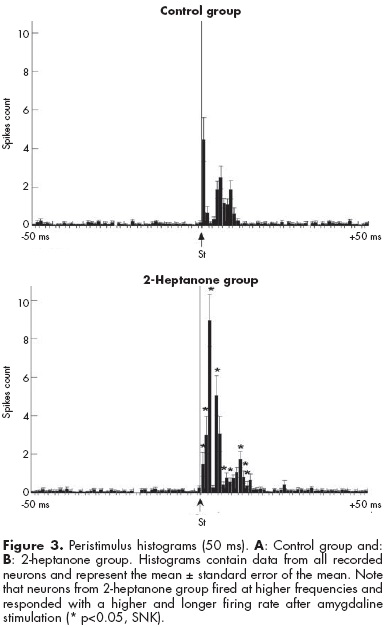
The two-way ANOVA illustrated significant differences in the factor group (F1,164=13.89, p<0.001). Regardless of the period of analysis (pre- or post-stimulus), the hippocampal neuronal firing rate was higher in the 2-heptanone group (3.8±0.2 spikes) than control group (2.3±0.27 spikes, p<0.05, SNK). The period factor also reached statistical significance (F1,164=107.16, p<0.001). Regardless of experimental group, all neurons increased their firing rate after the amygdaline stimulation. Interaction between group factor X period of stimulation factor (figure 3) also reached the criterion of significance (F1,164=13.18, p<0.001). The response of hippocampal neurons to amygdaline stimulation was significantly higher (p<0.05, SNK) in the 2-heptanone group than control group after stimulation, and longer (2-heptanone group, 16 ms; control group, 11 ms duration).
DISCUSSION
The aim of the present study was to explore the effect of a single exposure to an alarm pheromone, 2-heptanone, on the hippocampal neurons response to medial amygdala stimulation, the day after the exposure to this alarm pheromone. Regardless of group, most hippocampal neurons responded with an increased firing rate to the medial amygdaline stimulation. The previous exposition to 2-heptanone enhanced the hippocampal response to amygdaline stimulation.
According to our results, amygdaline-hippocampal neurons seem to be sensitive to one-assay learning. Behavioral studies using 2-heptanone as alarm pheromone are scarce, but the use of predator odors, i.e., 2,3,5-trimethyl-3-tiazoline, produces comparable results. A single exposure to this alarm pheromone contained in fox feces and cats increases the c-Fos expression in the lateral septal nucleus and central amygdala,28,29 among other structures. In consistence, as techniques of arterial spin labeling-based functional magnetic resonance imaging (fMRI) illustrate, during alarm pheromones exposure the neuronal activity increases in the dorsal periaqueductal gray, superior colliculus, and medial thalamus, but it is reduced in medial raphe, locus coeruleus, accumbens nucleis, ventral tegmental area, ventral pallidum and piriform and entorhinal cortex.30 In contrast, the exposure to odors from potential predators elicits fast waves in the dentate gyrus,31 and enhances the long-term-potentiation in dentate gyrus generated by the perforant pathway electrical stimulation.32 Consequently, profound changes must be expected in hippocampal neuronal activity after a single exposure to alarm substances, namely in structures related with emotional processing. This seems to be the case since we detected changes in amygdaline-hippocampal neurons after a single exposure to 2-heptanone. Albeit hippocampal neuronal firing rate did not change in basal condition, our results illustrate that the change lies on the responsivity of amygdaline-hippocampal connection, suggesting that changes occur on synaptic cleft, leading to an increased sensitization of amygdaline input to hippocampal neurons.
Both the main and the accessory olfactory systems are responsive to 2-heptanone;19 medial amygdala nucleus receives indirect inputs from the main olfactory system from the piriform cortex, the periamygdaloid cortex and cortical amygdala nucleus, and directly from the accessory olfactory system,4 and the hippocampus also receives odor information from both olfactory systems, through entorhinal cortex connections.33 Herein, neurons from medial and cortical amygdala nuclei activate in the presence of alarm pheromones.16 We found that most hippocampal neurons responded with an increased firing rate to amygdaline stimulation, and this characteristic was enhanced by the previous exposure to 2-heptanone and also changed the first order intervals distribution, at the expenses of a high amount of short intervals of firing, suggesting that the exposure to the alarm pheromone facilitated the medial amygdala-hippocampus connection probably representing a first step in the formation of a memory. This seems to be the case since medial amygdala is involved in the neuronal circuit associated to memory formation related to odors coming from predators leading further to the expression of unconditioned and conditioned fear behavior elicited by these odors.12,22
In conclusion, a single exposure to 2-heptanone elicits an increase in the medial amygdala-hippocampus connection responsivity. This could represent the formation of memories related with fear.
ACKNOWLEDGMENTS
This study was partially supported by grants from the Consejo Nacional de Ciencia y Tecnología, México (CONACyT: CB-2006-1, 61741), Universidad Nacional Autónoma de México (UNAM: DGA-PA-PAPIIT IN211111-3). During this investigation, TMJ received fellowships from the Consejo Nacional de Ciencia y Tecnología (CONACyT, México: Reg. 2057739).
REFERENCES
1. McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 2004;27:1-28. [ Links ]
2. Richter-Levin G, Akirav I. Emotional tagging of memory formation-in the search for neural mechanisms. Brain Res Brain Res Rev 2003;43:247-256. [ Links ]
3. Hughes M. Olfaction, emotion & the amygdala: arousal-dependent modulation of long-term autobiographical memory and its association with olfaction: beginning to unravel the Proust phenomenon? Premier J Undergraduate Publications Neurosciences 2004;1:1-58. [ Links ]
4. Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci 1998;2:323-331. [ Links ]
5. Luo M, Katz LC. Encoding pheromonal signals in the mammalian vomeronasal system. Neurobiol 2004;14:428-434. [ Links ]
6. Fleming AS, Suh EJ, Korsmit M, Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav Neurosci 1994;108:724-734. [ Links ]
7. Ferris CF, Stolberg T, Kulkarni P, Murugavel M et al. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci 2008;9:111. [ Links ]
8. Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol 2002;23:200-224. [ Links ]
9. Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci 1999;113:345-357. [ Links ]
10. Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn Mem 2005;12:120-129. [ Links ]
11. Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci 2004;118:324-332. [ Links ]
12. Müller M, Fendt M. Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rats. Behav Brain Res 2006;167:57-62. [ Links ]
13. Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 1995;360:213-245. [ Links ]
14. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000;23:115-184. [ Links ]
15. Kikusui T, Takigami S, Takeuchi Y, Mori Y. Alarm pheromone enhances stress-induced hyperthermia in rats. Physiol Behav 2001;72:45-50. [ Links ]
16. Kiyokawa Y, Shimozuru M, Kikusui T, Takeuchi Y et al. Alarm pheromone increases defensive and risk assessment behaviors in male rats. Physiol Behav 2006;87:383-387. [ Links ]
17. Hughes WO, Howse PE, Goulson D. Mandibular gland chemistry of grass-cutting ants: species, caste, and colony variation. J Chem Ecol 2001;27:109-124. [ Links ]
18. Balderrama N, Núñez J, Guerrieri F, Giurfa M. Different functions of two alarm substances in the honeybee. J Comp Physiol Neuroethol Sens Neural Behav Physiol 2002;188:485-491. [ Links ]
19. Xu F, Schaefer M, Kida I, Schafer J et al. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol 2005;489:491-500. [ Links ]
20. Gutiérrez-García AG, Contreras CM, Mendoza-López MR, Cruz-Sánchez S et al. A single session of emotional stress produces anxiety in Wistar rats. Behav Brain Res 2006;167:30-35. [ Links ]
21. Gutiérrez-García AG, Contreras CM, Mendoza-López MR, García-Barradas O et al. Urine from stressed rats increases immobility in receptor rats forced to swim: role of 2-heptanone. Physiol Behav 2007;91:166-172. [ Links ]
22. Takahashi LK, Hubbard DT, Lee I, Dar Y et al. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behav Neurosci 2007;121:100-110. [ Links ]
23. Campeau S, Nyhuis TJ, Sasse SK, Day HE et al. Acute and chronic effects of ferret odor exposure in Sprague-Dawley rats. Neurosci Biobehav Rev 2008;32:1277-1286. [ Links ]
24. Contreras CM, Gutiérrez-García AG, Molina-Jiménez T, Mendoza-López MR. 2-Heptanone increases the firing rate of the basal amygdala: Role of anterior olfactory epithelial organs. Neuropsychobiology 2012; 66:167-173. [ Links ]
25. National Research Council. Guide for the care and use of laboratory animals (NIH publication no. 85-23). Washington DC: National Academy Press; 1985. [ Links ]
26. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 1982. [ Links ]
27. Sánchez-Alvarez M, León-Olea M, Condés-Lara M, Briones M et al. Localization of the microelectrode tip combining a rapid procedure method and marking with pontamine sky blue. Bol Estud Med Biol 1988;36:55-59. [ Links ]
28. Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res 2004;1025:139-151. [ Links ]
29. Staples LG, Hunt GE, Cornish JL, McGregor IS. Neural activation during cat odor-induced conditioned fear and 'trial 2' fear in rats. Neurosci Biobehav Rev 2005;29:1265-1277. [ Links ]
30. Kessler MS, Debilly S, Schõppenthau S, Bielser T et al. fMRI fingerprint of unconditioned fear-like behavior in rats exposed to trimethylthiazoline. Eur Neuropsychopharmacol 2012;22:222-230. [ Links ]
31. Heale VR, Vanderwolf CH, Kavaliers M. Components of weasel and fox odors elicit fast wave bursts in the dentate gyrus of rats. Behav Brain Res 1994;63:159-165. [ Links ]
32. Dringenberg HC, Oliveira D, Habib D. Predator (cat hair)-induced enhancement of hippocampal long-term potentiation in rats: involvement of acetylcholine. Learn Mem 2008;15:112-116. [ Links ]
33. Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev 2001;38:247-289. [ Links ]
Declaration of conflict interest: None














