The constant growth of the population demands a guarantee for food production, making it a global challenge. It has been estimated that by 2050, food demand will have increased more than 50% (van Dijk et al., 2021), with the added responsibility of increasing productivity without causing a further environmental impact. The challenge is even greater, because pests and diseases are serious and constant constrains on food security. In particular, phytopathogenic fungi cause approximately 30% of all losses in agricultural crops in the world (Savary et al., 2019). Currently, the main control method is the use of chemical fungicides, which causes a strong pressure of selection on pathogens, favoring a greater genetic diversity and the appearance of more resistant strains in their populations, as well as problems in the environment and in human health (Brito et al., 2020). This makes it increasingly urgent to protect crops without depending on chemical fungicides. One option that offers great opportunities is the use of interference RNA (iRNA), which is the subject of this study.
Interference RNA is a conserved mechanism, present in most eukaryotic cells, and associated with the repression of the transcription (transcriptional regulation of the gene expression) and/or degradation of the messenger RNA (mRNA), or inhibition of the translation (post-transcriptional regulation). It also participates in the defense of organisms against foreign elements (Garcia-Ruiz et al., 2016; Rosa et al., 2018). This mechanism, known as gene silencing, is triggered by double-stranded RNA (dsRNA), which can have the structure of a double strand or a hairpin structure (Figure 1). The small RNAs (sRNAs) that trigger the silencing can be divided into two large groups, depending on their origin: micro-RNAs (miRNA) and small interfering RNA (siRNA).

Figure 1 Mechanisms of small RNA (sRNA) production in eukaryotes. sRNAs comprise the small interfering RNA (siRNA) and micro-RNA (miRNA). On the left, the scheme shows the different biosynthetic pathways to produce siRNA, which may have structure of lineal-double strand DNA, or hairpin (stem-loop) structure. These two structures (lineal double strand DNA and hairpin) are those imitated by the artificial double strand sRNA (dsRNA), it means, those designed for biotechnology. On the right, the picture shows the biosynthesis of miRNA which come from MIR genes, which are transcribed to pri-RNA. Silencing starts when the pri-RNA or siRNA precursors are recognized and cut by DCL (Dicer protein), producing siRNA and pre-RNA respectively. The last one is cut again by DCL into miRNA. siRNA and miRNA bind AGO (Argonaut protein) becoming into RISC (RNA-induced silencing complex). In RISC, one siRNA´ or miRNA´ strand is used to guide transcriptional or post-transcriptional silencing. In fungi, iRNA regulates processes of development, defense, and pathogenicity on transcriptional and post-transcriptional levels.
The miRNAs come from the transcription of the MIR genes; the initial transcript is called pri-miRNA and it forms a hairpin structure, recognized by a Dicer-like ribonuclease III (DCL), which cuts them into smaller structures called pre-miRNA. DCL cuts again on the pre-RNAs and the miRNAs are produced.
On the other hand, siRNAs are produced through the transcription of repeated sequences or of transposition elements, or through the replication in the genome of sequences of viral origin, or by the secondary amplification of dsRNA after the sRNA were cut by Dicer, or by hybridizations of complementary regions between two independent transcripts (that codify different proteins). The original dsRNAs, produced by either one of these paths, are processed by DCL in smaller fragments.
Both sRNAs (miRNA and siRNA) are fragments of approximately 19-24 nucleotides (nt) in length, and are recruited by the protein Argonaut (AGO) and processes in the RNA-induced silencing complex (RISC). The RISC complex uses one of the strands in the sRNA to guide the specific degradation of the target mRNA, which sRNA is complementary with; in this way, the system regulates the expression of genes in a sequence-specific way. Figure 1 outlines the production and processing of both types of sRNAs: miRNA and siRNA.
Plants have the natural ability to exchange sRNAs with their pathogens in a process called Crosskingdom iRNA, which plants can use to fight pathogens (Hudzik et al., 2020) (Figure 2). In biotechnology there are three approaches to using iRNA to protect crops against pathogens and pests: 1) the expression of transgenes in the plant, or host-induced gene silencing (HIGS). This is a strategy to silence the genes of pathogens through the production in vivo of sRNA complementary to the sequences of the pathogen, using genetic engineering and the transformation with Agrobacterium tumefaciens, resulting in a plant that is a genetically modified organism, or GMO (Halder et al., 2022; Ghag et al., 2019; Koch and Wassenegger, 2021). Most of the genes that have been targeted in fungi have been structural genes, development regulators and pathogenicity factors (Yin and Hulbert, 2015). 2) Use of recombinant viral vectors, which when replicated produce sRNAs in plants and these are directed to a fragment of the transcribed target, thus triggering its silencing. This procedure is known as virus-induced gene silencing (VIGS) (Villanueva-Alonzo et al., 2022). An example of this is the use of the Barley stripe mosaic virus (BSMV) to silence important genes of the fungus Puccinia striiformis f. sp. tritici to avoid the infection of wheat (Triticum aestivum) (Qi et al., 2018; Yin et al., 201). 3) The exogenous application of dsRNA, homologous to genes exclusive to the pathogen. This method is known as spray-induced gene silencing (SIGS) (Wang and Jin, 2017). Both HIGS and VIGS imply the use of genetically modified organisms (GMOs); the former, because it requires the genetic transformation of the plants of interest and the latter, because of the genetic modification of the viruses, with the constructs of expression, to replicate in plant cells and transcribe the sRNAs (Figure 3). Table 1 shows a summary of the comparison of the three techniques, as well as the advantages and disadvantages of each one. In several countries, the regulations on the use of GMOs in the field is still restricted, which means an opportunity for SIGS, since the exogenously applied dsRNA are temporary and does not require the creation or application of GMOs in the field. The aim of this manuscript is to present the potential of SIGS as the option for the use of iRNA in the field for the management of phytopathogenic fungi, particularly in Mexico and Latin America, and the aspects that still need to be overcome for this technology to be successfully used in the field.
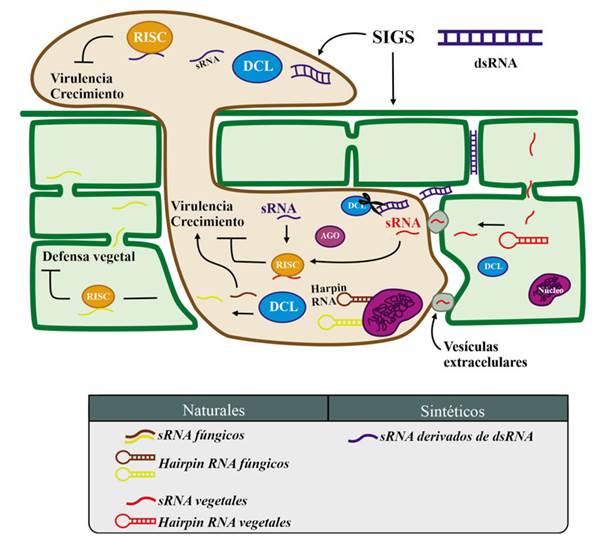
Figure 2 Global representation of sRNA movement and their functions between the fungus and plant host. The plant sRNA is delivered in extracellular vesicles, targeting the fungal cell to silence fungal genes involved in growth and virulence. The fungal sRNAs promote its virulence and growth, as well as they suppress the plant defense. This process of interchanging sRNA between the pathogen and the host is called “Cross-kingdom iRNA”. When exogenous double strand sRNA, dsRNA, (designed to target important fungal genes involved in growth and virulence) are sprayed directly on the fungus or on the leaf, they are later absorbed by the fungus resulting in siRNA that silence the targeted genes. The figure also shows that artificial silencing (designed with biotechnological purposes), occur in the pathosystem at same time as host genes silencing because natural sRNAs delivered by the fungus, as well as fungal genes silencing because natural sRNAs delivered by the host.
Table 1 Comparison of technologies based on interference RNA for the control of pests and agricultural diseases.
| Tecnología | Significado del acrónimo | Descripción | Ventajas | Desventajas | Referencias |
|---|---|---|---|---|---|
| HIGS | Host Induced Gene Silencing (silenciamiento génico por la producción de dsRNA por el hospedante). | La planta es transformada con un vector de ADN que integra el constructo de silenciamiento al genoma. | Producción estable y permanente de los dsRNA. Los dsRNA tienen mayor probabilidad de absorción por los patógenos de diferentes reinos. El procesamiento de los dsRNA depende de la maquinaria de silenciamiento de la planta. | Requiere protocolos de transformación y regeneración a planta de la especie que se quiera convertir en HIGS. La planta es OGM. Poca acceptación de consumidores. | Halder et al., 2022; Papadopoulou et al., 2020; Wang et. al., 2016 |
| VIGS | Virus Induced Gene Silencing (silenciamiento génico por dsRNA producidos con base en un vector viral). | La planta es inoculada con el vector de silenciamiento viral que contiene un fragmento del gen diana. El nuevo ADN no se integra al genoma vegetal. | No requiere de un protocolo de transformación de la planta de interés. El vector VIGS se inocula por bombardeo o agroinfiltración. El procesamiento de los dsRNA depende de la maquinaria de silenciamiento de la planta. | Requiere que el vector VIGS sea capaz de moverse, replicarse y transcribirse en la planta de interés. La producción de los dsRNA es temporal. No provee de una resistencia permanente hacia los patógenos. El vector viral es un OGM. | Halder et al., 2022; Villanueva-Alonzo et al., 2022 |
| SIGS | Spray Induced Gene Silencing (silenciamiento génico inducido por dsRNA asperjados). | Los dsRNAson producidos por transcripción in vitro o sintetizados químicamente. | Para la transcripción in vitro si se generan constructos de silenciamiento, pero los OGMs solo se manejan en el laboratorio. Las plantas asperjadas con dsRNA no son OGMs. | Los dsRNA entran de manera ineficiente a la planta y se degradan fácilmente sobre la superficie vegetal. La producción in vitro actualmente es costosa. El éxito del silenciamiento depende de que el patógeno posea una maquinaria de silenciamiento eficiente. | Gurusamy et al., 2020a, 2020b; Hoffle et al., 2020; Koch y Wassenegger, 2021; Qiao et al., 2021; Sarkar y Roy-Barman, 2021 |
In the cases of HIGS and VIGS, for the processing of the dsRNAs (that is, the cutting of the dsRNAs into siRNA by Dicer, the recognition of the siRNAs by Argonaut and the formulation of the RICS complex), depends almost entirely on the mechanism of the plant (Höfle et al., 2020), whereas in SIGS, it depends on the ability of the pathogen to absorb and process the dsRNAs into siRNA (Koch and Wassenegger, 2021; Qiao et al., 2021). Therefore, in order for the SIGS to be successful, a functional silencing machinery is required in the target organism. Consequently, it is important to analyze the components of the fungal silencing machinery.
Silencing machinery in fungi
As mentioned earlier, the silencing system is conserved in eukaryotes. In the first reports on Neurospora crassa and Schizosaccharomyces pombe, silencing was given the name of “quelling.” Thus, fungal proteins related to silencing were named QDE, or “quelling defective.” But these names may be confusing because while QDE1 is a DNA- dependent RNA polymerase, QDE2 is an argonaut protein. Similarly, SMS2 (Suppressor of meiotic silencing 2) is a homologous to argonaut protein in Neurospora crassa (Gaffar et al., 2019). Although narratives on the first investigations keep mentioning QDE proteins, the tendency is to name proteins of the silencing system like their plant counterparts, that is, dicer (DCL), argonaut (AGO) and RNA-dependent RNA polymerase (RDRP). In fungi, the number of members described in these families are lower than those described in the plants. For example, in the plant kingdom, more than 15 members of the AGO family have been described, along with at least 6 RDRP (García-Ruiz et al., 2016), while in fungi, three AGO members are known, along with two DCLs and five suppressors of ascus, or SAD (Lax et al., 2020).
Gene silencing in fungi, as in other eukaryotes, participates in multiple processes (Figure 1) such as the regulation of genetic expression, the formation of heterochromatin, defense against viruses, the control of transposable elements, adaptation to conditions of stress, cell division and development, as well as in pathogenesis (Lax et al., 2020; Zanini et al., 2021). Recently, Gaffar and collaborators (2019) studied the conidiation, ascoporogenesis, virulence and production of the metabolite deoxynivalenol in Fusarium graminearum and observed different combinations of AGO, DCL and RDRP in the different processes, which shows the complexity of the mechanism for the silencing, despite the fact that only a few members in the main families of the proteins involved are known in fungi.
The role of interference RNA in fungal virulence
Silencing plays an important role in the virulence of fungi (Gaffar et al., 2019; Weiberg et al., 2013; Yin et al., 2020; Zanini et al., 2021), therefore these proteins have been proposed as targets for the control of plant pathogens (Haile et al., 2021; Werner et al., 2020). For example, Botrytis cinerea is able to release sRNAs (Bc-sRNAs) during the infection of its host’s cells (Cai et al., 2018). These Bc-sRNAs act by sequestering the AGO protein of the host, such as Arabidopsis thaliana or Solanum lycopersicum, which is key in the defense system of both hosts (Weiberg et al., 2013). Interestingly, a single sRNA, named Bc-siR37, suppresses at least eight genes involved in the defense of A. thaliana. Among these, WRKY transcription factors, receptor kinases and cell wall modifying enzymes (Wang et al., 2017). Verticillium dahliae is another pathogen in which the role of the sRNAs has been studied regarding the invasion of the host, and it was found that it uses mechanisms similar to those described for B. cinerea (Wang et al., 2016). In another example, Fusarium graminearuma also releases Fg-sRNAs, which help silence defense genes in hosts, favoring their colonization (Jian and Liang, 2019; Werner et al., 2021). When F. graminearum invades wheat (T. aestivum), it secretes the sRNA Fg-sRNA1, which reduces the expression of a chitin elicitor binding protein (TaCEBiP) that has the function of eliciting the defense (Jian and Liang, 2019). In addition, it has been proven that DCL-dependent Fg-sRNAs generally regulate the expression of defense genes in grasses (Werner et al., 2021). The elimination of several components of the iRNA production system, particularly DCL1 and AGO2, results in a reduction of blight in wheat spikes, highlighting the importance of these proteins in the virulence of F. graminearum (Gaffar et al., 2019). Due to this, both genes (DCL1 and AGO2) have been proposed for the control of this pathogen in barley leaves (H. vulgare) (Werner et al., 2020). On the other hand, in Penicillium italicum, protein DCL2 regulates the expression of the microRNAs, and contributes in crucial ways to the pathogenesis (Yin et al., 2020), which shows that the importance of the different DCLs can vary between species. It is worth clarifying that, in the case of fungi, miRNAs are called micro-RNA like (milRNAs). Recently, Haile and collaborators (2021) created a chimera to silence the expression of both DCL genes in Plasmopara viticola, a strategy which suppressed the ability of this pathogen to colonize grapevines (Vitis vinifera).
In the fungus Valsa mali, virulence is regulated with a milRNA called VdmilR1, which operates at a transcriptional level on the extreme 3’ UTR of VdHy1 through the K9 methylation of histone H3; VdHy1 is necessary for the virulence of V. mali on Gossypium herbaceum (cotton) plants (Jin et al., 2019). Interestingly, unlike other canonical pathways for the biogenesis of milRNAs, the biogenesis of VdmilR1 does not depend on DCL or AGO, but on a protein called VdR3, which contains a domain of RNAse III (Jin et al., 2019), which highlights the importance of particularly characterizing the pathosystem to be controlled. Likewise, milRNAs play a crucial role in the posttranscriptional regulation of V. mali virulence genes during the infection in Malus domestica (apple) (Xu et al., 2020), as well as in the destabilization of the defense system of the host (Xu et al., 2022). These data suggest that the silencing machinery as a whole provides a mechanism maintained in fungi for the invasion of their hosts (Figure 2). Most sRNAs have the goal of inhibiting genes related to the plant defense system, and function in similar ways to the effector proteins of the pathogens (Todd et al., 2023).
Possibilities of interference rna in agriculture
It has been suggested that the use of iRNA can represent a powerful technological tool for the control of fungal diseases in plants (Wang and Jin, 2017; Zotti et al., 2018), and other pathogens such as viruses, insects and nematodes (Koch and Wassenegger, 2021). In the case of HIGS, most papers are based on the use of binary vectors that overexpress inverted sequences, separated by an intron, which help to form the hairpin (Figure 3). However, this approach is restricted to plants with established transformation methods, while most plants of agronomic interest do not have these protocols (Halder et al., 2022; Wang et al., 2016). The greatest limitation in many countries is the inherent use of GMOs, due to the limited acceptance and lack of regulations for their use (Papadopoulou et al., 2020). In the case of VIGS, it uses RNA or DNA viral vectors with the ability to transcribe foreign genes, without having to transform the entire plant (Halder et al., 2022; Villanueva-Alonzo et al., 2022). This approach is limited to the availability and efficiency of viral vectors for the plant of interest, but it also implies the management of genetically manipulated viral genomes (Figure 3). On the other hand, in the SIGS technology, dsRNAs are applied exogenously on the surface of the plant tissue, without the need for transformation (Koch et al., 2016; Sarkar and Roy-Barman, 2021). For the use of SIGS, the dsRNAs are produced by the chemical synthesis of each independent strand (sense and antisense), which are then mixed equimolarly, that is, in equal amounts, to generate the double strand. The chemical synthesis of each strand is analogous to the commercial synthesis of oligonucleotides, but using ribonucleotides as building blocks for the synthesis of the RNA strands. The dsRNAs can also be produced by in vitro transcription. In this case, the sequence chosen to silence in the target transcript is cloned in a circular DNA vector under the regulation of a promoter of bacteriophage origin such as T3, T7 or SP6. Two clones are generated, one of which has a sequence in a sense direction (5´-3´), and the other, in an antisense direction (3´-5´). For the transcription, both vectors are linearized with a restriction enzyme that has only one restriction site in these vectors. The in vitro transcription is carried out in a tube with a reaction mixture containing the linearized vectors, an RNA polymerase that recognizes the promotor being used (whether T3, T7 or SP6), and the ribonucleotides (Li and Zamore, 2019; Sun and Riggs, 2017). Figure 4A shows how the dsRNAs are synthesized in vitro.
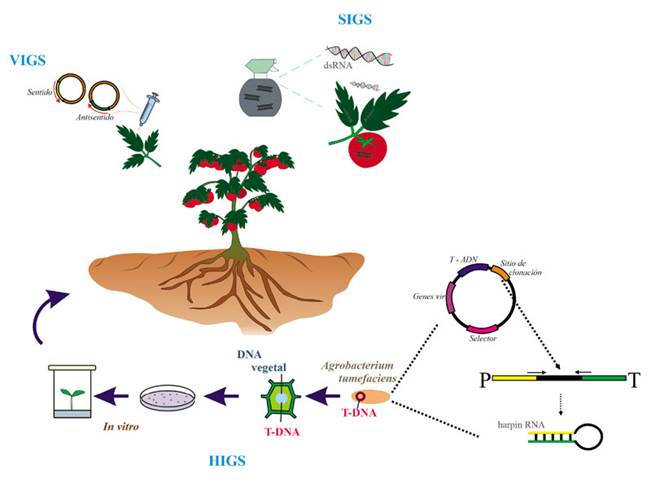
Figure 3 Schematic representation of the different control approaches based on the interfering RNA mechanism. HIGS (host induced gene silencing); in HIGS the host is transformed and genetically modified for endogenous generation of artificial sRNA; silencing cassette is introduced by Agrobacterium tumefasciens with a T-DNA cassette in which there are cloning the sense fragment (yellow square, arrow with right end), the antisense fragment (green square, arrow with left end), both flanking an independent sequence (black rectangle). Yellow and green sequences are the same sequences but inverted each other, then, they are complementary. In the expression cassette, P corresponds to the promoter and T to the terminator. When dsRNA is transcribed, it gets a hairpin structure. VIGS (virus induced gene silencing); the virus is genetically modified to produce each strand of sRNA (sense y antisense). SIGS (spray induced gene silencing) by exogenous sprayed dsRNA.

Figure 4 SIGS for controlling fungi, viruses and insects. A) in vitro production of dsRNA for exogenous spray. The target fragment is cloning in a circular vector under control of a bacteriophage promoter (T3, T7 o SP6); one vector is created for sense strand and another vector for antisense strand. For in vitro transcription, the vector is linearized with a restriction enzyme that cut one time on the vector (unique site), and that templete is mixed with the ribonucleotides and the RNA polymerase (T3, T7 o SP6 RNA polymerase, according the promoter in use), that carried out the in vitro synthesis of dsRNA. In the expression cassette, P corresponds to the promoter and T to the terminator. Both strands (sense and antisense) are synthesized and form the dsRNA B) The use of SIGS for controlling pathogens and pests on post-harvest fruit and vegetables is one of the applications that can be achieved soon.
SIGS has a regulatory advantage, since the crops sprayed with the dsRNAs are not considered as GMOs (Dalakouras et al., 2020; Taning et al., 2020). However, there are also limitations that must be overcome in order to apply SIGS in the field.
External applications on non-model plants and post-harvest products
The use of SIGS is an alternative to the classic plant genetic transformation. This approach has been described as eco-friendly, highly specific and with a wide range of crops it can be used on. It is worth mentioning that it can be applied on crops in the field, as well as on harvested agricultural products (Figure 4B).
The first analyses regarding its feasibility were performed on plants such as Nicotiana benthamiana and A. thaliana (Dalakouras et al., 2018; Wang et al., 2016), but it has also been evaluated on barley (H. vulgare), rice (Oryza sativa), canola (Brassica napus), and cucurbits (Haile et al., 2021; Kaldis et al., 2018; Qiao et al., 2021; Sarkar and Roy-Barman, 2021); in harvested strawberry (Fragaria vasca), grapevine (Vitis vinifera), tomato (S. lycopersicum) and apple (M. domestica) fruits (Qiao et al., 2021; Wang et al., 2016); as well as vegetables such as lettuce (Lactuca sativa) and onion (Allium cepa) (Qiao et al., 2021) and flowers (Qiao et al., 2021; Wang et al., 2016). The topical application of the dsRNAs has been observed to provide protection, not only in the area on which it is applied, but also in other parts of the plant. The ability of plants to absorb dsRNA through their leaves varies, although there are alternatives such as applying on the petioles (Dalakouras et al., 2018). Studies with Sclerotinia sclerotiorum suggest that the absorption of the dsRNAs in fungi takes place predominantly on the tip of the hypha, through endocytosis mediated by the protein clathrin (Wytinck et al., 2020). The results of all these recent investigations show the potential of the dsRNAs to control fungal diseases in agricultural crops.
Advantages and disadvantages of dsrnas in plant protection
Specificity of the dsRNAs. The greatest advantage of using dsRNA is that user can decide to silence a gene or a gene family, or several different genes in an organism, where virulence can be drastically reduced, or the fungal growth can even be drastically reduced. The user can also opt for the silencing the target in one single organism or many organisms simultaneously (Haile et al., 2021; Koch et al., 2019; Nerva et al., 2020; Werner et al., 2020). The specificity of the dsRNAs depends on the sequence used per se (Wang et al., 2016). Some fungal genes are monogenic, that is, they appear only once in the genome, for example, the LYS2 gene that encodes the enzyme a-aminoadipate reductase, or the HGMR gene, which encodes the enzyme hidroxymethylglutaryl CoA reductase. In these cases, silencing is only on one gene. Other genes have multiple members in the genome and they are said to form a gene family, such as the genes in catalases, chitinases, kinases, etc. For the design of silencing, the sequences of the messenger RNAs are compared and the user can choose a region that is identical for all the family members and silence them all, or silence them by groups, or choose regions that are different in each one and silence a specific member (Figure 5), whether to prove its relevance in the gene family, or because it contributes to the pathogenicity of the fungus. In order to achieve specificity and avoid unwanted targets, it is recommended to direct the design of the silencing towards divergent regions, whether in the encoding region or in the 5´and 3´UTR ends (Untranslated Regions) of the mRNA, particularly in the case of genes that encode highly conserved proteins. The UTRs are parts of the messenger RNA and they are found on its ends, respectively. They are important for its structure, although they are not translated into amino acids. The UTR sequences vary, even in the genes for highly conserved proteins.
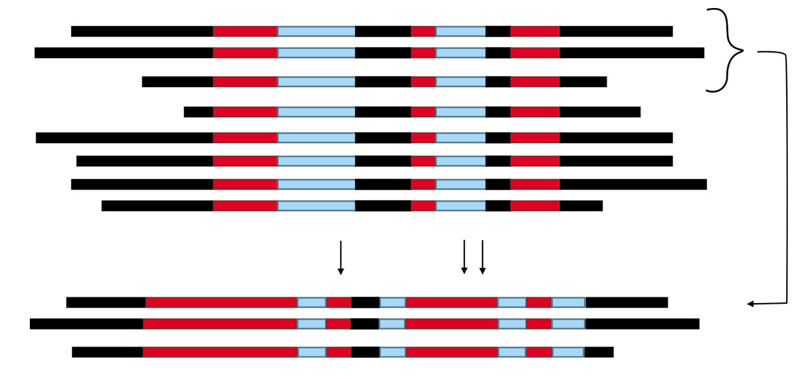
Figure 5 Strategies for designing dsRNA for either specific or multiple gene silenging. To achieve this, the nucleotide sequences are compared by using any bioinformatic tool for multialignment. The scheme may represent different situations: A) the same gene from different organisms. B) different genes belonging to the same gene family from the same organisms (for example, catalases family because there are multiple genes in the genome of a single organism). The red lines correspond to highly conserved regions, even 100%; the blue lines correspond to moderated conserved regions, and black lines to highly divergent regions. The dsRNA designed on the red regions in the alignment are able to silence the gen in different organisms (situation A), or all gene members in the gene family (situation B) in one organism (non-specific dsRNA). The dsRNA designed on the black regions will silence specifically the gene in a single organism with no silencing on others (situation A), or a single gene in a gene family (situation B) (specific dsRNA). The alignment below corresponds to: C) the same gene from close relative organisms, or D) members belonging to a gene subfamily; it is observed that some regions which were blue in the global alignment (above), in the close relatives (situation C) or the gene subfamily (situation D) become highly conserved (in red), and allow to design dsRNA able to silence that group of close relative organisms, but not others (situation C), or all members belonging to this subfamily, but with no interference on other members in this gene family (situation D).
The strategy is similar to apply silencing on a single organism or several species of organisms, a particular genus, etc. That is, it depends on whether regions are chosen that are highly conserved among all of them, or sequences that are exclusive to a particular organism. For example, Gutiérrez-Domínguez et al. (2022) recently reported a type of lipase that is only found in some genera of the Hypocreales and Glomerellales orders of filamentous fungi, including the genera Trichoderma, Fusarium, Nectria and Colletotrichum. If highly conserved regions are chosen in the gene of this lipase, silencing will occur in all these fungi, but if divergent sequences are chosen between them, silencing can be directed only at those we want to control, preventing them from affecting, for example, the species of the Trichoderma genus, which are beneficial fungi for agriculture, whereas the others are phytopathogenic.
The availability of constantly growing public databases of genomes and transcriptomes help to adequately design silencing strategies and prevent it from occurring with non-target organisms, that is, those that do not want to be affected.
dsRNA, low risk for humans. According to Fletcher et al. (2020), Jensen et al. (2013), and Rodrigues and Petrick (2020), the risk of consuming dsRNA in humans is low or none. For the dsRNAs applied on fruits or plants have a possible impact in human health, the dsRNAs must have to enter the cells and match with some of the human genes (Fletcher et al., 2020). However, in humans and mammals in general, there are many biological barriers in the gastrointestinal tract, blood flow and at a cellular level in which they are degraded due to the large amount of existing nucleases (Fletcher et al., 2020; Rodrigues and Petrick, 2020). In addition, man has consumed plant dsRNAs naturally by way of their conventional diet in maize (Zea mays), soybean (Glycine max), rice (O. sativa), lettuce and tomato (Jensen et al., 2013). For the suppression of the human gene, the sRNAs would need to be complementary and accumulated in biologically important concentrations in the target site, which does not occur (Jensen et al., 2013).
No persistance of dsRNAs in the plant. In a pioneering investigation by Koch and collaborators (2016), they applied in H. vulgare (barley) dsRNA designed against transcripts of the green fluorescent protein (GFP) and 48 h later, they infected the leaves with a transformed strain of F. graminearum, which produces the GFP. Interestingly, the silencing of GFP remained for 6 days post infection (dpi) in distal regions from where the dsRNA-GFP was applied, indicating its stability in the tissue and that the dsRNA moves inside the plant. In a similar fashion, the application on roses (Rosa chinensis) and tomato fruits of dsRNA that silence DCL1/2 of B. cinerea protected them against the pathogen for 8 -10 days. Reports on other plants with other fungal pathogens coincide with these times of stability of the dsRNAs (Nerva et al., 2020; Sarkar and Roy Barman, 2021).
Several papers report the application of the dsRNAs first and then they inoculate with the pathogen (Höfle et al., 2020; Koch et al., 2016; Koch et al., 2019; Werner et al., 2020; Sarkar and Roy-Barman, 2021), that is, a preventive effect of the infection. Recently, Haile and collaborators (2021) infected de Vitis vinifera leaves with Plasmopara viticola, they let the disease progress for 7 days, and later applied the dsRNA. This helped reduce the disease progress rate, implying that the control with dsRNAs could also be a curative treatment. However, the dsRNAs applied exogenously are extremely susceptible to degradation and have very low persistence in the environment (Bachman et al., 2020), therefore one of the main challenges for its success in agriculture is extending its stability in the environment.
Factors that affect the design of dsRNAs for SIGS. One of the main factors to be taken in consideration when designing the dsRNAs are the molecular targets themselves. Some of the targets that have been considered have included the most conventional ones, such as genes that encode enzymes that synthesize intermediaries in the synthesis of lanosterol (Cytochrome P450 lanosterol C-14α-demethylase, CYP51) (Koch et al., 2016), the silencing of effectors (Sarkar and Roy-Barman, 2021) and even the silencing components themselves (Wang et al., 2016) (Table 2). Both in experiments performed in vitro and in the applications on the surface of leaves, the effect of silencing has been observed to increase as the dose of the dsRNAs increases (Haile et al., 2021, McLoughlin et al., 2018, Wang et al., 2016). However, if the concentration is too high, better results are not obtained; this suggests that the iRNA machinery becomes saturated and it ceases to process the dsRNAs (McLoughlin et al., 2018). Consequently, to achieve the best results with the SIGS, characterizing every pathosystem and determining the most adequate length and concentration of dsRNA is crucial to control the disease.
Table 2 Successful examples of SIGSy in the inhibition of phytopathogenic fungi.
| Hospedante | Patógeno | Gen diana | Función | Longitud dsRNA (nt) | Máxima persistencia en la planta o in vitro | Referencias |
|---|---|---|---|---|---|---|
| Cebada (Hordeum vulgare) | Fusarium graminearum | FgCYP51A, FgCYP51B, FgCYP51C | Enzima clave en la biosíntesis de Ergosterol | 791 (20 ng µL-1) (CYP51A/CYP51B/CYP51C) | 8 días (6 dpi) z | Koch et al., 2016 |
| Cebada (H. vulgare) | F. graminearum | FgCYP51A, FgCYP51B, FgCYP51C | Enzima clave en la biosíntesis de Ergosterol | 294 (CYP1A), 220 (CYP1B), 238 (CYP1C), 514 (CYP1A/CYP1B), 532 (CYP1A/CYP51C), 458 (CYP1B/CYP51C) | 7 días (5 dpi) z | Koch et al., 2019 |
| Cebada (H. vulgare) | F. graminearum | DCL, AGO | Maquinaria de silenciamiento | 372 (AGO2/DCL1), 1782 (DCL1/DCL2), 1529 (AGO1/AGO2), 1741 (AGO2/DCL2) | 7 días z | Werner et al., 2020 |
| Cebada (H. vulgare) | F. graminearum | FgCYP51A, FgCYP51B, FgCYP51C | Enzima clave en la biosíntesis de Ergosterol | 500 (CYP51A), 400 (CYP51B), 400 (CYP51C), 800 (CYP51A), 800 (CYP51B), 800 (CYP51C), secuencia completa para CYP51A, B y C | 7 días z | Höfle et al., 2020 |
| Jitomate (Solanum lycopersicum) | Botrytis cinerea | DCL1, DCL2 | Maquinaria de silenciamiento | 7 días | Qiao et al., 2021 | |
| Vid (Vitis vinifera) | B. cinerea | BcCYP51, BcChs1, BcEF2 | Biosíntesis de ergosterol, quitina y factor de elongación | 732 (CYP51/Chs1/EF2) (300 µg en 3 mL, absorción por peciolo) | 7 días | Nerva et al., 2020 |
| Vid (V. vinifera) | Plasmopara viticola | DCL | Maquinaria de silenciamiento | 258 (DCL1), 257 (DCL2), 515 (DCL1/DCL2) (75, 100 o 125 ng µL-1) | 14 días | Haile et al., 2021 |
| Arroz (Oryza sativa) | Magnaporthe orizae | MoDES1 | Efector (patogenicidad) | 300 (MoDES1) 300 nM | 10 días z | Sarkar y Roy-Barman, 2021 |
| Fresa (Fragaria vasca) en post cosecha | Botryotinia fuckeliana | DCL, quitina sintasa clase III | Maquinaria de silenciamiento, estructura de la pared celular | dsRNA encapsulado en células de E. coli | 12 días | Islam et al., 2021 |
| Vid (V. vinifera ) en post cosecha | B. cinerea | BcCYP51, BcChs1, BcEF2 | Biosíntesis de ergosterol, quitina y factor de elongación | 732 (CYP51/Chs1/EF2) | 3 días | Nerva et al., 2020 |
| Sin hospedante. Cultivo del hongo en medio sólido (in vitro) | Macrophomina phaseolina | β-1,3-Glucano sintasa (GLS) | Enzima clave en la síntesis de β-1,3 glucano, componente de la pared celular | siRNA (100 nM) | 2.5 días | Forster y Shuai, 2020 |
| Sin hospedante. Cultivo del hongo en medio líquido (in vitro) | F. culmorum | CYP51A, CYP51B, CYP51C | Biosíntesis de Ergosterol | 791 (20 ng µL-1) | 2 días | Koch et al., 2018 |
y Spray Induced gene Silencing.
z The previous 48 h of treating with dsRNA were considered, along with the days of infection monitored.
Current limitations of SIGS in agriculture. In the SIGS system, success depends on the sRNAs being able to permeabilize into the tissue of the host, which depends on the presence or absence of stomata, the thickness of the cuticle and the degree of suberization of the epidermis, as well as on the efficiency of absorption and the processing of the dsRNA by the pathogen (Hoffle et al., 2020). Therefore, SIGS is effective on pathogens with an efficient absorption of dsRNAs and that have a functional iRNA machinery to process the sRNAs. Although this is a highly conserved process and present in most organisms, not all fungi have silencing mechanisms, an example being the basidiomycete Ustilago maydis. Likewise, it seems that iRNA is scarcely efficient in the control of Zymoseptoria tritici, one of the most devastating fungi for wheat (T. aestivum). Kettles and collaborators (2019) attempted to use HIGS to silence four key Z. tritici genes, but they were not successful, showing the inability of the fungus to absorb dsRNAs through the interaction with wheat. Later, Ma and collaborators (2020) reported that during the colonization of wheat by Z. tritici, they did not identify the presence of iRNA, therefore they concluded that the silencing mechanism does not participate in this infection. Despite Z. tritici maintaining the components of iRNA machinery, it does not seem to play a part in the infection of the host. This suggests that for the success of SIGS, it is crucial for a natural intercommunication to exist between the pathogen and the host via sRNA. Recently, Qiao and collaborators (2021) performed a test in which they used a fluorescent dsRNA to evaluate the ability of absorption of several fungi in vitro. They found that Colletotrichum gloeosporioides is unable to absorb them, whereas in B. cinerea, V. dahliae, Rhizoctonia solani, Aspergillus niger and S. sclerotiorum, the fluorescence was observed inside their cells after 6 h, indicating the internalization of the dsRNAs. Other microorganisms such as Trichoderma virens and Phytophthora infestans absorbed the dsRNA in a limited manner. Curiously, Mahto and collaborators (2020) silenced a C. gloeosporioides gene and control anthracnosis in chili pepper (Capsicum annuum) and tomato, where they used HIGS and proposed that the sRNA go through the haustorium of the fungus via extracellular vesicles secreted by the plant. However, it is important to remember that silencing via HIGS did not work in Z. tritici (Kettles et al., 2019), suggesting that the entry methods of the iRNAs can vary between microorganisms, and in some cases, may not exist. For most phytopathogenic fungi, their abilities of absorption and processing of dsRNAs are unknown, therefore these tests must be broadened to better establish the potential of SIGS for the control of phytopathogenic fungi.
Potential of interference rna in agricultural crops in mexico and latin america
Latin America and the Caribbean (LAC) is an important food-producing region in the world. Its main agricultural products are cereals, oily seeds, banana, coffee, sugar, fruits and vegetables. In addition, 26% of the world’s production of tropical fruit [banana (M. acuminata), mango (Mangifera indica), pineapple (Ananas comosus), avocado (Persea americana) and papaya (Carica papaya)] are grown in LAC, mainly in Brazil, Ecuador, Mexico and Costa Rica. LAC is an important exporting region and key in the world economy. For example, bananas and plantains (M. balbisiana) are among the most important food products in the world and their marketing is a cornerstone in the economies of many LAC countries. Despite this, their production is constantly threatened by diverse diseases, mainly black Sigatoka (SN), caused by the ascomycete fungus Pseudocercospora fijiensis, and wilting from Fusarium, also known as the Panama disease. SN is a foliar disease that significantly reduces the rate of photosynthesis in the plant, weakening it and eventually killing it (Chí-Manzanero et al., 2021). On the other hand, the Panama disease, caused by Fusarium oxysporum f. sp. cubense race 1 (Foc1), killed an entire Gros Michel banana crop, forcing farmers to change to a more resistant crop (cultivar Cavendish). However, with the appearance of Fusarium oxysporum f. sp. cubense race 4 (Foc4), the banana industry is in grave danger. The urgent need to control this dangerous race of Foc has led to an evaluation of the technology of silencing using iRNA via HIGS (Ghag and Ganapathi, 2019). These authors developed transgenic banana lines that express iRNA against the velvet gene and the Factor 1 of the transcription of Foc4, both genes related to growth, development and pathogenesis; the plants survived for six weeks and other for up to eight months without developing symptoms (Ghag et al., 2014). Likewise, transgenic banana lines have been generated that have been able to silence the transcripts of enzyme C-24 sterol methyltransferase (ERG6) and of the cytochrome P450 lanosterol C-14α dimethylase (ERG11), both of which are involved in the synthesis of ergosterol; both lines were evaluated for up to two years (Dou et al., 2020).
In other crops, it has been estimated that global mango (M. indica) and avocado (P. americana) production will continue to grow in LAC. Brazil and Mexico are the most important mango and avocado producers, and of the latter, Mexico is the main exporter, while Colombia, the Dominican Republic and Peru have increased their production; LAC covers 73% of the world’s avocado production, which highlights its importance in the agriculture in the region. Among these tropical fruits, the disease anthracnose (Colletotrichum sp.) is common under post-harvest conditions. In mango and avocado, anthracnose is caused by C. gloeosporioides and this fungus attacks fruits in pre- and post-harvest, and also damages leaves. The evidence available in literature suggests that C. gloeosporioides does not efficiently absorb the exogenous dsRNAs (Qiao et al., 2021). However, it has been proven that anthracnose in chili pepper and tomato may be controlled using HIGS (Mahto et al., 2020), suggesting that the use of vesicles as carriers may be an alternative for the control via SIGS. One of the main pests that attack important crops in LAC is the fall armyworm (Spodoptera frugiperda), a polyphagous pest that damages different crops, preferably maize, sorghum (Sorghum bicolor), cotton and soybean, onion, carrot (Daucus carota), lettuce, papaya, watermelon (Citrullus lanatus), melon (Cucumis melo), cucumber (Cucumis sativus), avocado, banana, rice, coffee (Coffea arabica), tomato, cacao (Theobroma cacao), sugarcane (Saccharum officinarum), and many others (Montezano et al., 2018). This pest is widely distributed in LAC and part of North America, and its larvae can cause losses of up to 100% of crops. Recently, Gurusamy and collaborators (2020a; 2020b) described the control of S. frugiperda via iRNA, using formulations of dsRNA with cationic lipids that protect the dsRNAs from degradation inside the insect, and make silencing more efficient (Gurusamy et al., 2020a). Another report by the same authors evaluated the use of nanoparticles for the protection of the dsRNAs, preferring the formulations with chitosan due to its protective abilities and for being a biodegradable, non-toxic, cheap and eco-friendly polymer, meaning an improvement in the efficiency of silencing by SIGS (Gurusamy et al., 2020b). Another important pest for LAC is the coffee berry borer (Hypothenemus hampei). This insect is responsible for most of the losses in coffee in the world (Jaramillo et al., 2006). The application of fragments of dsRNA in the preoral cavity of the borer larvae is effective for gene silencing, as well as the exogenous application for acquisition by ingestion (Aguilera et al., 2011). Among the target genes evaluated, the best results were obtained by silencing the mannanases or xylanases involved in the hydrolysis of the main carbohydrates of the coffee grain (Aguilera et al., 2011). The silverleaf whitefly (Bemicia tabaci) is another important pest insect, widely distributed throughout LAC. This insect attacks avocado, bean (Phaseolus vulgaris), tomato, chili pepper and other crops, since it feeds from the phloem of plants, causing physiological disorders. In addition, it is a Begomovirus vector. To date, there have been reports of the silencing of several B. tabaci target genes via HIGS or artificial diets that contain dsRNA (Grover et al., 2019), proving that the iRNA technology is a promising alternative for its control. However, there are still no reports of the evaluation in the field on the control of B. tabaci using any of these technologies, although the potential for their control using SIGS has recently been demonstrated, in combination with Mg-Fe layered double hydroxide nanoparticles (MgFe-LDH “Bioclay”) (Jain et al., 2022). Likewise, the potential of homologous sequences derived from the Pepper golden mosaic virus (PepGMV) and heterologous derived from the Tomato chino La Paz virus (ToChLPV) has been proven, opening the possibility of protection against the Pepper golden mosaic virus (PepGMV) (Medina-Hernández et al., 2013; Vargas-Salinas et al., 2021). Despite being promising for an integrated and eco- and human-friendly management, the potential of the dsRNAs, and particularly of SIGS, in the agriculture of LAC is unknown.
In Mexico, iRNA have been used in the application of VIGS to study plant genes. Álvarez-Venegas et al. (2011) silenced genes related to flowering in canola (B. napus) and they obtained plants that flower without vernalization. In another study, Villanueva-Hernández et al. (2013), created a VIGS vector using an isolated Begomovirus in Yucatán in plants of the Euphorbia genus, which they called pEuMV-YP, with which they silenced ChlI as a reporter gene to evaluate the functionality of the VIGS vector. ChlI encodes a protein that protects chlorophyll and its silencing generates a photobleaching phenotype. The authors showed that pEuMV-YP is adequate for silencing genes in N. benthamiana and C. annuum plants. Similarly, Arce-Rodríguez and Ochoa-Alejo (2020) created a viral vector based on the Tobacco rattle virus (TRV), in which they incorporated a Gateway technology cassette in order to clone in it the DNA by recombination and they also incorporated the T-ADN region of A. tumefasciens to introduce the VIGS construct via agroinfiltration. This VIGS was used as a proof of concept to silence the gene that encodes the phytoene desaturase in chili pepper, which reduced the synthesis of phytoene, a precursor of the carotenes, and generated a phenotype of photobleaching in the leaves. Recently, Villanueva-Hernández et al. (2022) used pEuMV-YP to express in N. benthamiana an siRNA against the Krt18 gene in mice (Mus musculus), showing that the expression in plants mediated by VIGS can be used to silence genes in different kingdoms, in this case, from plants to mammals. We naturally consume small RNAs from some plants that we use as food or medications. For example, in China, the Lonicera japonica plant is used against influenza A and the SARS-CoV-2 virus, and its active ingredient has been proven to be a small RNA, miRNA-type, miRNA2911 (Zhou et al., 2015; 2020), therefore the production in plants of dsRNA directed against human pathogens can be of medical interest, although there are many points still to solve, such as the low stability of sRNAs and reaching a sufficient concentration for it to be therapeutic. Although the number of studies on the silencing in plants or phytopathogens in Mexico is still limited, the methodological tools that have been created help expect that the number of investigations in this field continue to increase.
Challenges to establish sigs in the field
The potential of the iRNAs in tropical crops using the strategy of producing transgenic plant (HIGS) has been evaluated in several investigations. Although results have been promising, there are limitations in the legislations of most LAC countries for the agronomic use of genetically modified materials. However, the results have revealed the potential of iRNA to control, for example, Foc4, and pave the way for the evaluation of non-transgenic alternatives, SIGS (Dou et al., 2020; Ghag et al., 2014; Koch et al., 2019; Koch and Wassenegger, 2021; Mahto et al., 2020; Montezano et al., 2018; Qi et al., 2018; Qiao et al., 2021; Zhu et al., 2017).
In order to successfully protect plants and post-harvest fruits of agronomic interest using SIGS, the functionality of the dsRNAs in the plant-pathogen interaction must be studied. One of the challenges is the construction of nanovehicles that protect and extend the stability of the dsRNAs, which must be innocuous to humans and other living beings, as well as biodegradable. A nanoparticle that has these characteristics is bioclay, a layered double hydroxide clay that forms nanofilms, that nanoencaspulates the dsRNAs and keeps them stable for up to 20 days (Gebremichael et al., 2021). It was initially developed as a form of plant protection against phytopathogenic viruses, but it is currently being evaluated for protection against fungal diseases in plants. Another challenge to overcome is the improvement of the absorption of the dsRNAs in the fungi that have silencing machinery, but are recalcitrant, such as C. glosporoides. As well as finding more profitable dsRNA production costs. The next investigations should focus on solving these challenges to turn SIGS from a promise into a reality. The normativity also requires work, so that it can eventually become a commercial technology.
Perspectives of sigs
It is important to focus efforts on overcoming the technical limitations of SIGS, since is perspective is huge. The selection of possible targets is very broad. Practically any gene involved in the viability or the pathogenicity of an organism is susceptible of being a target, and the ease of production of dsRNAs makes this technology friendly for large-scale studies. Its nearest commercial applications are the control of plant and human disease vector insects (Castellanos et al., 2019; Zheng et al., 2019; Zotti et al., 2018), the control of post-harvest fruit and vegetable diseases (de Oliveira et al., 2021; Qiao et al., 2021) and the control of weeds (Zabala-Pardo et al., 2022), because there are already reports that prove the conditions under which they can be effective for these cases.
Conclusions
The iRNA technology has great potential for the control of pests and diseases caused by fungi, particularly the SIGS strategy, the use of which is safe and eco-friendly. Further investigations in this direction on the agronomically important pathosystems in Mexico and Latin America is crucial to create a catalogue of the pathogens that can be controlled using SIGS.











 texto en
texto en 


