Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Salud mental
versão impressa ISSN 0185-3325
Salud Ment vol.36 no.2 México Mar./Abr. 2013
Artículo original
Endogenous opioids participation in the effect of Rosmarinus officinalis L. in the visceral, inflammatory and gout arthritis nociception in rodents
Participación de los opioides endógenos en el efecto de Rosmarinus officinalis L. en la nocicepción visceral, inflamatoria y artritis gotosa en roedores
Ana Laura Martínez,1,2 Ma. Eva González-Trujano,1 Francisco J. López-Muñoz2
1 Laboratorio de Neurofarmacología de Productos Naturales. Dirección de Investigaciones en Neurociencias del Instituto Nacional de Psiquiatría Ramón de la Fuente Muñíz.
2 Departamento de Farmacobiología, Cinvestav-Sede Sur. INP.
Corresponding:
Ma. Eva González-Trujano.
Laboratorio de Neurofarmacología de Productos Naturales.
Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz.
Calz. México-Xochimilco 101, Sn Lorenzo Huipulco,
Tlalpan, 14370, México, DF.
Tel: (+52 55) 4160-5085. Fax: (+52 55) 5655-9980.
E-mail: evag@imp.edu.mx
Recibido: 9 de mayo de 2012.
Aceptado: 20 de noviembre de 2012.
ABSTRACT
The aim of this study was to investigate the endogenous opioid participation in the antinociceptive effect of R. officinalis aerial parts in experimental models of visceral, inflammatory and gout arthritis nociception. Acid-acetic induced writhing and formalin tests as well as the pain-induced functional impairment model in the rat (PIFIR) assay were studied. Antinociceptive doses of R. officinalis via oral, alone and in presence of an opioid antagonist were evaluated in comparison to the reference analgesic drug tramadol (31.6 and 50mg/kg i.p., in mice and rats, respectively). The antinociceptive effect of R. officinalis at a 300mg/kg dosage was significantly reverted in presence of 1.0mg/ kg s.c. of naloxone in writhing and formalin tests. Concerning PIFIR model, significant antinociceptive response produced for 1000 and 3000mg/kg was not inhibited in presence of 1.0 or 3.16mg/kg, s.c. of naloxone. In the antinociceptive effect of tramadol, naloxone produced partial inhibition in all models tested. These results suggest that antinociceptive and anti-inflammatory activities of R. officinalis aerial parts involve endogenous opioids, but activation of these mediators depends on the experimental model and the physiological process of the induced nociception.
Key words: Antinociceptive, nociception, Rosmarinus officinalis, traditional medicine, tramadol.
RESUMEN
El objetivo de este estudio fue investigar la participación de los opioides endógenos en el efecto antinociceptivo producido por un extracto preparado con las partes aéreas de Rosmarinus officinalis en modelos experimentales de nocicepción visceral, inflamatoria y tipo artritis gotosa. Para la inducción de nocicepción visceral e inflamatoria se utilizaron los modelos de estiramiento abdominal "writhing" y de formalina intraplantar al 1 %, respectivamente, en ratones. A su vez, para la nocicepción de tipo artritis gotosa se utilizó el modelo de disfunción inducida por ácido úrico al 20% intraarticular en ratas conocido como PIFIR (por sus siglas en inglés). Dosis antinociceptivas de R. officinalis vía oral se evaluaron solas y en presencia del antagonista de opioides endógenos naloxona. Adicionalmente, dicho efecto se comparó con el fármaco analgésico de referencia tramadol (31.6 y 50mg/kg i.p., en ratones y ratas, respectivamente). El efecto antinociceptivo de R. officinalis significativo en la dosis de 300mg/kg se revirtió en presencia de 1mg/kg s.c. de naloxona en las pruebas de estiramiento abdominal y formalina. En cuanto al modelo PIFIR, la respuesta antinociceptiva producida por 1000 y 3000mg/kg no se inhibió en presencia de 1 o 3.16mg/kg, s.c. de naloxona. En el efecto de tramadol, opioide atípico, la naloxona produjo inhibición parcial de la respuesta antinociceptiva en todos los modelos probados. Los resultados sugieren que la actividad antinociceptiva producida por el extracto de las partes aéreas de R. officinalis involucra al sistema de opioides endógenos, pero la presencia de estos mediadores depende del tipo de estímulo y del proceso fisiológico involucrado en la nocicepción inducida.
Palabras clave: Antinociceptivo, nocicepción, medicina tradicional, Rosmarinus officinalis, tramadol.
INTRODUCTION
It is known that systemically administered opioid drugs may produce antinociceptive effects through inhibition of the ascending nociceptive transmission and activation of descending pathways.1-3 Opioid drugs induce antinociception by activating opioid receptors not only within the Central Nervous System but also on peripheral sensory neurons.4,5
Many species of plants are used by humans throughout the world to achieve central psycholeptic activities, such as analgesic action. Rosmarinus officinalis (Lamiaceae) is a common spice and household plant widely used around the world for medicinal purposes. In folk medicine, its aerial parts are used in oral administration to cure renal colic and dysmenorrhoea and as antispasmodic.6 In Mexico, it is prepared as maceration in ethanol and used as topical administration to cure rheumatic pain in humans. A tisane made of the boiled leaves is used to improve digestion and to cure stomachache.7-9 In experimental studies, it has been reported a reduction in the morphine withdrawal syndrome at 0.96g/kg dosage in mice.10 Phytochemical studies showed that the ethanol extract of R. officinalis aerial parts contains flavonoids, tannins and saponins constituents.9 It has been reported that some of these constituents, mainly flavonoids, produce opioid analgesic effect.11-13 In a previous study we demonstrated the dose-dependent antino-ciceptive effect of an ethanol extract of R. officinalis using either the writhing and formalin tests in mice or the PIFIR assay in rats.14 However, there is a lack of studies analysing the mechanism of action in the antinociceptive effect of this plant. The purpose of the present study was to evaluate the participation of the endogenous opiods in the effect of R. officinalis using some experimental models of nociception in rodents.
MATERIAL AND METHODS
Animals
Male Swiss Webster mice weighing 25-30g (Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz) and male Wi-star rats weighing 180-200g [Crl(WI)fBR] (Cinvestav-Sede Sur) were used in this study. They were housed in a temperature- and light controlled room under a 12:12h light: dark cycle (light on at 7:00 a.m.) with water and food provided ad libitum. Twelve hours before the experiments, food was withheld, but the animals had free access to tap drinking water. All experimental procedures followed the Guidelines on Ethical Standards for Investigations of Experimental Pain in Animals,15 and were carried out according to a protocol approved by the local Animal Ethics Committee. The number of experimental animals was kept to a minimum and they were used only once. All animals were adapted to manipulation through a daily saline solution (s.s.) injection (10ml/kg) for five days before extract or vehicle solutions were administered. For each experimental procedure, animal groups consisted of six mice or rats each.
Plant material
The R. officinalis aerial parts were collected in June 2004 in Morelos, Mexico. MSc Abigail Aguilar identified a specimen and a voucher specimen (IMSSM-15005) was deposited in the herbarium of the Instituto Mexicano del Seguro Social, Mexico, for future reference. This project (number INP3280) was approved by the ethical committee on September 2006.
Preparation of the extract
The dried mature aerial parts of R. officinalis were cut into small bits (330g) and kept in a container for extraction by successive maceration at room temperature (22°C±1) for 48h. A first extraction with hexane (1200mL x 3) was carried out followed by filtration. The residue was extracted with ethanol (1200mL x 3) and after filtration it was discarded and the final filtrate was concentrated under vacuum to eliminate ethanol solvent and to yield 111g of a green solid ethanol extract (33.6%).
Drugs preparation
The R. officinalis ethanol extract was suspended in vehicle (0.2% or 0.5% tween 80 in s.s. for mice and rats, respectively). Tramadol chloridrate (Grünenthal de Mexico, S.A. de C.V. 98% purity) was diluted in s.s. and used as antinociceptive reference drug. The extract and tramadol were administered via oral (p.o.) and via intraperitoneal (i.p.), respectively, in a volume of 0.1mL/10g body weight. Control animals received the same volume of vehicle or s.s. alone by the respective route of administration. To induce noci-ception, acetic acid (Merck), formalin (Baker) and uric acid (Sigma) were used in solution at 0.6%, 1% and 20%, correspondingly. Acetic acid and formalin were diluted in s.s. and uric acid was suspended in mineral oil. Naloxone hydrochloride (Sigma) was used as an opioid antagonist and dissolved with s.s. Drugs were freshly prepared on the day of the experiments.
Antinociceptive activity evaluation
Different groups of mice were administrated with R. officinalis ethanol extract (300mg/kg, p.o.), tramadol (50mg/kg, i.p.) or the respective vehicle (p.o. or i.p.) 30 minutes before writhing and formalin tests. Other groups of mice were treated with naloxone (1 or 3.16mg/kg, s.c.) and after 15 min these animals received the same doses of extract, tramadol or the respective vehicle.
Writhing Test
This test consists in to induce nociception by an i.p. acetic acid at 0.6% administration in a volume of 10mg/kg in mice. The induced nociceptive behaviour is characterized by abdominal contraction defined as an exaggerated extension of the abdomen, combined with the outstretching of the hind limbs known as writhing.16 The accumulated number of writhes manifested by each mouse was recorded in the following periods: 0-5, 5-10, 10-15, 15-20, 20-25 and 25-30min immediately after the injection of acetic acid 0.6%. These data were expressed as a temporal course to observe changes in the maximal number of writhes induced, and in a dose-response curve to determine the significant antinociceptive dose.
Formalin test
The method used was similar to that described by Hun-skaar and Hole.17 To induce nociception, mice were injected under the skin of the dorsal surface of the right hind paw with 20|iL of dilute formalin (1% in s.s.) by using a 30-gauge needle. Immediately after, each single mouse was led into a cylinder of glass, with two mirrors behind of it, to have a total panorama of nociceptive behavior. Number of shakings and the accumulated time spent in licking the injected paw was taken as nociceptive response. Two periods of high licking and shaking activity were considered: the first one was obtained immediately after injection for a period of 5 min known as "early phase". A second period was observed from 20-25 min after formalin injection and determined as the "late phase".
PIFIR assay
Antinociceptive activity was measured using the PIFIR model.18 Nociception was induced by injection of 50|iL of 20% uric acid into the knee joint of the right hind limb (i.art.), under light anaesthesia with ether. After uric acid injection, the animals developed a progressive dysfunction of the injured limb. The time of contact of the injured hind limb reached a zero value at 2-2.5h after the uric acid injection. Rats were forced to walk on the rotating cylinder for periods of 2min, and then, rats were allowed to rest between recording periods. Data are expressed as the percentage of the functionality index (FI%), i.e., the time of contact of injected foot divided by the time of contact of the control left foot and multiplied by 100. Once the FI% was zero, different groups of rats received one of the following treatments: vehicle (0.5% tween 80 in s.s., p.o.), R. officinalis extract (1000 and 3000mg/kg, p.o) or tramadol (31.6mg/kg, i.p.). Other groups of rats received naloxone (1.0 or 3.16mg/kg s.c.) 15 min previous to R. officinalis extract (1000 and 3000mg/kg, p.o.), tramadol (31.6mg/ kg) or the respective vehicle administration. Recordings were taken every 15 min in the first 2h, and after this time every 30 min until 4h were completed. Recovery of the FI% was considered as expression of the antinociceptive effect. Time-response curves were constructed to observe the onset of the antinociceptive effect, but also dose-response curves were obtained to determine the significant antinociceptive dose. For the purpose of this study, inducing nociception in the experimental animals was unavoidable. However, care was taken to avoid unnecessary suffering.
Data analysis
Data are expressed as the mean ± standard error of the mean (S.E.M.). The area under the curve (AUC) values were calculated from the respective time course curves of writhing and PIFIR assays and considered as an expression of the overall antinociceptive activity during the 30min or 4h observation period, respectively, using the trapezoidal rule.19 All data were compared by analysis of variance (ANOVA) followed by Dunnett's test or by Student's t test using SIGMA STAT® software, version 2.3. P<0.05 was considered statistically significant.
RESULTS
Effect of R. officinalis in the writing test
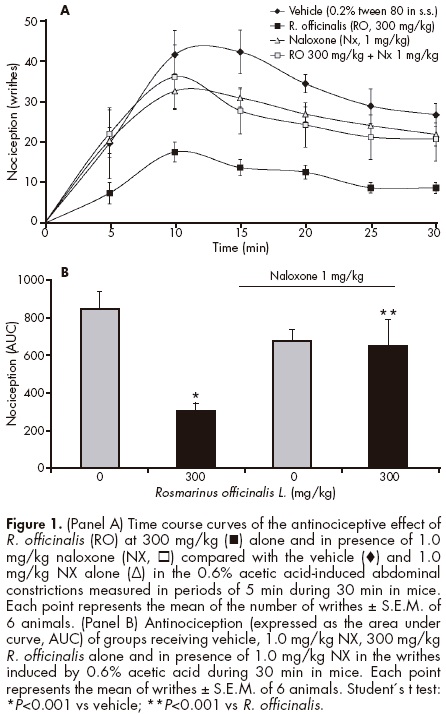
The number of writhes (nociception) induced by acetic acid 0.6% in the vehicle group (0.2% tween 80 in s.s.) was diminished from the first five minutes of the R. officinalis administration (300mg/kg); this diminution was kept until the end of the test (figure 1A). The nociceptive activity in the R. officinalis group measured as AUC=306±39 area units (au) was significantly (t=5.56, df=10, P<0.001) diminished in comparison with AUC=854±91 au of nociceptive activity observed in the vehicle group (figure 1B). Presence of tramadol (50 mg/kg) produced a total inhibition (AUC=15±4au) on the number of writhes induced by acetic acid 0.6% in the vehicle group (s.s., AUC=802±61au); this inhibition was observed from the beginning until the end of the test (figure 2A). The antinociceptive effect of R. officinalis was almost totally reverted in presence of 1 mg/kg of naloxone (AUC=656±138au) (t=-8.09, df=10, P<0.001) (figures 1A and 1B). Whereas, a partial inhibition was observed with 50mg/kg of tramadol in presence of the same dose of naloxone (AUC=434±28au) (t=-14.69, fd=10, P<0.001) (figures 2A and 2B).

Effect of R. officinalis in the formalin test
Significant antinociceptive responses were observed in the shaking and in the licking time spent in mice with R. officinalis (300mg/kg) using formalin test. Both phases (early and late phases) were reverted in presence of 1mg/kg of naloxone (table 1). In the case of tramadol, the antinociceptive effect produced for 50mg/kg was totally reverted in presence of 1mg/kg of naloxone in the early phase and partially on shaking behavior in the late phase. Both R. officinalis and tramadol showed a partial inhibition in the licking behavior of the late phase (table 1).
Effect of R. officinalis in the PIFIR assay
Both controls (receiving vehicle: s.s. and 0.5% tween 80 in s.s.) showed a FI%=0 during the lasting 4h of the experiment alone or in presence of 1 and/or 3.16 mg/kg naloxone (figures 3A and 4A). Whereas, a significant and in a dosedependent antinociceptive effect observed with R. officinalis at 1000 and 3000 mg/kg was not reverted in presence of 1 or 3.16mg/kg naloxone, respectively (figures 3A and 3B). While as it was expected, the antinociceptive effect produced for 31.6mg/kg of tramadol was partially inhibited by naloxone at 3.16mg/kg in this experimental model (F5,30=110.6.05, P <0.001) (figures 4A and 4B).


DISCUSSION
In the present study, it was investigated the endogenous opioid participation in the antinociceptive effect obtained with R. officinalis in three different kinds of induced nociception in rodents as previously observed.14 As we demonstrate in our present research, presence of naloxone, an opioid antagonist, reverted the effect of R. officinalis in writhing test, an experimental model used in the screening of analgesic drugs.20 These results are in agreement with a previous report where higher doses as 640 and 1120mg/kg of R. officinalis inhibited the number of writhes in mice and these effects were totally reverted with naloxone 1mg/kg s.c.10 Additionally, antinociceptive effects of R. officinalis, observed in the formalin test,21 were reverted in both licking and shaking behavior in either early (neurogenic) or late (inflammatory) phases in presence of naloxone. It is known that centrally acting drugs, such as opioids, inhibit both phases of nociception equally22 involving the effect produced by prostaglandins released at this level in response to inflammation17,23 or by endogenous opioid through their action on the Central Nervous System.24 Inhibition of the antinociceptive effects of R. officinalis in writhing and in both phases of the formalin test in presence of naloxone may involve participation of endogenous opioids at central level. However, in spite of it, it has been thought that opioid drugs act exclusively within the Central Nervous System. Both animal4,25,26 and human27 studies have demonstrated that peripheral opioid mechanism plays a role in antinociception, particularly prominent under painful inflammatory conditions.28
During inflammatory processes, opioid receptors are transported from dorsal root ganglia towards the peripheral sensory nerve endings. At the same time, immune cells containing endogenous opioid peptides accumulate within the inflamed tissue, which can liberate them to interact with the neuronal opioid receptors and elicit local analgesia.29 DAMGO, a µ-opioid receptor agonist, was able to suppress nociception on the late behaviour induced by formalin, but also decreases post-surgical pain after instillation into the knee joint in humans.27 Rodrigues and Duarte30 reported that peripheral antinociceptive effect induced by morphine might result from activation of ATP-sensitive K+ channels, which may cause a hyperpolarization of peripheral terminal of primary afferents, leading to a decrease in action potential generation. But also, in the Central Nervous System, the opening of K+ channels plays a role in opioid-mediated antinociception.31 In the late phase of formalin test, it was observed an increase on the formalin-induced nociception when a 300mg/kg dosage of R. officinalis was tested after 15min of 1mg/kg of naloxone. This pro-nociceptive effect has been previously observed when local treatment with naloxone increases post-surgical pain in humans.32 It may be interesting in the future to evaluate the specific central and/or peripheral participation of the opioid system in the antinociceptive effects of R. officinalis.
In the PIFIR assay, naloxone administration did not modify the R. officinalis antinociception; this suggests that an endogenous opioid participation is not the main mechanism of action involved in this kind of induced nociception. These results suggest that R. officinalis activity may be associated with different vias to produce antinociceptive effects depending on the kind and intensity of the induced nociception, but also different constituents of the R. officinalis may also be implicated.
Tramadol was used as a reference drug producing antinociceptive effect in all models used in this study. The specific mechanism of action of this analgesic drug is unknown. However, it has been described that tramadol produces analgesic activity by modulation of monoamines like noradrenaline and serotonin, but also by GABAergic neuro-transmission.33 Moreover, it is considered that mechanisms of action of tramadol involve a partial action through u.-opi-oid receptors,34 which is in accord with our results because the presence of naloxone partially reverted the antinociceptive effect of this analgesic drug in all models experienced in our study.
In conclusion, antinociceptive effects of an ethanol extract of R. officinalis were reverted in presence of naloxone in visceral and inflammatory nociception, but not in the gout arthritis assay in rats. The outcome of the present study demonstrates that opioid participation is involved in the mechanism of action of R. officinalis antinociceptive activity which depends on the nociceptive stimulus and likely to the participation of different constituents in the plant.
ACKNOWLEDGEMENTS
We wish to thank Froylán Sánchez, Luis Oliva and Rubén Luviano for their technical assistance. This work was partially supported by CONACYT-80811 and INP3280 grants. Ana Laura Martínez received fellowship CONACYT-203600.
REFERENCES
1. Herz A, Teschemacher H. Activities and sites of antinociceptive action of morphine-like analgesics. Adv Drug Res 1971;6:79-119. [ Links ]
2. Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science 1976;192:1357-1358. [ Links ]
3. Chen SR, Pan HL. Blocking mu opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res 2006;1081:119-125. [ Links ]
4. Ferreira SH, Nakamura M. Prostaglandin hyperalgesia: The peripheral analgesic activity of morphine, enkephalins and opioid antagonists. Prostaglandins 1979a;18:191-200. [ Links ]
5. Stein C, Gramsch C, Herz A. Intrinsic mechanisms of antinociception in inflammation: local opioid receptors and ß-endorphin. J Neurosci 1990;10:1292-1298. [ Links ]
6. Al-Sereiti MR, Abu-Amer KM, Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J Exp Biol 1999;37:124-130. [ Links ]
7. Romo de Vivar A. Productos Naturales de la Flora Mexicana. México: Limusa; 1985. [ Links ]
8. Martínez M. Las plantas medicinales de México. 6th Ed. Mexico City: Botas; 1989. [ Links ]
9. Argueta VA. Atlas de las plantas medicinales tradicionales mexicanas. México: Instituto Indigenista; 1994. [ Links ]
10. Hosseinzadeh H, Nourbakhsh M. Effect of Rosmarinus officinalis L. aerial parts extract on morphine withdrawal syndrome in mice. Phytother Res 2003;17:938-941. [ Links ]
11. Capasso A, Picacente S, Pizza C, Sorrentino L. Flavonoids reduce morphine withdrawal in vitro. J Pharm Pharmacol 1998;50:561-564. [ Links ]
12. Capasso A, Saturnino P, Simone FD, Aquino R. Flavonol glycosides from Aristeguietia discolor reduce morphine withdrawal in vitro. Phytother Res 2000;14:538-540. [ Links ]
13. Rajendran NN, Thirugnanasambandam P, Viswanathan S, Parvathavarthini S et al. Antinociceptive pattern of flavone and its mechanism as tested by formalin assay. Indian J Exp Biol 2000;38:182-185. [ Links ]
14. González-Trujano ME, Peña EI, Martínez AL, Guevara-Fefer P et al. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J Ethnopharmacol 2007;111:476-482. [ Links ]
15. Ferrada M. Etnografía, un enfoque para la investigación de Weblogs en Biblioteconomía y documentación. Biblios [edición online]. 2006;7(23) [9 páginas]. Accesible en URL: http://eprints.rclis.org/7395/:1/2005_19.pdf. Consultada el 11 de mayo de 2011. [ Links ]
16. Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother 1968;32:295-310. [ Links ]
17. Mayring P. Qualitative Content Analysis. Forum Qual Soc Res [edición online]. 2000;1(2) [10 páginas]. Accesible en URL: http://www.qualitative-research.nel/index.php/fqs/article/view/1089. Consultada el 6 de enero de 2011. [ Links ]
18. López-Muñoz FJ, Salazar LA, Castañeda-Hernández G, Villarreal JE. A new model to assess analgesic activity: pain-induced functional impairment in the rat (PIFIR). Drug Dev Res 1993;28:169-175. [ Links ]
19. Rowland M, Toser TN. Clinical Pharmacokinetics: concepts and applications. 2nd Ed. Philadelphia: Lea & Febiger; 1989. [ Links ]
20. Koster R, Anderson M, De Beer EJ. Acetic acid analgesic screening. Fed Proc 1959;18:418-420. [ Links ]
21. Abbott FV, Franklin KBJ, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain 1995;60:91-102. [ Links ]
22. Shibata M, Okhubo T, Takahashi H, Inoki R. Modified formalin test: Characteristics biphasic pain response. Pain 1989;38:346-352. [ Links ]
23. Ferreira SH. Inflammatory pain, prostaglandin hyperalgesia and the development of peripheral analgesics. Trends Pharmacol Sci 1981;2:183-186. [ Links ]
24. Jaffe JH, Martin WR. Opioid analgesics and antagonists. En: Gilman AG, Rall TW, Nies AS, Taylor P (eds.). Goodman and Gilman's the pharmacological basis of therapeutics. 9th Ed. New York: MacMillan; 1990. [ Links ]
25. Ferreira SH, Nakamura M. Prostaglandin hyperalgesia: a cAMP/Ca+2-dependent process. Prostaglandins 1979b;18:179-190. [ Links ]
26. Stein C, Millan MJ, Stüppenberg TS, Peter K et al. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther 1989;248:1269-1275. [ Links ]
27. Khoury GF, Chen ACN, Garland DE, Stein C. Intraarticular morphine, bupivacaine and morphine/bupivacaine for pain control after knee videoarthroscopy. Anesthesiology 1992;77:263-266. [ Links ]
28. Hong Y, Abbott FV. Peripheral opioid modulation of pain and inflammation in the formalin test. Eur J Pharmacol 1995;277:21-28. [ Links ]
29. Janson W, Stein C. Peripheral opioid analgesia. Curr Pharm Biotechnol 2003;4:270-274. [ Links ]
30. Rodrigues ARA, Duarte IDG. The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K+ channels. Br J Pharmacol 2000;129:110-114. [ Links ]
31. Roane DS, Boyd NE. Reduction of food intake and morphine analgesia by central glybenclamide. Pharmacol Biochem Behav 1993;46:205-207. [ Links ]
32. Stein C, Hassan AHS, Lehrberger K, Giefing J. Local analgesic effect of endogenous opioid peptides. Lancet 1993;342:321-324. [ Links ]
33. Hara K, Minami K, Sata T. The effects of tramadol and its metabolite on glycine, gamma-aminobutyric acid A, and N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Anesth Analg 2005;100:1400-1405. [ Links ]
34. Koga A, Fujita T, Totoki T, Kumamoto E. Tramadol produces outward currents by activating mu-opioid receptors in adult rat substantia gelatinosa neurones. Br J Pharmacol 2005;145:602-607. [ Links ]














