Services on Demand
Journal
Article
Indicators
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Salud mental
Print version ISSN 0185-3325
Salud Ment vol.34 n.4 México Jul./Aug. 2011
Artículo original
Clinical and electrophysiological effect of right and left repetitive transcranial magnetic stimulation in patients with major depressive disorder
Efecto clínico y electrofisiológico de la estimulación magnética transcraneal repetitiva derecha e izquierda en pacientes con transtorno depresivo mayor
María García–Anaya,1 Jorge González–Olvera,1 Josefina Ricardo–Garcell,2,3 Gabriela Armas,2 Edgar Miranda,2 Ernesto Reyes,2 Gloria Adelina Otero4
1 Clinical Research Division, National Institute of Psychiatry.
2 Clinical Services Division, National Institute of Psychiatry.
3 Neurobiology Institute, National Autonomous University of Mexico.
4 School of Medicine, State of Mexico Autonomous University.
Correspondence:
J. Ricardo–Garcell.
Neurodevelopment Research Unit Dr. Augusto Fernández Guardiola,
Neurobiology Institute, UNAM, Blvd. Juriquilla 3001, Querétaro 76230, México.
Phone: (525)5623–4207.
E–mail: oojrg@yahoo.com
Recibido primera versión: 22 de diciembre de 2010.
Segunda versión: 5 de abril de 2011.
Aceptado: 12 de abril de 2011.
ABSTRACTS
Major Depressive Disorder (MDD) is a common psychiatric disorder that represents one of the main public health problems worldwide. It has been projected that for 2020 it will be the second cause of disability–adjusted life years just below ischemic heart disease.
Quantitative electroencephalogram provides the opportunity to study cortical oscillatory activity across the different frequency bands. It constitutes an accessible tool to explore the clinical and neurophysiologic correlates underlying psychiatric disorders as well as the effect of diverse therapeutic options and the performance through cognitive tasks.
Repetitive transcranial magnetic stimulation is a technique that allows the stimulation of the cerebral cortex noninvasively, relatively painlessly and with fairly few side effects.
The vast majority of rTMS studies target left dorsolateral prefrontal cortex (DLPFC) based on imaging studies showing that left prefrontal cortex dysfunction is pathophysiologically linked to depression. However, there is some evidence implicating right PFC in the pathophysiology of depression.
Comparison of antidepressant efficacy of diverse stimulation frequencies is relevant since a main concern around rTMS is its potential to induce seizures; hence we consider that frequency of stimulation is an important aspect to be studied.
For this study we aimed to elucidate the clinical efficacy of rTMS comparing two groups of depressed patients stimulated over DLPFC, one over the left (at 5 Hz) and other over the right (at 1 Hz). We also meant to know if there were clinical and electroencephalographic differential long–term after–effects between those groups of treatment.
We included twenty right–handed patients with a DSM–IVR diagnosis of MDD. They were assigned into two groups of treatment. Group 1 received 5Hz rTMS over the left DLPFC. Group 2 received 1Hz rTMS over the right DLPFC.
We obtained two EEG measurements in order to analyze Z score of broad–band spectral parameters and cross–spectral.
No statistical differences among groups were found in response to treatment after weekly comparisons of clinimetric scores and significant differences between baseline and final assessment by HDRS, MADRS, BDI and HARS.
The major rTMS effect on EEG was observed in the group that received 1 Hz over the right DLPFC and no significant effects were observed for the group that received 5 Hz over the left DLPFC.
Our results propose that administration of 15 sessions on either left (5 Hz) or right (1 Hz) rTMS over DLPFC is sufficient to reach response to treatment, assessed by HDRS, MADRS, BDI and HARS in subjects with MDD. Moreover, in both cases rTMS was able to induce an equivalent antidepressant effect.
The major effect of rTMS on EEG was observed in the right 1 Hz rTMS group where changes were elicited mainly over frontal, central and temporal regions on alpha and particularly beta frequency bands. In a lesser extent for left 5 Hz rTMS group the main effect was observed on anterior regions for beta and particularly alpha frequency bands.
We believe it is pertinent to continue exploring the therapeutic potential of lower stimulation frequencies, for what further research including larger samples is still necessary to confirm these trends.
Key words: Major depressive disorder, rTMS, EEG, laterality, 5Hz, 1Hz.
RESUMEN
El trastorno depresivo mayor es una entidad psiquiátrica que representa uno de los principales problemas de salud pública a nivel mundial. Se ha proyectado que para el año 2020 será la segunda causa de discapacidad únicamente por debajo de la cardiopatía isquémica.
La utilización del electroencefalograma cuantitativo ofrece la oportunidad de estudiar la actividad oscilatoria cortical a través de las diferentes bandas de frecuencias.
Éste constituye una herramienta para explorar las características clínicas y neurofisiológicas que subyacen a los trastornos psiquiátricos, así como un instrumento para evaluar el efecto de diversas opciones terapéuticas y el desempeño de los sujetos durante la realización de tareas cognitivas.
La estimulación magnética transcraneal repetitiva (EMTr) es una técnica que permite la estimulación de la corteza cerebral de manera no invasiva, relativamente sin dolor y con pocos efectos secundarios.
Con base en los estudios de neuroimagen que vinculan la fisiopatología de la depresión con disfunción en la corteza prefrontal dorsolateral (CPFDL), la mayoría de las investigaciones se han enfocado en estimular dicha corteza en el hemisferio izquierdo.
No obstante, existen pruebas que implican a la corteza prefrontal derecha con la fisiopatología de la depresión.
La relevancia de comparar la eficacia antidepresiva de diversas frecuencias radica en el hecho de que un tema de interés particular alrededor de la EMTr es su potencial para inducir convulsiones, por ello consideramos que la exploración de las diversas frecuencias de estimulación con efecto terapéutico constituye un aspecto importante de estudio.
Para este trabajo nos propusimos determinar la eficacia antidepresiva de la EMTr comparando dos grupos de pacientes que fueron estimulados en la CPFDL derecha a 1 Hz o en la izquierda a 5 Hz. También buscamos dilucidar si existen diferencias clínicas y electroencefalográficas a largo plazo entre grupos de tratamiento.
Para este estudio se incluyeron 20 pacientes con diagnóstico de trastorno depresivo mayor de acuerdo con los criterios del DSM–I V. Los sujetos fueron asignados aleatoriamente a uno de dos grupos de tratamiento. Un grupo recibió EMTr sobre la CPFDL izquierda a 5Hz; el otro recibió estimulación a 1 Hz sobre la corteza contralateral.
Se obtuvieron dos registros electroencefalográficos, uno basal y otro final, con el fin de comparar las medidas espectrales de banda ancha y estrecha, pretratamiento y postratamiento. Se realizaron evaluaciones clinimétricas con las escalas de Hamilton para Depresión y Ansiedad, la escala de Depresión de Montgomery–Asberg y el Inventario de Beck. No encontramos diferencias significativas al comparar la respuesta a la EMTr entre ambos grupos. Los pacientes de ambos grupos presentaron respuesta a tratamiento (disminución de ≥50% de la sintomatología depresiva) medida por clinimetría.
El efecto más importante de la EMTr sobre el EEG se observó en el grupo de estimulación derecha a 1 Hz donde encontramos disminución postratamiento en los valores Z de banda estrecha alfa y beta, principalmente en regiones fronto–centro–temporales. Aunque en menor proporción, en el grupo de estimulación izquierda a 5 Hz encontramos incrementos significativos post EMTr, predominantemente en las bandas beta y alfa sobre todo en regiones anteriores. No se encontraron resultados significativos en el análisis de banda ancha.
Nuestros resultados sugieren que la administración de 15 sesiones de EMTr ya sea sobre la CPFDL derecha (1 Hz) o izquierda (5 Hz) es capaz de lograr respuesta antidepresiva.
Nuestros hallazgos electrofisiológicos sugieren que uno de los efectos a largo plazo de la EMTr es la reorganización de los circuitos neuronales implicados en la depresión.
Palabras clave: Trastorno depresivo mayor, EMTr, EEG, lateralidad, 5Hz, 1Hz.
INTRODUCTION
Major depressive disorder is a common psychiatric disorder that represents one of the main public health problems worldwide. It has been projected that for 2020 it will be the second cause of disability–adjusted life years just below ischemic heart disease.1 MDD has a lifetime prevalence estimated at 5.8% in women and 2.5% in men in Mexican population,2 while in the USA the lifetime prevalence of CIDI/DSM–IV MDD for adult population is 16.2%.3
In a real life scenario, these numbers represent a huge social and economical burden;4 therefore, research focused on successful diagnostic and therapeutic tools should be a priority of mental health systems.
In that sense, quantitative electroencephalogram (QEEG) provides the opportunity to study cortical oscillatory activity across the different frequency bands. It constitutes an accessible tool to explore the clinical and neurophysiologic correlates underlying psychiatric disorders as well as the effect of diverse therapeutic options and the performance through cognitive tasks.5
On the other hand, currently, the pharmacological approach still constitutes the first election treatment for MDD. However, it is not exempt from having a significant percentage of failures6 and an important amount of drop outs is explained in part by adverse and side effects of pharmacological antidepressants.7
Repetitive transcranial magnetic stimulation (rTMS) has emerged as an alternative to standard pharmacological therapies. Its antidepressant efficacy has been examined by several meta–analyses which in general have shown evidence of statistical clinical benefit that has importantly improved from the first studies8–10 until the most recent ones.11,12 These findings remarkably point out how the more the rTMS therapeutic potential is studied the better results are achieved. In relation to this, it has been demonstrated how the larger amount of total pulses is administered, the stronger antidepressant effect is achieved.13
Repetitive transcranial magnetic stimulation is a technique that allows the stimulation of the cerebral cortex noninvasively, relatively painlessly and with fairly few side effects.14 The focused magnetic field over the surface of the head induces electrical currents in the brain that as a result depolarizes the underlying superficial neurons.15,16 Even when rTMS effects vary depending on the stimulus frequency, intensity and duration, as well as on the number of sessions, it is generally accepted that rTMS involves a wide range of excitatory, inhibitory and plastic neuronal processes.17–22
One pathophysiological hypothesis of MDD stands on evidence from imaging studies showing that decreased left prefrontal cortex (PFC) function with respect to the right is linked to depression.23,24 On that basis, stimulation parameters chosen as treatment are selected from evidence of how high frequency rTMS (≥1 Hz) increases excitability below the underlying cortex just as low frequency rTMS (≤1 Hz) does it so.25
Thus, the vast majority of rTMS studies target left dorsolateral prefrontal cortex (DLPFC). However, there is some evidence implicating right PFC in the pathophysiology of depression. In a double–blind placebo controlled clinical trial no significant differences emerged when comparing high–frequency left rTMS with low–frequency right rTMS.26 In addition, there is a study where abnormal EEG sources (increase in current density) were observed in both hemispheres but with maximal inverse solution located mainly over right frontal lobe.27
Numerous authors26,28–35 have reported results of antidepressant effect for left high–frequency rTMS and few less for right low–frequency rTMS, but only one has compared these two stimulation strategies, finding no significant differences between left 10 Hz rTMS and right 1 Hz rTMS, both applied over DLPFC.26
Comparison of antidepressant efficacy of diverse stimulation frequencies is relevant since a main concern around rTMS is its potential to induce seizures;36 hence we consider that frequency of stimulation is an important aspect to be studied. In regards to that, a plausible rationale to target 5 Hz rTMS for antidepressant purposes, besides its promising effectiveness, is the fact that it has been poorly studied and also represents a lower risk for induced seizures besides of a more tolerable sensation for patients.
For this study we aimed to elucidate the clinical efficacy of rTMS comparing two groups of depressed patients stimulated over DLPFC, one over the left (at 5 Hz) and other over the right (at 1 Hz). We also meant to know if there were clinical and electroencephalographic differential long–term after–effects between those groups of treatment.
MATERIALS AND METHOD
Participants
Twenty right–handed patients (14 female, range 19–46 years, mean age=31.7, S.D.=7.38) with a DSM–IVR diagnosis of MDD37 were included in this study. Diagnosis was made by a psychiatrist using the Structured Clinical Interview for DSM–IVR (SCID–I). All patients scored higher than 18 points in the Hamilton Depression Rating Scale at baseline (mean score=28.3, S.D.=6.20).
Subjects with epilepsy, convulsive antecedents, drug abuse history, actual suicidal ideation or any axis I psychiatric disorder, excepting anxiety disorders were excluded. In order to reduce rTMS–induced seizure risk, the subjects showing epileptiform activity in the EEG recording were also excluded. In this sample, 15 patients were virgin to treatment. The remaining were medication–resistant patients; four of them were under a third–course and one under a second–course trial, having no response after at least eight weeks of treatment at maximum dose. Three of them were assigned to group 1 and two to group 2 (see below for assignment to group of treatment). A complete description of the study was given to every patient and afterwards an informed consent was signed by them on a form approved by the National Institute of Psychiatry (NIP) Research Ethics Committee. All subjects were recruited from the outpatient unit of the NIP in Mexico City.
Clinical assessment
A psychiatrist performed clinimetric assessment blinded to rTMS treatment by means of Hamilton Depression Rating Scale 21–item version (HDRS), Montgomery–Asberg Depression Rating Scale (MADRS), Beck Depression Inventory 21–item version (BDI) and Hamilton Anxiety Rating Scale (HARS).
Evaluation with those instruments was made at baseline and after sessions 5, 10 and 15. Response to treatment was considered as the reduction ≥50% in clinimetric scores.
Treatment
Repetitive Transcranial Magnetic Stimulation at 100% of motor threshold was administered using a Dantec MagPro rapid magnetic stimulator with a 50mm diameter figure–eight–shaped MC–B70 coil (Dantec; Skovlunde, Denmark). Motor threshold was determined at the beginning of every session using visual inspection method, assessing the motor response of the abductor of pollicis brevis muscle. The site of stimulation was defined as the region 5cm anterior to the point of maximum stimulation of the abductor pollicis brevis muscle.30,31,38,39 At every moment, the imaginary axis in the middle of the coil was held matching with the scalp parasagital line. Patients were randomly assigned into one of two groups of treatment. Group 1 received 5Hz rTMS over the left DLPFC (30 trains of 10 sec duration separated by 10sec; 22500 pulses per session). Group 2 received 1Hz rTMS over the right DLPFC (1 train of 15 min duration, 900 pulses per session). One daily session was administered from Monday to Friday until they all accomplished a total of 15.
EEG acquisition
We obtained two EEG studies, at enrollment and three days after the last rTMS session. EEG recordings were acquired with subjects awake with eyes closed, lying on a couch in a dimly lighted and not acoustically shielded room. The participants wore a polyester cap with surface electrodes distributed according to the 10–20 International System (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, Cz, Pz), using linked earlobes as reference. EOG was recorded from a supraorbital electrode and from an electrode on the extermal canthus of the left eye. Impedance levels were ≤5 KΩ The amplifier bandwidth was set between 0.5 and 30 Hz and the EEG was sampled every 5 ms using Trackwalker v 2.0 and Medicid IV system from NeuronicTM. A photostimulator was used in order to reject subjects where photosensitive epileptiform activity was present. Total recording time was from 20 to 30 min.
EEG analysis
Two expert electroencephalographists selected 24 independent artifact–free EEG segments of 2.56 sec by visual inspection. Segments were included only if both professionals agreed on the selection. EEG analysis was carried out off–line. Data sample spectral analysis was calculated by Fast Fourier Transform (FFT) and cross–segment averaging.
Cross–spectral matrices were calculated every 0.39 Hz, from 0.39 to 19.11 Hz, and Narrow Band Spectral Parameters (NBSP) were obtained (absolute power from 0.39 to 19.11 Hz). We also got Broad Band Spectral Parameters (BBSP: absolute power, relative power and total absolute power) for delta (1.5–19.0 Hz) (1.5–3.5 Hz), theta (3.5–7.5 Hz), alpha (7.5–12.5 Hz), and beta (12.5–19.0) bands. In order to calculate Z values on both NBSP and BBSP, each individual EEG recording was compared to normal subject parameters data bases for each frequency and electrode position.40,41 All this analysis was done using Neuronic Quantitative and Tomographic EEG v 6.0 software.
Data analysis
Repeated measures ANOVA was performed to estimate decreases in HDRS, MSDRS, BDI and HARS scores over sessions. U–Mann Whitney tests were performed to investigate differences among the two groups in clinical and demographical variables. Post–hoc Bonferroni tests were only calculated where a significant effect was found in the analysis of variance. Significance was set at α=0.05. This statistical analysis was computed with PASW Statistics 18 software.
To explore differences after left or right rTMS, two factors (1dep/1indep) univariate ANOVA for Z values of BBSP and NBSP was performed. Post–hoc paired–t tests were only calculated when a significant effect was found in the analysis of variance. Significance was set at α=0.005. For this analysis we utilized Neuronic Statistics v4.0 software.
RESULTS
Clinical and demographic characteristics were compared by Mann–Whitney U (α=0.05), no statistical differences at baseline among groups were found (table 1). We performed repeated measures ANOVA to estimate decreases in HDRS, MSDRS, BDI and HARS scores over sessions. Effects are presented in graphic 1. We observed response to treatment (reduction of ≥50% in clinical scores) for both groups of treatment. There was an overall effect group with significant differences between baseline and session 5 up to 15 assessed by HDRS (F=65.57; P= 0.001), MADRS (F=60.22; P=0.001) and HARS (F=58.79; P=0.001), and between baseline and session 10 and 15 assessed by BDI (F=46.84; P=0.001).

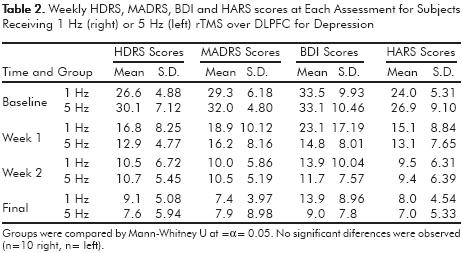
No statistical differences in response to treatment were observed when weekly comparisons of clinimetric scores among groups were performed using Mann–Whitney U test (α=0.05) (table 2).
After a two factor univariate ANOVA analysis carried out on BBSP Z values we found no significant results (α=0.05).
Regarding NBSP Z values, we got significances for interaction (α=0.005; DF=1.18; F=10.218). Subsequent paired–t tests showed a significant effect on pre–post differences for both groups of treatment. The major effect was observed in the right 1 Hz rTMS group where changes were elicited mainly over frontal, central and temporal regions on alpha and beta frequency bands, particularly on the last one; interestingly all t–scores for this group were positive (α=0.025) (figure 1). For the left 5 Hz rTMS group, significant pre–post changes were spread across all frequency bands and topographies although the main effect was observed on anterior regions for fast frequency bands. Remarkably and opposite to the right 1 Hz rTMS group, the majority of t–scores (17/21) were negative (α=0.025) (figure 2).

As side–effects, three subjects reported headaches along the first three days of treatment. Pain responded to treatment with aspirin. One of those patients also experienced facial paresthesia that spontaneously disappeared after the third session of treatment.
Since all subjects achieved response to treatment after 15 rTMS sessions, they all were invited to receive maintenance treatment with one session per week for eight weeks (data to be published later). After the follow up, all patients were referred to the NIP outpatient service for their further management. At the moment patients were referred, pharmacological treatment was left unchanged in subjects who formerly had it and no pharmacological adding was made on the remaining ones.
DISCUSSION
After slightly more than two decades of studies focused on rTMS therapeutic usages, an important amount of evidence points out the antidepressant effect of this technique.8–11,32,42 According to those findings, significant decreases on weekly assessed scores in HDRS, MADRS, BDI and HARS observed in our study show that both prefrontal left and right rTMS are able to reach response to treatment after 15 sessions.
Joint together with the study of Fitzgerald et al.,26 where right (1 Hz) and left (10 Hz) rTMS resulted in the same antidepressant effect, our results support that rTMS applied in either one or other PFC is equivalent in terms of efficacy. Unlike Fitzgerald's study, we administered 5 Hz rTMS for left PFC, which raises an important clinical consideration. Since the unpleasant feeling and induced seizure risk increases at higher stimulation frequencies,16 the relevance of our results lies in the need to highlight that lower frequencies applied into rTMS protocols could bring benefits to patients in terms of safety and comfort.
The study of rTMS by means of EEG or neuroimaging techniques has brought traces of its effect on brain connectivity. The number of reports combining QEEG and rTMS is not extensive35,43–48 and they all differ in terms of hypothesis, aims and stimulation parameters. Among them all, only three assessed the long–term effect of rTMS (considered as more than 1 session)35,45,49 and just two of them included depressed subjects.35,49
Griskova et al.45 administered, on separate days, one real and one sham rTMS session at 10 Hz over left DLPFC in healthy subjects. They found a significant increase in delta power over frontal, central and parietal regions after real stimulation.
Spronk et al.35 applied a scheme of sessions varying from 15 to 25 over the left DLPFC at 10 Hz, depending on the clinical course of each patient. Besides a highly significant clinical improvement evidenced by a decrease in BDI scores from baseline to end of treatment (p<0.001), they found a trend to increase in power of delta and alfa–2 bands, as well as a decrease in theta 2 power after treatment. In a preliminary analysis of 10 Hz rTMS effect, Funk et al.49 calculated a hemispheric ratio for each frequency band considering values pre–rTMS and post–rTMS every treatment session. For all bands, EEG power was stronger in the right hemisphere at baseline and throughout the course of treatment it tended to become stronger in left hemisphere until reaching a reversal of pre and post values, except for theta were the pre–post ratio reached equilibrium.
In contrast with the aforementioned studies, we did not find significant results in BBSP data when intra– and inter–group rTMS effect was compared. Nevertheless, NBSP analysis showed significant changes after rTMS treatment for both grups, mainly on frontal, central and temporal regions for alpha and beta frequency bands. Our results can hardly be compared to studies mentioned above mostly for three reasons: 1. they do not comprise the whole spectrum of every frequency band, 2. we explored different stimulation parameters and laterality and 3. none of the studies previously reported assessed rTMS long–term EEG after effects.
However, we observed an interesting pattern in relation to the effect of the frequency of stimulation used on each hemisphere. Remarkably, the direction of change of Z values was the same for all t scores for each group of treatment. With regard to right 1 Hz rTMS group, all t scores were positive for electrodes where significances were observed, which means an after treatment decrease in spectral power on alpha and beta frequency bands. As for the left 5Hz rTMS group, except for FZ and F8 (on delta frequency band) and T5 and T6 (on beta band), all t scores were negative, meaning an increase on spectral power for theta, alpha and partially beta frequency bands after treatment.
In consequence, those decreases and increases on spectral power could be explained according to this major hypothesis concerning the underlying neural mechanisms of rTMS that points at the excitatory effect of high stimulation frequencies (≥1 Hz), as well as the inhibitory effect of low stimulation frequencies (≤1 Hz).25,50–56
Additional supporting evidence for this rationale may stand on studies where rTMS at high44,57–59 and low57,60–64 frequency has been administered over either left or right prefrontal57,61,63 and primary motor cortex62,64 has induced either increased or decreased regional cerebral blood flow (rCBF) on ipsilateral and contralateral functional–related areas to the site of stimulation.
Based on all these data, it is feasible to expect an after–rTMS resultant cascade of effects throughout the brain which can also be measured at more locations,65 suggesting an underlying interhemispheric modulatory effect53 that probably entails the reorganization of neural circuits.
If such interhemispheric modulatory effect is so, a plausible explanation to the underlying neural mechanism related to changes observed in our study could be partially found in a way analogous to single–cell long–term depression and long–term potentiation56 acting over the stimulated hemisphere and perhaps giving place to those after–rTMS changes on spectral power.
CONCLUSION
To our knowledge, this is the first study to examine rTMS effect on EEG by NBSP, to explore the effect of 5 Hz rTMS on EEG and also to investigate long–term after effects of laterality of rTMS.
Clinically, our results demonstrate that administration of 15 sessions on either left (5 Hz) or right (1 Hz) rTMS over DLPFC is sufficient to reach response to treatment, assessed by HDRS, MADRS, BDI and HARS in subjects with MDD. Moreover, in both cases rTMS was able to induce an equivalent antidepressant effect.
Electrophysiologically, right 1 Hz rTMS was able to elicit more changes than left 5 Hz rTMS. Those changes were observed mainly on beta frequency band for right 1 Hz group and alpha frequency band for left 5 Hz group. Additionally, NBSP were more useful to reflect EEG changes induced by rTMS. These findings suggest a reorganization of neural circuits secondary to long–term stimulation.
Despite that the main limitation of this study was the small number of patients included, its strength laid in our contribution to clinical and electrophysiological characterization of long–term effects of right (1 Hz) and left (5 Hz) rTMS, as currently there is a lack of studies comparing laterality of stimulation, especially with lower frequencies, and the neurophysiological mechanisms underlying to this promising therapeutic tool.
Finally, it is pertinent to continue exploring the therapeutic potential of lower stimulation frequencies, for what further research including larger samples is still necessary to confirm these trends.
ACKNOWLEDGMENTS
We thank Ms. Lidice Galán from the Neurosciences Center of Cuba for statistical consultation and generous support during Maria's stay in La Habana. We also thank the National Council of Science and Technology (CONACYT) for the grant to María García–Anaya (CONACYT scholar: 162249).
REFERENCES
1. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden Disease Study. Lancet 1997;349: 1498–1504. [ Links ]
2. Belló M, Puentes–Rosas E, Medina–Mora M, Lozano R. Prevalence and diagnosis of depression in Mexico. Salud Pública México 2005;47:4–11. [ Links ]
3. Kessler RC, Berglund P, Demler O et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS–R). JAMA 2003;289:3095–3105. [ Links ]
4. Broadhead WE, Blazer DG, George LK, Tse CK. Depression, disability days, and days lost from work in a prospective epidemiologic survey. JAMA 1990;264:2524–2528. [ Links ]
5. Boutros NN, Berman RM, Hoffman R, Miano AP et al. Electroencephalogram and repetitive transcranial magnetic stimulation. Depression Anxiety 2000;12:166–2169. [ Links ]
6. Fava M. Diagnosis and definition of treatment–resistant depression. Biol Psychiatry 2003;53:649–659. [ Links ]
7. Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: a meta–analysis of comparative outcome studies. J Consult Clin Psychol 2008;76:909–922. [ Links ]
8. Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol 2002;5:73–103. [ Links ]
9. Holtzheimer PE, 3rd, Russo J, Avery DH. A meta–analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull 2001;35:149–169. [ Links ]
10. Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E et al. Repetitive transcranial magnetic stimulation for the treatment of depression. Systematic review and meta–analysis. Br J Psychiatry 2003;182:480–491. [ Links ]
11. Gross M, Nakamura L, Pascual–Leone A, Fregni F. Has repetitive transcranial magnetic stimulation (rTMS) treatment for depression improved? A systematic review and meta–analysis comparing the recent vs. the earlier rTMS studies. Acta Psychiatr Scand 2007;116:165–173. [ Links ]
12. Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta–analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry 2010;71:873–884. [ Links ]
13. Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry 2003;160:835–845. [ Links ]
14. Kobayashi M, Pascual–Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003;2:145–156. [ Links ]
15. George MS, Belmaker RH. Transcranial magnetic stimulation in clinical psychiatry. Washington, D.C.: American Psychiatric Press; 2000. [ Links ]
16. George MS, Lisanby SH, Sackeim HA. Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry 1999;56:300–311. [ Links ]
17. Chen R, Classen J, Gerloff C et al. Depression of motor cortex excitability by low–frequency transcranial magnetic stimulation. Neurology 1997; 48:1398–1403. [ Links ]
18. Hallett M. Transcranial magnetic stimulation: a primer. Neuron 2007;55:187–199. [ Links ]
19. Kujirai T, Caramia MD, Rothwell JC et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;471:501–519. [ Links ]
20. Pascual–Leone A, Davey N, Rothwell J, Wassermann EM. Handbook of transcranial magnetic stimulation. London: Hodder Arnold; 2002. [ Links ]
21. Pascual–Leone A, Valls–Sole J, Wassermann EM, Hallett M. Responses to rapid–rate transcranial magnetic stimulation of the human motor cortex. Brain 1994;117(Pt 4):847–858. [ Links ]
22. Wagner T, Valero–Cabre A, Pascual–Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng 2007;9:527–565. [ Links ]
23. Bench CJ, Frackowiak RS, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med 1995;25:247–261. [ Links ]
24. Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 2000;126:413–431. [ Links ]
25. Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nature Reviews Neurscience 2007;8:559–567. [ Links ]
26. Fitzgerald PB, Brown TL, Marston NA, Daskalakis et al. Transcranial magnetic stimulation in the treatment of depression: a double–blind, placebo–controlled trial. Archives General Psychiatry 2003;60:1002–1008. [ Links ]
27. Ricardo–Garcell J, Gonzalez–Olvera JJ, Miranda E et al. EEG sources in a group of patients with major depressive disorders. Int J Psychophysiol 2009;71:70–74. [ Links ]
28. Fitzgerald PB, Benitez J, De Castella A, Daskalakis ZJ et al. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment–resistant depression. Am J Psychiatry 2006;163:88–94. [ Links ]
29. Fitzgerald PB, Huntsman S, Gunewardene R, Kulkarni J et al. A randomized trial of low–frequency right–prefrontal–cortex transcranial magnetic stimulation as augmentation in treatment–resistant major depression. Int J Neuropsychopharmacol 2006;9:655–666. [ Links ]
30. George MS, Wassermann EM, Williams WA et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995;6:1853–1856. [ Links ]
31. George MW E, Kimbrell T, Little J, Williams W et al. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo–controlled crossover trial. American J Psychiatry 1997;154:1752–1756. [ Links ]
32. Kozel FA, George MS. Meta–analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002;8:270–275. [ Links ]
33. Lisanby SH, Husain MM, Rosenquist PB et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology 2009;34:522–534. [ Links ]
34. Padberg F, Haag C, Zwanzger P et al. Rapid and slow transcranial magnetic stimulation are equally effective in medication–resistant depression: a placebo–controlled study. In: CINP Abstracts 21st Congress; 1998, 1998; p. 103–st0306. [ Links ]
35. Spronk D, Arns M, Bootsma A, van Ruth R et al. Long–term effects of left frontal rTMS on EEG and ERPs in patients with depression. Clin EEG Neurosci 2008;39:118–124. [ Links ]
36. Pascual–Leone A, Valls–Sole J, Brasil–Neto JP, Cohen LG, Hallett M. Seizure induction and transcranial magnetic stimulation. Lancet 1992;339:997. [ Links ]
37. APA. Diagnostic and Statistic Manual of Mental Disorders. Fourth edition, text revision (DSM–IV–TR), 4ta. ed. Arlington VA: American Psychiatric Publishing, Inc.; 2000. [ Links ]
38. George MS, Wassermann EM, Williams WA et al. Changes in mood and hormone levels after rapid–rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci 1996;8:172–180. [ Links ]
39. Pascual–Leone A, Catala MD, Pascual–Leone Pascual A. Lateralized effect of rapid–rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology 1996;46:499–502. [ Links ]
40. Szava S, Valdés P, Biscay R et al. Hihg resolution quantitative EEG analysis. Brain Topography 1994;6:211–219. [ Links ]
41. Valdés P, Valdés M, Carballo JA et al. QEEG in a public health system. Brain Topography 1992;4:259–266. [ Links ]
42. Couturier JL. Efficacy of rapid–rate repetitive transcranial magnetic stimulation in the treatment of depression: a systematic review and meta–analysis. J Psychiatry Neurosci 2005;30:83–90. [ Links ]
43. Chen WH, Mima T, Siebner HR et al. Low–frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin Neurophysiol 2003;114:1628–1637. [ Links ]
44. Fuggetta G, Pavone EF, Fiaschi A, Manganotti P. Acute modulation of cortical oscillatory activities during short trains of high–frequency repetitive transcranial magnetic stimulation of the human motor cortex: a combined EEG and TMS study. Hum Brain Mapp 2008;29:1–13. [ Links ]
45. Griskova I, Ruksenas O, Dapsys K, Herpertz S et al. The effects of 10 Hz repetitive transcranial magnetic stimulation on resting EEG power spectrum in healthy subjects. Neurosci Lett 2007;419:162–167. [ Links ]
46. Hamidi M, Slagter HA, Tononi G, Postle BR. Repetitive Transcranial Magnetic Stimulation Affects behavior by Biasing Endogenous Cortical Oscillations. Front Integr Neurosci 2009;3:14. [ Links ]
47. Okamura H, Jing H, Takigawa M. EEG modification induced by repetitive transcranial magnetic stimulation. J Clin Neurophysiol 2001;18:318–325. [ Links ]
48. Strens LH, Oliviero A, Bloem BR, Gerschlager W et al. The effects of subthreshold 1 Hz repetitive TMS on cortico–cortical and interhemispheric coherence. Clin Neurophysiol 2002;113:1279–1285. [ Links ]
49. Funk AP, George MS. Prefrontal EEG asymmetry as a potential biomarker of antidepressant treatment response with transcranial magnetic stimulation (TMS): a case series. Clin EEG Neurosci 2008;39:125–130. [ Links ]
50. Fierro B, Brighina F, Vitello G et al. Modulatory effects of low– and high–frequency repetitive transcranial magnetic stimulation on visual cortex of healthy subjects undergoing light deprivation. J Physiology 2005;565:659–665. [ Links ]
51. Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J Neuroengineering Rehabilitation 2009;6:7. [ Links ]
52. Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms Progress Neurobiology 2010;93:59–98. [ Links ]
53. Salerno A, Georgesco M. Interhemispheric facilitation and inhibition studies in man with double magnetic stimulation. Electroencephalogr Clin Neurophysiol 1996;101:395–403. [ Links ]
54. Suppa A, Bologna M, Gilio F, Lorenzano C et al. Preconditioning repetitive transcranial magnetic stimulation of premotor cortez can reduce but not enhance short–term facilitation of primary motor cortex. J Neurophysiology 2008;99:564–570. [ Links ]
55. Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clinical Neurophysiology 2002;19:322–343. [ Links ]
56. Wang H, Wang X, SCheich H. LTD and LTP induced by transcranial magnetic stimulation in auditory cortex. Neuroreport 1996;7:521–525. [ Links ]
57. Loo CK, Sachdev PS, Haindl W et al. High (15 Hz) and low (1 Hz) frequency transcranial magnetic stimulation have different acute effects on regional cerebral blood flow in depressed patients. Psychological Medicine 2003;33:997–1006. [ Links ]
58. Nadeau SE, McCoy KJ, Crucian GP et al. Cerebral blood flow changes in depressed patients after treatment with repetitive transcranial magnetic stimulation: evidence of individual variability. Neuropsychiatry Neuropsychol Behav Neurol 2002;15:159–175. [ Links ]
59. Speer AM, Benson BE, Kimbrell TK et al. Opposite effects of high and low frequency rTMS on mood in depressed patients: Relationship to baseline cerebral activity on PET. J Affective Disorders 2009;115:386–394. [ Links ]
60. Eisenegger C, Treyer V, Knoch D. Time–course of «off–line» prefrontal rTMS effects – a PET study. Neuroimage 2008;42:379–384. [ Links ]
61. Kito S, Fujita K, Koga F. Regional cerebral blood flow changes after low–frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment–resistant depression. Neurpsychobiology 2008;58:29–36. [ Links ]
62. Okabe S, Hanajima R, Ohnishi T et al. Functional connectivity revealed by single–photon emission computed tomography (SPECT) during repetitive transcranial magnetic stimulation (rTMS) of the motor cortex. Clinical Neurophysiology 2003;114:450–457. [ Links ]
63. Speer AM, Willis MW, Herscovitch P, Daube–Witherspoon M et al. Intensity–dependent regional cerebral blood flow during 1–Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H2 15Opositron emission tomography: I. Effects of primary motor cortex rTMS. Biol Psychiatry 2003;54:818–825. [ Links ]
64. Speer AM, Willis MW, Herscovitch P et al. Intensity–dependent regional cerebral blood flow during 1–Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H215O positron emission tomography: I. Effects of primary motor cortex rTMS. Biol Psychiatry 2003;54:818–825. [ Links ]
65. Fitzgerald PB, Sritharan A, Daskalakis ZJ, De Castella AR et al. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clinical Psychopharmacology 2007;27:488–492. [ Links ]














