Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Salud mental
versão impressa ISSN 0185-3325
Salud Ment vol.31 no.2 México Mar./Abr. 2008
Editorial
How important is psychoneuroimmunology?
Brian E.Leonard1
1 Pharmacology Department, National University of Ireland, Galway. Brain and Behaviour Research Institute, University of Maastricht, The Netherlands.
Psychoneuroimmunology is a recent hybrid science that encompasses immunology, endocrinology, neuroscience, psychiatry and psychology. However, the concept that the brain has a profound effect on the physical, as well as the mental, health is not new. For example, the Roman physician Galen, 1800 years B.P., reported that only 20% of his patients had a major physical basis for their medical symptoms. He also noted that melancholic women were more prone to develop cancer than those with a sanguine temperament. Anecdotal reports occurred in the medical literature regarding the association of different types of cancer with depression. Later, it was observed that the survival time of a patient with a serious, life–threatening infection (such as TB or AIDs) is significantly affected by the psychological state of the patient. The more positive the attitude to the infection, the longer the survival time. Thus, by the late 20th century there were numerous clinical observations suggesting that the brain has a major effect on the immune system, and conversely, the immune system can affect the mental state.
The fundamental basis of psychoneuroimmunology was a serious subject of basic research before clinically relevant studies were undertaken. Hans Seyle, in the 1930's, developed the concept of homeostasis to stress in which he showed that the hypothalamic–pituitary–adrenal (HPA) axis, and the immune system, played an essential role in the adaptive response. Later, investigators in the Soviet Union, the USA and Europe demonstrated that changes in the immune system could be classically conditioned in the Pavlovian sense.1 Thus in rodents, an immune response elicited by an unpleasant (painful or noxious) stimulus, termed the unconditioned stimulus, when paired with a neutral conditioning stimulus (for example, light, sound, scratching the skin of the animal) could be observed at a later occasion when the conditioning stimulus alone was applied. By the 1980's, it was well–established that specific brain lesions could influence the immune system. For example, lesions of the cortex were associated with a decrease in natural killer cell activity, T–cell proliferation and antibody synthesis, while the newly discovered immunotransmitters, the interleukins, were shown to profoundly influence the HPA axis and brain neurotransmitter function.2
By the late 1990's, it was evident that there is a constant <<cross–talk>> between the central and peripheral neurotransmitters, the endocrine and immune systems. Some 30+ cytokines and chemokines had been identified, and cytokine receptors were shown to be widely distributed not only on immune cells but also neurons and accessory cells in the brain (microglia and astrocytes). It is now well–established that:
1. The brain, the HPA axis and immune systems are closely inter–related.3
2. Stress and emotional states significantly affect immune function and can precipitate physical as well as causing psychological changes.
3. Cytokines play a key role as immunotransmitters and coordinate the activity of the endocrine, immune and neurotransmitter systems.4,5
IS IT ALL IN THE BRAIN?
It is now well–established that in addition to immune cells in the blood and spleen that produce pro–inflammatory [interleukin (IL)–1,–6, tumour necrosis factor (TNFalpha), interferon gamma (INF) and anti–inflammatory (IL–4,–10,–13, transforming growth factor (TGF)beta] cytokines, astrocytes, microglia and neurons in the brain also produce these immunotransmitters.4 In addition, endogenous opioids (endorphins and enkephalins) and cannabinoids (such as anadamide) inhibit cellular immunity. In depression, there is an increase in pro–inflammatory cytokines in the blood and brain. Similar changes occur after chronic stress and following brain lesions resulting from stroke.6 These changes are associated with an increased release of glucocorticoids and an increase in such inflammatory mediators as prostaglandin (PG), E2 and nitric oxide, events that reflect the actions of the pro–inflammatory cytokines on the hypothalamus (increase in HPA activity), cyclooxygenase (increased PGE2) and inducible nitric oxide synthase (NO).7 Thus depression is associated with a chronic, low grade inflammation that ultimately contributes to increased apoptosis and cortical atrophy. The macrophage theory of depression, proposed by Smith in 1991, arose from these observations. In essence, this theory stated that the increase in pro–inflammatory cytokines and a reduction in anti–inflammatory cytokines was a reflection of a chronic, low grade inflammatory process that was ultimately responsible for the symptoms of depression.8 In support of this theory, it was found that the therapeutic use of IFN alpha for the treatment of hepatitis or multiple sclerosis, for example, is associated with depressive symptoms in a substantial minority of otherwise non–depressed patients.9 Similar changes can be initiated in animals and man by a lipopolysaccharide (LPS) challenge. LPS is derived from the coat of pathogenic bacteria and activates macrophages thereby increasing the blood and brain concentrations of pro–inflammatory cytokines. Thus the main biological markers of depression (dysfunctional monoamine neurotransmitters, hypercortisolaemia, depressed mood, increased inflammatory status) can be ascribed to an increase in peripheral and central macrophage activity from which the cytokines are derived.
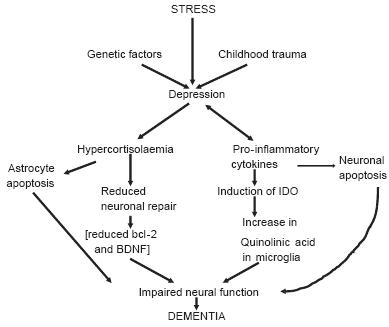
A possible consequence of the chronic inflammation is an increase in apoptosis leading to neurodegenerative changes in the brain.7 There is evidence that such changes occur in both depression and schizophrenia.10 The inflammatory changes shown to occur in major depression are qualitatively similar to those reported in patients with Alzheimer's disease. This has led to the suggestion that chronic depression can predispose patients to dementia in later life. Figure 1 summarises the immune/endocrine cascades that may form the basis for the progression from chronic depression to dementia.
PSYCHOLOGICAL–IMMUNE INTERACTIONS
The lack of adaptation to stress underlies most major psychiatric disorders. From the psychological perspective, a defective coping strategy may be associated with the personality of the individual (neuroticism, extraversion, etc.), the coping ability, gender and mood state.11,12 Clearly the nature of the stress, its severity and duration are also important factors.
It is now apparent that cerebral lateralization also plays a role in the response to stress. Thus those individuals showing predominantly right cortical activation to a stressful stimulus are more likely to experience negative symptoms than those showing left cortical activation to the same stimulus. Such individuals also show a greater degree of behavioural inhibition, and respond more strongly to negative than positive challenges. In studies in monkeys it has been shown that animals showing right cortical activation tend to be more emotional, have raised basal cortisol and a reduction in the activity of natural killer cells.
WHAT ARE THE LIMITATIONS OF PSYCHONEUROIMMUNOLOGY (PNI)?
There are several problems that currently limit the advance of PNI in clinical and experimental neuroscience.
Firstly, there is a large variation in the immune parameters following a specific stressor due to genetic, environmental background, adaptive capacity and, in clinical studies, personality of the subject or patient.12
Secondly, even in well–controlled experimental situations there is a need to distinguish between statistical and biologically important changes. For example, it is generally unknown if a statistically large and significant change in an immune parameter has any biological relevance for the health of the subject. Even a large change may still be within the normal range of values and the subject is not therefore immunocompromised. A brief survey of the published literature clearly indicates that qualitatively and quantitatively different results are obtained, often within the same laboratory, for patients supposedly suffering from the same condition and using the same methods to determine the immune parameter. Problems of non–replication of results can be attributed to the heterogeneity of the patients studied and the controls used (age, gender, whether hospitalised or in the community, time of blood or tissue sampling, type of medication used, etc.).
Thirdly, there is often a high degree of variability due to the different assays used and, to date, there is no agreement regarding the standard methods that should be used.
Fourthly, most clinical studies are based on single point analyses. All immune parameters change according to the circadian rhythm so, unless the samples are taken at exactly the same time of day, it is difficult to draw any conclusions regarding the relevance of the findings. Ideally, the immune changes should be assessed at intervals over the 24–hour period but this is, in practice, difficult to achieve particularly with psychiatric patients.
Fifthly, it is often assumed that there is only one immune compartment outside of the Central Nervous System. This is untrue! For example, in the blood compartment the mitogen induced proliferation of lymphocytes is mediated by glucocorticoids, but in the spleen compartment the catecholamines are the mediators. This could result in significant differences in interpretation of the results of a study.
Finally, few clinical studies take the psychological state of the patient into account at the time of sampling. All animals can learn to immunosuppress or immunoenhance their T–cell, B–cell and Natural Killer cell function. Such factors are often ignored in both clinical and experimental studies that explore the relationship between different types of stressor and/or psychological status and immune function.
HOW USEFUL IS PSYCHONEUROIMMUNOLGY?
PNI is still in its infancy as a method for exploring the interface between behaviour, brain function, the immune and endocrine systems. For that reason, reliable data collection is essential in order to develop hypotheses that are necessary to establish PNI as a valuable contributor to understanding normal and abnormal mental processes. It is therefore essential to better standardize the experimental procedures and to take into account the complexity of factors that influence immune function. Unless this is achieved, PNI research will continue to be treated as a relatively obscure, minority interest.
REFERENCES
1. Ader R, Cohen N. Conditioning the immune system. Neth J Med 1991;39:263–276. [ Links ]
2. Ballieux RE. Bidirectional communication between the brain and the immune system. Eur J Clin Invest 1992;22:6–9. [ Links ]
3. Besedovsky HO, del Rey AE, Sorkin E. Immune–neuroendocrine interations. J Immunol 1985;135(suppl.1):750S–754S. [ Links ]
4. Dantzer R. How do cytokines say hello to the brain? Neural verses humoral mediation. Eur C network 1994;5:271–273. [ Links ]
5. Connor T, Leonard BE. Depression,stress and immunological activation: the role of cytokines in depressive disorders. Life Sci 1998;62:583–606. [ Links ]
6. Maier SF, Watkins LR, Fleshner M. Psychoneuroimmunology–the interface between behaviour. Brain and immunity. Amer Psychol 1994;49: 1004–1017. [ Links ]
7. Leonard BE, Myint AM. Changes in the immune system in depression and dementia: causal or co–incidental effects? Dialog Clin Neurosci 2006; 8:163–174. [ Links ]
8. Smith RS. The macrophage theory of depression. Med Hypoth 1991;35: 97–104. [ Links ]
9. Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S et al. IDO and interferon alpha induced depression symptoms; a shift in hypothesis from tryptophan depletion. Med Hypoth 2005;10:538–544. [ Links ]
10. Leonard BE. Is there an immunologic basis for schizophrenia? Expert Rev Clin Immunol 2005;1:103–112. [ Links ]
11. Glaser GR, Kielcolt–Glaser JK, Stout JC, Tarr KL, Speicher CE et al. Stress related impairments in cellular immunity. Psychiat Res 1985;16:233–239. [ Links ]
12. Kielcolt–Glaser JK, Glaser R. Methological issues in behavioral immunology research in humans. Brain Behav Immun 1988;2:67–78. [ Links ]














