Services on Demand
Journal
Article
Indicators
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Salud mental
Print version ISSN 0185-3325
Salud Ment vol.32 n.2 México Mar./Apr. 2009
Artículo original
Systemic amantadine diminishes inflammatory and neuropathic nociception in the rat
La amantadina sistémica disminuye la nocicepción inflamatoria y neuropática en la rata
Ulises Coffeen,1,3 Alberto López–Ávila,2,4 Francisco Pellicer1
1 Laboratorio de Neurofisiología Integrativa, Dirección de Investigaciones en Neurociencias, Instituto Nacional de Psiquiatría Ramón de la Fuente.
2 Laboratorio de Neurofisiología de la Percepción, Instituto Nacional de Psiquiatría Ramón de la Fuente.
3 Departamento de Farmacobiología, Cinvestav Sede Sur. México, D.F.
4 Departamento de Atención a la Salud. Universidad Autónoma Metropolitana. México, D.F.
*Correspondence:
Alberto López–Avila.
Instituto Nacional de Psiquiatría Ramón de la Fuente,
Calz. México–Xochimilco 101,
San Lorenzo Huipulco,Tlalpan,
14370, México, D.F.
Fax: (5255) 5655 99 80.
Email: alopez@imp.edu.mx
Received first version: 14–03–2008
Second version: 30–09–2008
Accepted for publication: 13–11 –2008.
Abstract
The role of dopamine as a possible central inhibitory mediator of pain processes has been demonstrated. The administration of L–Dopa diminishes pain perception in humans as well as the response to nociceptive stimuli in animals. Also, the intracerebral microinjection of dopamine in inflammatory and neuropathic pain models (formalin test and deafferentation, respectively) reduces nociceptive response. In this sense, the selective activation of dopamine D2 receptors and the blockade of D1 in the insular cortex and spinal cord diminish nociception. Furthermore, the microinjection of dopamine or amantadine (dopamine releaser) in the anterior cingulate cortex (ACC) also reduces chronic nociception.
The efficacy of amantadine has been tested in the treatment of neuropathic pain, even when given as a single dose. There is evidence of the role of amantadine as a releaser of dopamine (DA) calcium channel (N type) dependent in the striatum as well as a low affinity non–competitive antagonist of blockade/non–blockade kinetics of the NMDA receptor. This compound has also been described as a DA agonist and an inhibitor of its reuptake. With this background, we decided to test if the effects of systemically given amantadine related to acute nociception, hyperalgesia and neuropathic nociception can be reverted by a dopaminergic blockade (using haloperidol) within the ACC.
The experiments were conducted in agreement with the Ethics Committee of the International Association for the Study of Pain and the approval of the Projects Commission of the Instituto Nacional de Psiquiatría Ramón de la Fuente (INPRF).
Male Wistar rats (250–300 g) were housed in the INPRF. During the observation period, the animals were maintained in transparent acrylic individual cages with light–dark cycles of 12 X 12 h, with feeding and hydration ad libitum.
For all surgical procedures, rats were anaesthetised with halothane 2% mixed with O2 98%.
In order to test the dopaminergic effect of amantadine within the ACC in nociception, we used the hyperalgesia model as well as a neuropathic nociception model induced by denervation. In the model of hyperalgesia, carrageenan was injected in the plantar region (50 µl at 1%), followed by a thermonociception test in which paw withdrawal latency was measured. In the neuropathic nociception model, the right sciatic nerve was denervated and chronic nociception was measured as autotomy behaviour.
Moreover, in another series of experiments, haloperidol (3 mg/ 200 nl) was microinjected into the ACC before the induction of hyperalgesia and neuropathic nociception. Amantadine was then injected (90 mg/kg i.p.) and the behavioural development was observed in both models.
Systemic amantadine was able to reduce both neuropathic nociception and hyperalgesia. Also, the results show, on the one hand, that haloperidol significantly decreases the antinociceptive effect of amantadine measured as paw withdrawal latency. On the other hand, amantadine can reduce nociception when administered systemically and, according to what has been published previously, when administered directly into the ACC.
Our results show that amantadine is effective in diminishing hyperalgesia and nociception induced by deafferentation. This suggests that amantadine can be a therapeutic alternative for the treatment and prevention of neuropathic pain such as phantom limb pain or pain due to deafferentation, among others.
Key words: Amantadine, dopamine, NMDA, chronic pain, anterior cingulate cortex.
Resumen
Se ha demostrado el papel de la dopamina como posible mediador inhibitorio central de procesos dolorosos. La administración de L–dopa disminuye la percepción de dolor en los seres humanos, así como la respuesta ante estímulos nociceptivos en los animales. Además, la microinyección intracerebral de dopamina en modelos de dolor inflamatorio y neuropático (prueba de formalina y deaferentación) reduce la respuesta nociceptiva. En este sentido, la activación selectiva de los receptores dopaminérgicos D2 y el bloqueo de los receptores D1 en la corteza insular y la médula espinal disminuyen la nocicepción. La microinyección de dopamina o de amantadina (liberador dopaminérgico) en la corteza anterior del cíngulo (CAC) reduce también la nocicepción crónica.
Se ha probado la eficacia de la amantadina en el tratamiento del dolor neuropático, incluso cuando se administra en una sola dosis. También se ha demostrado el papel de la amantadina como liberador de dopamina dependiente del canal del calcio (tipo N) en el estriado, así como el de antagonista no competitivo de baja afinidad de cinética de bloqueo y desbloqueo rápido del receptor de NMDA. Este compuesto también se ha descrito como un agonista de dopamina e inhibidor de su recaptura.
Con estos antecedentes decidimos probar si los efectos de la amantadina sistémica relacionados con la nocicepción aguda, la hiperalgesia y la nocicepción neuropática, pueden ser revertidos por el bloqueo dopaminérgico mediante la micro inyección de haloperidol en la corteza anterior del cíngulo.
Los experimentos se realizaron de acuerdo con las normas del Comité de Ética de la Asociación Internacional para el Estudio del Dolor y con la aprobación de la Comisión de Proyectos del Instituto Nacional de Psiquiatría Ramón de Fuente (INPRF).
Se utilizaron ratas Wistar macho (250–300 g) mantenidas en el bioterio del INPRF. Durante el periodo de observación, los animales se mantuvieron en jaulas individuales de acrílico transparente, con ciclos de luz–oscuridad de 12 X 12 h, con alimentación e hidratación ad libitum.
Para todos los procedimientos quirúrgicos, las ratas se anestesiaron con halotano al 2%, mezclado con 98% de O2.
Para probar el efecto dopaminérgico de la amantadina en la nocicepción en la corteza anterior del cíngulo, utilizamos un modelo de hiperalgesia y un modelo de nocicepción neuropática inducida por denervación. En el modelo de hiperalgesia, se inyectó carragenina en la región plantar (50 µl al 1%), seguida por una prueba de termonocicepción para posteriormente medir la latencia de retiro de la pata. En el modelo de nocicepción neuropática, se denervó el ciático derecho y se midió la nocicepción crónica mediante la conducta de autotomía.
Asimismo, en otra serie experimental se microinyectó haloperidol (3mg/200nl) en la CAC antes de la inducción de la hiperalgesia y de la nocicepción neuropática, y posteriormente se inyectó amantadina (90 mg/kg i.p.) y se observó el desarrollo conductual en ambos modelos.
La administración sistémica de amantadina logró reducir tanto la nocicepción neuropática como la hiperalgesia. Además, los resultados muestran, por un lado, que el haloperidol disminuye significativamente el efecto antinociceptivo de la amantadina medido como la latencia de retiro de la pata. Por otro, la amantadina puede reducir la nocicepción cuando se administra sistémicamente y, según lo publicado previamente, directamente en la CAC.
Nuestros resultados muestran que la amantadina es efectiva en la reducción de la hiperalgesia y la nocicepción por deaferentación. Este hecho sitúa a la amantadina como una alternativa terapéutica para el tratamiento y prevención del dolor neuropático, como el miembro fantasma doloroso o el dolor por deaferentación.
Palabras clave: Amantadina, dopamina, NMDA, dolor crónico, corteza anterior del cíngulo.
INTRODUCTION
The role of dopamine as a possible central inhibitory mediator of painful processes has been documented.1–3 The administration of L–dopa diminishes pain perception in humans4 as well as behavioural nociceptive responses in animals.5 Additionally, intracerebral microinjection of dopamine in inflammatory and neuropathic pain models (formalin assay and deafferentation) reduces the nociceptive response.6–8 In this sense, our group has shown that the lesion and electrical stimulation of the ventral tegmental area (A10 dopaminergic nucleus) diminish persistent nociceptive behaviour in the rat.9 Moreover, the selective activation of dopamine D2 and blockade of D1 receptors in the insular cortex and spinal cord diminish nociception.10,11 Furthermore, the microinjection of dopamine or the dopamine releaser amantadine into the anterior cingulate cortex (ACC) also reduces chronic nociception.12
The efficacy of amantadine has been tested in the treatment of chronic neuropathic pain, even when administered systemically in a single dose.13,14 Furthermore, a microdyalisis study in rats has demonstrated that amantadine has an effective central concentration 20 min after intraperitoneal administration.15 Also, there is evidence of the role of amantadine as calcium channel (Ntype) dependent dopamine (DA) releaser in the striatum,15 as well as a low affinity non–competitive antagonist of rapid block and unblock kinetics of the NMDA receptor.16,17 This compound has also been described as a DA agonist18,19 and reuptake inhibitor.20,21
With this framework, we decided to test if the systemic effects of amantadine related to acute nociception, inflammatory hyperalgesia and neuropathic nociception can be reverted with a dopaminergic blockade (haloperidol) within the ACC.
METHODS
The experiments were conducted in agreement with the Ethics Committee regulations of the International Association for the Study of Pain22 and with the approval of the Projects Commission of the Instituto Nacional de Psiquiatría Ramón de la Fuente (INPRF).
Male Wistar rats (250–300 g) were raised, housed and maintained in the INPRF. During the observation period, the animals were kept in transparent acrylic individual cages with light–dark cycles of 12 x 12 h, and with ad libitum feeding and hydration.
For all surgical procedures, the rats were anaesthetized with halothane 2% mixed with 98% O2.
Thermonociceptive testing
After 7 days of habituation to the testing equipment and personnel (10min/day), rats were weighed and properly marked. Thermonociceptive response was measured in a Plantar Test Apparatus (Ugo Basile mod.7370). Rats were placed in a clear plastic chamber (17cm x 22cm x 13cm) with a glass floor. After acclimation, the radiant heat source was positioned under the glass floor beneath the right hind paw. Paw withdrawal latency (PWL) was determined to the nearest 0.1 second by the electronic clock of the device for acute and inflammatory hyperalgesia groups. All groups had the thermonociceptive test performed 1 and 24 h after i.p. injection.
Inflammatory hyperalgesia
Groups: control (n=25) only with thermonociceptive testing (vide supra). Vehicle (n=15) injected with saline 1 ml/kg i.p. after the intraplantar injection of 50µl of saline (0.9% NaCl). Carrageenan (Car) (n=15), injected with saline 1ml/ kg i.p. after the intraplantar inflammatory process (vide infra). Car + amantadine groups (n=15 each), injected with amantadine (adamantanamine hydrochloride, Sigma Chemical Co., St. Louis MO, USA) 3, 30 and 90mg/kg i.p., respectively, after the intraplantar inflammatory process. Hal ACC + amantadine group (n=15), microinjected with haloperidol (haloperidol, Sigma Chemical Co., St. Louis MO, USA) (3µg/200nl) directly into the ACC followed by the intraplantar inflammatory process and a i.p. injection of amantadine 90mg/kg i.p.
Induction of the intraplantar inflammatory process
An inflammatory process was induced by an intraplantar infiltration of carrageenan lambda (Car: 50µl at 1% saline solution, Sigma Chemical Co., St. Louis MO, USA) in the rat's right hindpaw.
Acute nociception
This experiment evaluated the action of amantadine on the acute thermonociceptive response only. The same procedure was used as in the inflammatory hyperalgesia experiment, except for the induction of the intraplantar inflammatory process and its vehicle, which consisted of an i.p. injection of saline solution only.
Statistical analysis for acute nociception and inflammatory hyperalgesia experiments
In order to determine differences in paw withdrawal latencies per test (1 and 24h) between groups, analysis of variance (ANOVA) and a Tukey's test as a post hoc were applied. A repeated measures ANOVA analysis was used to determine differences in paw withdrawal latency along time between groups.
Neuropathic nociception
Groups: vehicle (n=14) with the induction of an inflammatory process (vide infra) 30min previous to a right sciatic denervation described by Wall et al. (1979), followed by a single 1 ml/kg i.p. saline injection. Amantadine groups (n=10 each), with the induction of an inflammatory process 30 min previous to denervation, followed by the injection of amantadine, 3, 30 and 90mg/kg i.p., respectively, for each group. Haloperidol plus amantadine 90 mg/kg group, with the microinjection of haloperidol (3µg/200nl) in the ACC, followed by the same procedure as the previous groups in this section.
Inflammatory process
In order to enhance autotomy behaviour (AB), we induced an inflammatory process, 30min previous to ipsilateral denervation, by intraplantar infiltration of 250µl of carrageenan lambda in 1% saline solution in the rat's right hind paw.23
Autotomy behaviour (AB)
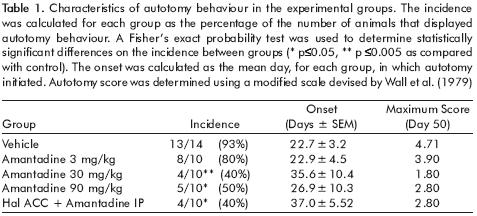
Daily autotomy scores were recorded for 50 days, using a modified scale devised by Wall et al.24 This scale gives the following scores: 1 point for the removal of one or more nails; 1 additional point for each distal half digit attacked and a further point for each proximal half digit attacked. If the distal or proximal half of the paw was attacked, one additional point was added for each one. We also recorded the autotomy onset time and the number of animals that presented autotomy behaviour by group (incidence). On day 50, the animals were sacrificed by an overdose of pentobarbital and denervation was verified.
Statistical analysis for neuropathic nociception experiments
In order to analyze autotomy evolution between groups, we used a repeated measures ANOVA test. Autotomy onset differences were established by a Student t–test. For the autotomy incidence, a Fisher exact probability test was used. Differences between treatments per day were analyzed using Tukey's as a post hoc. The significance for all statistical analysis was established at p<0.05.
RESULTS
Inflammatory hyperalgesia
This experiment tested the thermonociceptive response measured as PWL, 1 and 24h after the induction of an inflammatory process with different doses of amantadine and if this effect could be reverted by a dopaminergic blockade with haloperidol directly in the ACC.
The results show that a decreased PWL induced by previous inflammation (carrageenan infiltration in the hind paw), followed by a thermonociceptive stimulus an hour later, is subject of being reverted by systemic amantadine (3, 30 and 90mg/kg, i.p.), in a dose dependent manner. The antihyperalgesic effect of amantadine is reverted by a dopaminergic blockade with haloperidol (3µg/200nl) microinjected in the ACC.
PWL was measured in seconds for all groups. The Car group (4.54 ± 0.39) was significantly diminished as compared to the control, vehicle and amantadine 90mg/kg groups (6.22 ± 0.43). The Car + amantadine 3mg/kg (4.76 ± 0.32) group showed a significantly diminished latency compared with the control group (p=0.016) and vehicle (p=0.014). The Car + amantadine 30mg/kg (5.72 ± 0.48) and 90mg/kg groups showed similar PWL when compared to control and vehicle. The Car + amantadine groups presented a dose–dependent antinociceptive tendency, with a statistical difference in the 90mg/kg group as compared to the Car + amantadine 3mg/kg group (p=0.045). In the group in which a microinjection of haloperidol was applied prior to the administration of i.p. amantadine, there was a significant difference when compared to the Car + amantadine 90mg/ kg, vehicle and control groups (p<0.05) (Figure 1A).

There were no statistical significant differences between the groups tested 24h after the induction of the inflammatory process nor in the paw withdrawal evolution 1 vs 24h post inflammatory process (Figure 1B).
Acute nociception
This experiment evaluated the action of amantadine on acute thermonociceptive response.
There were no statistically significant differences in PWL between groups when tested both after 1h and 24h of the inflammatory process (data not shown).
Neuropathic nociception
This experiment evaluated the action of single i.p. doses of amantadine on the development of a neuropathic nociceptive process (autotomy) and if its effect could be reverted by the single microinjection of haloperidol in the ACC.
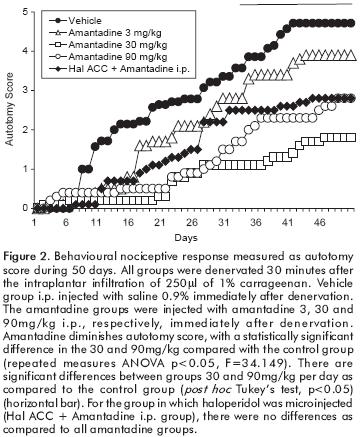
A single i.p. injection of amantadine diminishes autotomy behaviour, showing statistically significant differences in the amantadine 30 and 90mg/kg groups compared with vehicle (repeated measures ANOVA p<0.05, F=34.149). Significant differences were obtained in the incidence of 30 and 90mg/kg groups as compared with vehicle (Fisher exact test, p<0.05). No statistical differences were established in the onset of autotomy (table 1, figure 2). For the group in which haloperidol was microinjected, there were no differences as compared to all amantadine groups.
DISCUSSION
Our results show that the intraperitoneal administration of amantadine (dopaminergic agonist and NMDA antagonist) diminishes, in a dose–dependent manner, the nociceptive response in inflammatory nociception. The results support the fact that the mesolimbic dopaminergic system plays a key role in the inhibition of inflammatory and neuropathic nociception and it is subject of being enhanced by systemic amantadine whose effect can be reverted by a dopaminergic blockade in the ACC.
Inflammatory hyperalgesia
Carrageenan injection into soft tissues has been used as a chemically induced chronic pain model,25,26 which triggers a mild inflammatory process with hyperalgesia.27 The infiltration of 50µl 1% Car followed 1h later by a thermo–nociceptive test causes a decrease in the withdrawal latency reflex with respect to the control and the vehicle (figure 1A). This phenomenon is called hyperalgesic behaviour and it is lost 24h later (figure 1B).
The systemic administration of amantadine is able to partially (30mg/kg) or totally (90mg/kg) revert the hyperalgesic behaviour in a dose–dependent manner (figure 1A). This antihyperalgesic effect of systemic amantadine can be diminished by the microinjection of haloperidol within the ACC. This remarkable effect of a dopaminergic blockade in a precise locus in the CNS is highly suggestive of the possible site of action where the dopaminergic release produced by amantadine exerts its function. Also, it supports the relationship between the ACC and nociceptive modulation.28–33
Acute nociception
In the groups in which acute thermonociception was used, amantadine elicited no antinociceptive effect. Due to the fact that acute nociception is a process mainly integrated in the spinal cord34 and that the dopaminergic antinociceptive effects have been mainly related to cortical areas associated with tonic and chronic nociceptive process,1,11,12 it is not surprising that amantadine has no effect in reverting them.
Neuropathic nociception
It has been vastly documented that nervous transection in the rat produces autotomy24,35 associated to a process of chronic nociception.25 In addition, the nociceptive stimulus previous to denervation notably increases autotomy.36 Activation of receptors and related pathways with nociceptive stimuli previous to denervation within minutes produces central changes related to sensitization processes that increase the level of nociception or dysesthesia.23 In the present study, we used the infiltration of carrageenan into the paw as nociceptive stimulus previous to denervation, which increases autotomy behaviour. This model seems adequate to demonstrate the effect of amantadine, since it mimics similar human pathologies associated to a painful phantom limb and to pain by denervation, among others.
Our results show that a single dose of amantadine produces a decrease in autotomy behaviour. Also, the 30mg/kg dose may have reached a pharmacological ceiling effect, since the 90mg/kg dose did not produce any further antinociceptive effect.
Considering the results in the inflammatory model, we decided to test if a dopaminergic blockade with haloperidol in the ACC could revert the antihyperalgesic effect of amantadine on autotomy behaviour. Nevertheless, we did not find this effect on autotomy behaviour in amantadine (90mg/kg) treated rats. This neuropathic pain model is complex and involves several neurotransmission systems. Besides, amantadine acts both as a dopamine releaser and an NMDA antagonist. We do not have elements to discard this or other effects and further studies are required in order to explain this result.
CONCLUSION
Our results show that amantadine is effective in the reduction of nociception by deafferentation and inflammatory hyperalgesia, and therefore suggest it as a therapeutic alternative in the treatment and prevention of neuropathic chronic pain such as a painful phantom limb.
ACKNOWLEDGMENTS
This research was partially supported by CONACyT FP–P62433. Scholarship CONACyT for UC 185496. CONACyT ALA–61027. INP–RFM–110 and Miguel Alemán Foundation.
REFERENCES
1. Saade NE, Atweh SF, Bahuth NB, Jabbur SJ. Augmentation of nociceptive reflexes and chronic deafferentation pain by chemical lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain Res 1997;751:1–12. [ Links ]
2. Magnusson JE, Fisher K. The involvement of dopamine in nociception: the role of D1 and D2 receptors in the dorsolateral striatum. Brain Res 2000;855:260–6. [ Links ]
3. Wood PB. Mesolimbic dopaminergic mechanisms and pain control. Pain 2006;120:230–4. [ Links ]
4. Ertas M, Sagduyu A, Arac N, Uludag B, Ertekin C. Use of levodopa to relieve pain from painful symmetrical diabetic polyneuropathy. Pain 1998;75:257–9. [ Links ]
5. Paalzow GH. L–dopa induces opposing effects on pain in intact rats: (–)–sulpiride, SCH 23390 or alpha–methyl–DL–p–tyrosine methylester hydrochloride reveals profound hyperalgesia in large antinociceptive doses. J Pharmacol Exp Ther 1992;263:470–9. [ Links ]
6. Franklin KB. Analgesia and the neural substrate of reward. Neurosci Biobehav Rev 1989;13:149–54. [ Links ]
7. Lyerly MA, Rossitch E Jr., Ovelmen–Levitt J, Nashold BS Jr. The deafferentation syndrome in the rat: effects of intraventricular apomorphine. Exp Neurol 1988;100:188–202. [ Links ]
8. Morgan MJ, Franklin KB. Dopamine receptor subtypes and formalin test analgesia. Pharmacol Biochem Behav 1991;40: 317–22. [ Links ]
9. Sotres–Bayon F, Torres–Lopez E, Lopez–Avila A, Del Angel R, Pellicer F. Lesion and electrical stimulation of the ventral tegmental area modify persistent nociceptive behavior in the rat. Brain Res 2001;898:342–9. [ Links ]
10. Gao X, Zhang Y, Wu G. Effects of dopaminergic agents on carrageenan hyperalgesia after intrathecal administration to rats. Eur J Pharmacol 2001;418:73–7. [ Links ]
11. Coffeen U, Lopez–Avila A, Ortega–Legaspi JM, Del Angel R, Lopez–Munoz FJ et al. Dopamine receptors in the anterior insular cortex modulate long–term nociception in the rat. Eur J Pain 2008;12:535–43. [ Links ]
12. Lopez–Avila A, Coffeen U, Ortega–Legaspi JM, Del Angel R, Pellicer F. Dopamine and NMDA systems modulate long–term nociception in the rat anterior cingulate cortex. Pain 2004; 111:136–43. [ Links ]
13. Pud D, Eisenberg E, Spitzer A, Adler R, Fried G et al. The NMDA receptor antagonist amantadine reduces surgical neuropathic pain in cancer patients: a double blind, randomized, placebo controlled trial. Pain 1998;75:349–54. [ Links ]
14. Eisenberg E, Pud D. Can patients with chronic neuropathic pain be cured by acute administration of the NMDA receptor antagonist amantadine? Pain 1998;74:337–9. [ Links ]
15. Takahashi T, Yamashita H, Zhang YX, Nakamura S. Inhibitory effect of MK–801 on amantadine–induced dopamine release in the rat striatum. Brain Res Bull 1996;41:363–7. [ Links ]
16. Kornhuber J, Quack G, Danysz W, Jellinger K, Danielczyk W et al. Therapeutic brain concentration of the NMDA receptor antagonist amantadine. Neuropharmacol 1995;34:713–21. [ Links ]
17. Kornhuber J, Bormann J, Hubers M, Rusche K, Riederer P. Effects of the 1–amino–adamantanes at the MK–801–binding site of the NMDA–receptor–gated ion channel: a human postmortem brain study. Eur J Pharmacol 1991;206:297–300. [ Links ]
18. Karobath M, Leitich H. Antipsychotic drugs and dopamine–stimulated adenylate cyclase prepared from corpus striatum of rat brain. Proc Nat Acad Sci 1974;71:2915–8. [ Links ]
19. Vernier VG, Harmon JB, Stump JM, Lynes TE, Marvel JP et al. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol Appl Pharmacol 1969;15: 642–65. [ Links ]
20. Fletcher EA, Redfern PH. The effect of amantadine on the uptake of dopamine and noradrenaline by rat brain homogenates. J Pharm Pharmacol 1970;22:957–9. [ Links ]
21. Heikkila RE, Cohen G. Evaluation of amantadine as a releasing agent or uptake blocker for H 3 –dopamine in rat brain slices. Eur J Pharmacol 1972;20:156–60. [ Links ]
22. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983;16:109–10. [ Links ]
23. Lopez–Avila A, Sotres–Bayon F, Del Angel R, Pellicer F. Time span between nociceptive stimulus and denervation modifies autotomy behavior in the rat. Analgesia 1999;4:475–78. [ Links ]
24. Wall PD, Scadding JW, Tomkiewicz MM. The production and prevention of experimental anesthesia dolorosa. Pain 1979;6:175–82. [ Links ]
25. Albe–Fessard D, Giamberardino MA, Rampin O. Comparison of different animal models of chronic pain. In: S. L (ed.). Ad Pain Res Ther. New York: Raven Press; 1990; p. 11–27. [ Links ]
26. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988;32:77–88. [ Links ]
27. Lopez–Avila A, Rodriguez–Manzo G, Coffeen U, Del Angel R, Pellicer F. Self–injury behaviour induced by intraplantar carrageenan infiltration: a model of tonic nociception. Brain Res Prot 2004;13:37–44. [ Links ]
28. Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Nat Acad Sci 2001;98:8077–82. [ Links ]
29. Pellicer F, Torres–López E, Sotres–Bayón F, López–Avila A, Coffeen U et al. The affective and cognitive dimension of nociception in an animal model: The role of the anterior cingulate cortex. In: Lucas A (ed.). Prog Pain Res; 2006. [ Links ]
30. Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain 1999;79:105–11. [ Links ]
31. Treede RD. Assessment of pain as an emotion in animals and in humans [comment]. Exp Neurol 2006;197:1–3. [ Links ]
32. Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000;288:1769–72. [ Links ]
33. Zhuo M. Neuronal mechanism for neuropathic pain. Molecular Pain 2007;3:14. [ Links ]
34. Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord. Plenum Press; 1991. [ Links ]
35. Basbaum AI. Effects of central lesions on disorders produced by multiple dorsal rhizotomy in rats. Exp Neurol 1974;42:490–501. [ Links ]
36. Coderre TJ, Melzack R. Procedures which increase acute pain sensitivity also increase autotomy. Exp Neurol 1986;92: 713–22. [ Links ]














