Plant diseases caused by fungal pathogens are a significant threat to food security (Fernandez-San Millan et al., 2021; Delgado-Baquerizo et al., 2020). Fungi are estimated to be accountable for losses exceeding 65% in global crop yield, an annual escalation attributed to factors like shifts in agricultural practices and excessive usage of synthetic fungicides. These elements contribute to the emergence of resistant strains, complicating the control and early-stage detection of fungal infections (Delgado-Baquerizo et al., 2020; Fernandez-San Millan et al., 2021; Sharma and Sharma, 2020).
Prominent among the soil-borne fungal pathogens affecting plants are those belonging to the Fusarium genus, known for their pronounced pathogenicity. These fungi have been associated with numerous diseases across more than 200 crop species worldwide, causing significant economic losses (Fernandez-San Millan et al., 2021; Sampaio et al., 2020; Summerell, 2019). Their invasion of plants’vascular tissue leads to symptoms such as root and stem discoloration, decay, wilting, growth inhibition, and defoliation. These effects result from the blockage of xylem, impeding the movement of water and nutrients within the plant and ultimately leading to its death (Fernandez-San Millan et al., 2021; Sampaio et al., 2020).
The primary challenge in controlling these pathogens lies in their capability to reside within host organisms and form structures that enable them to remain dormant in the soil in the absence of hosts (Fernandez-San Millan et al., 2021; Panth et al., 2020).
An alternative approach for pest and disease control involves the utilization of RNA interference (RNAi), a natural mechanism in plants. Plants employ RNAi, which originates from viral infections, to generate specific resistance as a defense against viral attacks (Baulcombe, 2004). RNAi is generated through a process known as post-transcriptional gene silencing. This mechanism involves the recognition of double-stranded RNA (dsRNA) intermediates from the virus replication stage, which are cleaved into short RNAs of 21-26 nucleotides by an RNase III-like enzyme called Dicer. These small RNAs are incorporated into a RNA-induced silencing complex (RISC), containing an Argonaute (AGO) protein with endonucleolytic activity for cleaving the target RNA (Baulcombe, 2004; Van Kammen, 1997; Zhu et al., 2019). The tool known as virus-induced gene silencing (VIGS) was developed based on this natural mechanism. VIGS employs viral vectors designed to carry the fragment of the gene of interest for silencing, enabling the analysis of gene functions in plants (Baulcombe, 1999; Bekele et al., 2019). Additionally, it aids in pathogen control by systemic replication alongside the viral genome (Lange et al., 2013; Becker and Lange, 2010). Gene silencing has demonstrated effectiveness in reducing symptoms caused by different Fusarium species in Arabidopsis thaliana plants, through the silencing of the lanosterol C14α-demethylase gene (Koch et al., 2013). It has also been employed in banana (Musa spp.) by targeting genes related to conidial development and the production of velvet and FTF toxins (Ghag et al., 2014), in wheat (Triticum aestivum) by silencing the CHS3 gene of the chitin synthase family (Cheng et al., 2015), and in barley (Hordeum vulgare) through silencing AGO and DICER genes essential for the silencing mechanism (Werner et al., 2020).
Other potential target genes for the control of plant pathogenic fungi by gene silencing include chitin synthases (CHS), which are enzymes involved in the biosynthesis of chitin, an important structural component of the fungal cell wall (Roncero et al., 2003; Lenardon et al., 2010). Chitin and CHS are absent in plants and mammals, so this gene family has been considered a safe and attractive target for antifungal development (Martín-Udíroz et al., 2004; Cheng et al., 2015). Plant pathogenic fungi have multiple CHS genes and all of them have different roles in development and virulence (Lenardon et al., 2012; Geoghegan et al., 2017). It has been reported that mutation of chitin synthase V can cause alterations such as decreased infective capacity of Fusarium oxysporum in tomato (Solanum lycopersicum) plants (Madrid et al., 2003), but silencing of this same gene in Fusarium culmorum is not associated with reduced symptoms in maize plants (Chen et al., 2016). Likewise, in some Fusarium species, the evaluation of CHS4 has not been possible by generating mutants, but in silico evaluation has reported the possible existence of one or more CHS4 genes that have been related to a high identity with the CHS7 gene; however, analysis of CHS4 activity in Saccharomyces cerevisiae showed that they do not present the same cellular localization nor the same function in development (Larson et al., 2011; Martín-Udíroz et al., 2004; Roncero et al., 2003). However, in Penicillium chrysogenum it was observed that mutation of this gene reduces conidia formation and presents atrophied hyphae, causing changes in penicillin production (Liu et al., 2013). These reports demonstrate that the function and characteristics of each chitin synthase differ depending on the fungal species and this also varies according to the interactions that the pathogen has with the host plant and its environment.
Gene silencing across organisms from different biological kingdoms is achievable through bidirectional molecule exchange. This exchange occurs, for instance, between pathogens and host plants (Wang et al., 2016) and through cell-cell molecule movement. This inter-kingdom communication also involves transport mechanisms such as vesicles or receptor proteins (Majumdar et al., 2017; Weiberg et al., 2015). Moreover, certain fungal pathogens like Botrytis cinerea have the capacity to produce RNA interference (RNAi), enabling them to impede plant defense genes (Wang et al., 2017). Conversely, plant microRNA production initiates fungal gene silencing. For instance, in cotton (Gossypium hirsutum) plants expressing miR166 and miR159, specific genes of Verticillium dahliae are silenced, resulting in pathogen resistance (Zhang et al., 2016). While this approach is effective, it necessitates the use of genetically modified organisms (GMOs) (Cai et al., 2019).
An alternative strategy involves RNAi production in plants and subsequent transport to fungi via plant-replicating viral vectors, known as virus-induced gene silencing (VIGS). The systemic movement of RNAi from plants to fungi highlights the biotechnological potential of RNAi silencing models (Cai et al., 2018). This study aims to assess a VIGS vector carrying a fragment of the Fusarium sp. CHS4 gene for its effectiveness in controlling fungal infection in Nicotiana benthamiana.
Materials and methods
Plant material. Seeds of Nicotiana benthamiana were germinated in 120 mL unicel containers with Sunshine #3 special fine mix, which contains short fiber peatmoss, dolomitic lime and vermiculite, at 25±2 °C, with a photoperiod of 16/8 hours light/dark and weekly fertilization with 4 mL L-1 of Bayfolan® Forte (Bayer de México, S.A. de C.V. CropScience Division).
Fungal material. The fungus Fusarium sp. strain INECOL_BM-06, provided by the Institute of Ecology A.C. (INECOL), was used. This strain was isolated from the ambrosial insect Xylosandrus morigerus in the Jaguaroundi ecological park in Coatzacoalcos, Veracruz (Carreras-Villaseñor et al., 2022). The strain was maintained on Potato Dextrose Agar (PDA) plates (BD, DifcoTM, Franklin Lakes, NJ, USA), with subcultures every 30 days at room temperature, and was also preserved in 20% glycerol at -80 °C for 6 to 24 months.
Cloning of the chitin synthase 4 gene fragment of Fusarium sp. The fungal CHS4 gene fragment was amplified from DNA using the oligonucleotides CHS4F (5’-CAAATTATCCTCCTCATGTCGTTC-3’) and CHS4R (5’-TCAGGATCTTTCACCATGGC-3’). These oligonucleotides amplify a 204 bp region encompassing the distinctive domain of chitin synthase 4 found in filamentous fungi. The design of these oligonucleotides was based on the sequences from Fusarium graminearum (XM_011318750.1), F. oxysporum (XM_018376225.1), and F. verticillioides (XM_018888350.1), using the Primer designing tool provided by the National Center for Biotechnology Information (NCBI).
To ensure standardization, a series of tests were conducted, including gradient temperature alignment, annealing concentration, and oligonucleotide concentration assessments. The optimal conditions for the polymerase chain reaction (PCR) were as follows: initial denaturation at 95 °C for 1 min, followed by 30 cycles of denaturation at 95 °C for 20 s, annealing at 58 °C for 30 s, extension at 72 °C for 20 s, and a final extension step at 72 °C for 5 min. The resulting PCR product was then inserted into the pGEM-T Easy cloning vector (Promega®, Madison, WI, USA), in accordance with the manufacturer’s guidelines. Subsequently, the ligation products were employed to transform Escherichia coli TOP10 competent cells.
Construction of the VIGS EuMV-YP∆CHS4 Vector. For the development of the VIGS EuMV-YP∆CHS4 vector, we employed the VIGS vector pEuMV-YP:ΔAV1, which was designed based on the A genomic component of the bipartite geminivirus Euphorbia mosaic virus-Yucatan peninsula (EuMV-YP; DQ318937) (Villanueva-Alonzo et al., 2013). The 204 bp segment of the CHS4 gene, previously integrated into the pGEM:GCHS4 vector, was isolated using SphI and PstI restriction enzymes. Subsequently, it was fused with the VIGS pEuMV-YP:ΔAV1 vector, which had been previously treated with the same restriction enzymes. The resultant construct underwent sequencing by Macrogen Inc (Seoul, South Korea). Sequence analysis was performed using the NCBI BLASTn platform. The sequences were edited utilizing the Mega 6 (Tamura et al., 2013) and Biedit (Hall, 1999) software to eliminate vector-specific elements.
Inoculation of N. benthamiana plants with Fusarium sp. A fungal culture grown for 15-30 days in Potato Dextrose Broth (PDB) liquid medium (BD Difco™, Franklin Lakes, NJ, USA) was utilized. The mycelium was gathered and homogenized in a blender at maximum speed for 1 minute. The concentration of the mycelial fragments was adjusted to 106 mL-1 and combined with grenetin to reach a final concentration of 1% (v/v). This mixture of mycelial fragments was applied to a previously created scalpel cut of approximately 0.5 mm on the stem, positioned between the root and the first petiole of each plant. Uninoculated control plants (SI) harboring Fusarium sp. were inoculated with 1% (v/v) grenetin at the incision point. The plants were then maintained under the photoperiod conditions outlined earlier, either for 14 days or until plant mortality occurred.
Silencing induced by the VIGS EuMV-YP∆CHS4 vector in the context of Fusarium sp. and N. benthamiana interaction. To assess the influence of VIGS vector treatments during the infection process, symptoms of leaf drop and stem necrosis were monitored in N. benthamiana plants. Two groups were examined, each comprising six 3-week-old plants per treatment and collection day: the initial group of N. benthamiana plants was exposed to fungal inoculation five days prior to being subjected to bombardment with gold microprojectiles coated with VIGS vector DNA (as detailed later). Stem samples were obtained at 7 and 14 days post bombardment (dpB), corresponding to 12 and 19 days post inoculation (dpI) with Fusarium sp. These plants were labeled as E-CHS4 (7 dpB/12 dpI and 14 dpB/19 dpI, respectively). In the second group of N. benthamiana plants, bombardment with the VIGS vector preceded fungal inoculation by 5 days, adhering to the previously detailed conditions. Stem samples were collected at 7 and 14 dpI. These plants were designated as O-CHS4 (7 dpI/12 dpB and 14 dpI/19 dpB). All experiments were conducted in triplicate with 6 plants per replicate, yielding a total of 18 plants per treatment/collection day over the study period. For both examined plant groups, bombardment was performed using 1 μm gold microprojectiles (BioRad, Hercules, California, USA) coated with 10 μg of DNA for each viral component. In particular, 5 μg of the VIGS vector EuMV-YPΔCHS4 and 5 μg of the wild-type EuMV-YP component B were used for component A. Bombardment was conducted from a 2 cm distance from the plant, employing 30 PSI of helium gas. Control groups consisted of SI plants and plants solely inoculated with Fusarium sp (I). The number of lost leaves and the extent of damage caused by the fungus during the initial week were documented.
Quantitative PCR (qPCR) analysis of the relative expression of the CHS4 gene during the Fusarium sp. -N. benthamiana interaction. To assess the relative expression of the CHS4 gene, RNA extraction from collected stems was realized using TRIzolTM Reagent (Invitrogen, Carlsbad, CA, USA) according to the provided guidelines. Subsequently, for cDNA synthesis, 100-500 ng of total RNA and SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) were employed. In brief, 200 ng of oligo dT (Invitrogen, Carlsbad, CA, USA) was incubated for 5 minutes at 65 °C, followed by 1 minute on ice. Then, 4 μL of first-strand buffer, 1 μL of 0.1M DTT, and 1 μL of RNAseOUTTM were added and incubated at 25 °C for 5 minutes, followed by 50 °C for 60 minutes; finally, the reaction was deactivated by incubation at 70 °C for 15 minutes (modified from Gerard et al., 1997). Subsequently, 10-20 ng of cDNA was utilized for qPCR reactions. These reactions were conducted using the Applied Biosystems StepOne Real-Time PCR system (Applied Biosystems, Life Technologies, NY, USA) with a 48-well setup, along with SYBR Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA, USA). The qPCR conditions were as follows: one cycle of 50 °C for 2 minutes and 95 °C for 5 minutes, followed by 40 cycles of 95 °C for 30 seconds, 58 °C for 1 minute, and 72 °C for 30 seconds. The oligos used were CHS4F, 5’-CAAATTATCCTCATGTCGTTC-3’, and CHS4R 5’-TCAGGATCTTTCACCATGGC-3’ as previously mentioned. The results were normalized to the actin gene of Fusarium solani (XM_046282608.1). The treatments used to assess expression were as follows: (1) Uninoculated plants, (2) Plants inoculated with the fungus, (3) Plants inoculated with the vector, (4) E-CHS4 plants 7dpB/12dpI, (5) E-CHS14 plants 14dpB/19dpI, (6) O-CHS4 plants 7dpI/12dpB, and (7) Plants 14dpI/19dpB. Amplifications were performed in quadruplicate, and relative expression levels were calculated using the 2-ΔΔCT method (Livak and Schmittgen, 2001).
Statistical analysis. The data acquired concerning leaf loss and lesion size were subjected to ANOVA, followed by the Tukey post hoc test, using a significance level of p=0.05, through the employment of RStudio Team (2022) software. Before the ANOVA test, the normality of the data was assessed using the Shapiro-Wilk test, and the homogeneity of variances was verified through the Levene test.
Results
Analysis of the cloned CHS4 gene fragment. The obtained results indicated that the cloned gene fragment exhibited a 95% identity with the chitin synthase 4 gene of Fusarium vanettenii (XM_003053353.1), a 95% identity with the CHS4 gene of F. facilforme (CP104050.1), and a 94% identity with the CHS4 gene of a F. solani strain (CP090036.1). Upon translating the 204-nucleotide fragment into a protein, a sequence of 68 amino acid residues was discerned (Figure 1), within which the DADT domain was identified. This domain is among those characterizing the chitin synthase family 4, which is the most prevalent among filamentous fungi (Pacheco-Arjona and Ramirez-Prado, 2014).

Figure 1 Amino acid and nucleotide sequence of the partial CHS4 fragment from Fusarium sp. The inset shows the DADT domain, which characterizes family 4 of fungal chitin synthases
Evaluation of the symptomatology of Fusarium sp. inoculation in N. benthamiana. A comparison was made between the symptoms observed in the two groups of plants. The results revealed that within the E-CHS4 treatment, the average leaf loss amounted to 4 per plant during the initial week of Fusarium sp. infection, whereas for the O-CHS4 treatment, the average leaf loss per plant was 3. In the case of healthy SI plants, the average count of shed leaves was 2, a natural occurrence stemming from mechanical damage inflicted on the plants. Among the infection control plants (I), the average leaf loss reached 6. In the course of the statistical analysis, a noteworthy disparity in leaf drop emerged between the E-CHS4, O-CHS4, and SI treatments compared to the I treatment. Nevertheless, no significant difference materialized between the E-CHS4 and O-CHS4 treatments, and similarly, no significant difference was evident between the O-CHS4 treatment and the SI control. However, a marked variance was discerned between the E-CHS4 treatment and the SI control (Figure 2A). Another variable subjected to analysis for gauging the efficacy of VIGS treatments was the size of lesions arising from the fungus on plant stems. The average lesion size on N. benthamiana stems with E-CHS4 and O-CHS4 treatments measured 2 cm, whereas plants subject to the I treatment displayed lesions averaging 3 cm. SI control plants exhibited an average lesion size of 1 cm due to scalpel-induced mechanical damage. Statistical evaluation indicated that the mean lesion size did not display a significant difference between the E-CHS4 and O-CHS4 treatments. However, both treatments showed a substantial disparity when compared to the I plants solely infected with Fusarium sp. (Figure 2B). Collectively, these findings imply that treatments involving the EuMV-YPΔCHS4 VIGS vector can mitigate the symptoms triggered by Fusarium sp. in N. benthamiana. During the course of the experiment, other symptoms caused by the fungus were analyzed, such as loss of turgor, increased wilting and reduced plant growth. In Figure 3A line 1, it was observed that after 7dpB/12dpI, the I plants showed symptoms such as loss of turgor in the upper leaves and wrinkling and wilting of the same, while the plants with the E-CHS4 treatment showed physiological characteristics similar to the SI control plants (Figure 3A, line 1); however, wrinkling was presented in the upper leaves and a decrease in growth was observed compared to the plants with the SI control treatment. Stem necrosis was also observed (Figure 3A line 2). The effect on symptom attenuation was not permanent, since at 14 dpB/19 dpI (Figure 3B), the plants with the E-CHS4 treatment showed symptoms of wilting and wrinkling of the upper leaves, loss of leaf turgor and stem necrosis (Figure 3B line 1), specifically the stem necrosis presented characteristics similar to those observed in the I plants (Figure 3B line 2).

Figure 2 Number of leaves lost and average lesion size in cm, during E-CHS4 and O-CHS4 treatment with the VIGS vector EuMV-YP∆CHS4. A. Average number of leaves lost in N. benthamiana plants with E-CHS4 and O-CHS4 treatment, healthy control plants (SI) and plants inoculated with Fusarium sp. (I). B. Average lesion size in cm in plants with treatment E-CHS4, O-CHS4, SI and I. Letters above the bar indicate significant differences, with a Tukey’s analysis test with a p < 0.05.
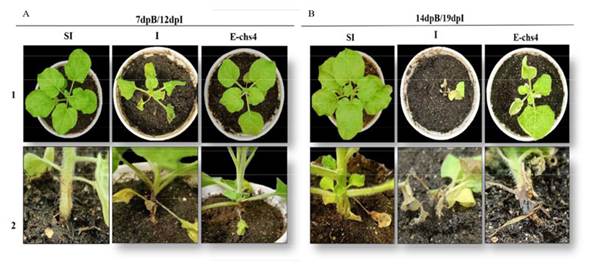
Figure 3 Symptom analysis in N. benthamiana plants inoculated with Fusarium sp. and subsequently inoculated with the VIGS vector EuMV-YP∆CHS4 (treatment E-CHS4). A. N. benthamiana plants at 7dpB/12dpI and B. N. benthamiana plants at 14dpB/19 dpI; SI: healthy control plants, I: plants inoculated with Fusarium sp. and E-CHS4: plants inoculated with Fusarium sp. and inoculated with the VIGS vector EuMV-YP∆CHS4. 1: Top view and 2: Side view of plants.
In the O-CHS4 treatment, it was observed that the plants at 7 dpI/12 dpB showed a lower level of symptoms compared to the I plants and showed characteristics similar to the SI control plants (Figure 4A line 1), only damage was observed at the base of the stem (Figure 4A line 2). The decrease in symptoms in this treatment was not a permanent effect, since at 14 dpI/19 dpB (Figure 4B line 1), the symptoms of wilting and loss of leaf turgor increased and were similar to that observed in the fungal infection control plants I. The damage to the base of the stem of the plants with the O-CHS4 treatment was similar to that observed in the I plants (Figure 4B line 2). These results showed that the EuMV-YPΔCHS4 VIGS vector is capable of delaying symptoms in the Fusarium sp.-N. benthamiana interaction during the establishment of infection, but this effect is transient.

Figure 4 Symptom analysis in N. benthamiana plants inoculated with the VIGS vector EuMV-YP∆CHS4 and subsequently inoculated with Fusarium sp. (treatment O-CHS4). A. Plants at 7dpI/12dpB. B. Plants at 14 dpI and 19 dpB; SI: healthy control plants, I: plants inoculated with Fusarium sp and E-chs4: plants inoculated with Fusarium sp. and inoculated with the VIGS vector EuMV-YP∆CHS4. 1: Top view and 2: Side view of plants.
Relative expression of the CHS4 gene of Fusarium sp. during its interaction with N. benthamiana plants. In plants subjected to the E-CHS4 treatment, it was observed that the expression of the CHS4 gene increased fourfold after 7 dpB compared to the I control. However, at 14 dpB using the VIGS EuMV-YPΔCHS4 vector, the relative expression of the CHS4 gene decreased by 20% in comparison to the infected control (Figure 5). For plants treated with the O-CHS4 regimen, a reduction in the relative expression of CHS4 by 30% was noted after 7 dpI in comparison to infected control plants. Nevertheless, at 14 dpI, the relative expression of the CHS4 gene increased 2.5 times relative to the infected control (Figure 6). These findings suggest that the observed effects on the symptoms of E-CHS4 and O-CHS4 plants may be linked to the transient silencing of the CHS4 gene, stemming from the presence of the EuMV-YPΔCHS4 VIGS vector and its replication within N. benthamiana plants.

Figure 5 Relative quantification by qPCR analysis of the CHS4 gene fragment of Fusarium sp. in plant stems with the E-CHS4 treatment. The Fusarium actin gene was used to normalize the data. E-CHS4 7dpB: relative CHS4 expression in Fusarium sp. inoculated plants at 7 days with E-CHS4 treatment; E-CHS4 14dpB: relative CHS4 expression in N. benthamiana plants with Fusarium sp. at 14 days with E-CHS4 treatment. I: Inoculated control plants.
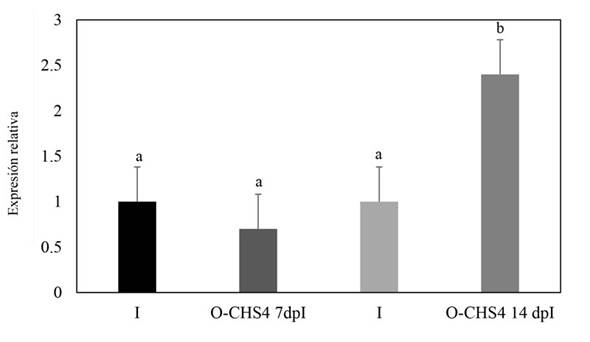
Figure 6 Relative quantification by qPCR analysis of the CHS4 gene fragment of Fusarium sp. in plant stems with the O-CHS4 treatment. The Fusarium actin gene was used to normalize the data. O-CHS4 7dpI: relative CHS4 expression in N. benthamiana plants with EuMV-YP∆CHS4 VIGS vector treatment seven days post inoculation with Fusarium sp.; O-CHS4 14 dpI: relative CHS4 expression in N. benthamiana plants with EuMV-YP∆CHS4
Discussion
In the plant-pathogen interaction, bidirectional exchange facilitates the establishment of pathogen infection and activation of host defense mechanisms (Cai et al., 2019). This study assessed the impact of employing a VIGS vector based on Euphorbia mosaic virus-Yucatan Peninsula (EuMV-YP) to induce silencing of the CHS4 gene of Fusarium sp. during its interaction with N. benthamiana. The results obtained demonstrated a reduction in symptoms among plants infected with Fusarium sp. and subjected to bombardment with the VIGS EuMV-YPΔCHS4 vector. Importantly, this effect was achieved without generating stable transgenic plants or relying on fungal mutants, which are conventional methods employed for the analysis of fungal genes.
VIGS systems initially evolved as tools for plant gene analysis. However, the evolution of novel protocols and viral vectors has demonstrated their applicability in fungi. These systems offer a direct and facile strategy, effectively curtailing costs and labor time (Tinoco et al., 2010; Mascia et al., 2014). Application of the VIGS EuMV-YPΔCHS4 vector yielded a reduction in the distinct symptoms induced by Fusarium sp. infection in N. benthamiana plants, including leaf drop and lesion size (Figure 2). Parallel outcomes were documented in studies leveraging HIGS (host-induced gene silencing) technology. For instance, in A. thaliana, plants expressing dsRNA against the lanosterol C-14α-demethylase (CYP5) gene of F. graminearum remained symptom-free after infection, unlike their non-transformed dsRNA-lacking counterparts. These plants displayed a mere 0.9% leaf area infected, in stark contrast to the 77% presented by untreated, infected controls, three days post-inoculation (Koch et al., 2013). Conversely, wheat plants expressing dsRNA featuring a hairpin structure targeted at the chitin synthase 3 (Chs3b) gene of F. graminearum demonstrated subdued infection symptoms. Additionally, lesion length diminished from 8.5 mm to values ranging between 6.7 mm and 4.5 mm across all gene constructions evaluated (Cheng et al., 2015). In the context of silencing technologies such as SIGS (Spray-induced gene silencing), Höfle et al. (2020) illustrated that applying dsRNA of varying lengths derived from the CYP5 gene onto A. thaliana leaves resulted in up to an 82% reduction in F. graminearum infection symptoms, compared to controls. This outcome echoes our observations in this study utilizing VIGS. Here, the EuMV-YPΔCHS4 VIGS vector led to a decrease in lesion size from 3 cm, as seen in plants solely inoculated with Fusarium sp., to 2 cm. From these data, it is reasonable to infer that silencing mechanisms may elicit disparate responses and phenotypes, even when targeting genes with related functions or within the same fungal species.
While the HIGS technique has been introduced as a strategy for exploring fungal-plant interactions, it operates through the generation of transgenic plants capable of producing RNAi (Nowara et al., 2010; Panwar et al., 2013; Cheng et al., 2015). This characteristic has limited its acceptance among consumers due to potential unknown environmental consequences (Cai et al., 2019; Wang et al., 2016). Consequently, the use of VIGS vectors emerges as an alternative mechanism to the HIGS system, forming part of the new generation of RNA-based fungicides (Padilla-Roji et al., 2023).
The application of VIGS to silence the expression of fungal genes has been previously documented. Barley stripe mosaic virus (BSMV) has served as a VIGS vehicle to express dsRNA targeting genes associated with the haustorium of Puccinia triticina f. sp. tritici (Pst) (Yin et al., 2011). These findings revealed that silencing was most effective in haustorium cells with greater contact with host cells (Yin et al., 2011). Similarly, wheat plants employing BSMV as a VIGS tool were used to evaluate dsRNA targeting three Puccinia triticina (Pt) genes: PtCYC1 (cyclophilin), PtMAPK1 (a protein kinase), and PtCNB (a calcineurin regulatory subunit), all associated with pathogenicity. The study indicated that silencing any of these three target genes led to a reduction in lesion size during the infection process (Panwar et al., 2013). In another instance, BSMV-mediated silencing of the PstCFEM effector gene in wheat plants resulted in decreased sporulation, leading to a significant reduction in fungal biomass and infection area (Bai et al., 2022). In this study, the utilization of EuMV-YP as a VIGS vector resulted in observed reductions in foliar damage (leaf drop and wrinkling), as well as diminished lesion size following Fusarium sp. infection. These effects were attributed to the presence of the EuMV-YPΔCHS4 VIGS vector. This suggests that VIGS vectors offer non-transgenic, environmentally friendly alternatives for studying and analyzing new tools against plant pathogens and insect pests (Coleman, 2016; Dinolfo et al., 2017; Martín-Udíroz et al., 2004), while also highlighting the application potential of EuMV-YP-based VIGS vectors.
Apart from the observed disparities in symptomatology between plants infected with Fusarium sp. and those treated with the VIGS EuMV-YPΔCHS4 vector, compared to plants solely infected by Fusarium sp., notable silencing effects of up to 20% on the CHS4 gene expression were evident in plants subjected to the E-CHS4 treatment (Figure 5). Likewise, in plants treated with the O-CHS4 regimen, a significant silencing of 30% in the CHS4 gene expression was evident (Figure 6). Nevertheless, the exclusive silencing of the chitin synthase 4 gene proved insufficient to avert the progression of fungal infection, consequently leading to an inability for plant survival or recovery.
This loss of silencing effect could be attributed to the presence of multiple chitin synthases. It has been reported that disruptions in cell wall synthesis can trigger a compensatory response to safeguard fungal cell wall integrity. This response often involves heightened chitin synthesis through alternative chitin synthase members (Kappel et al., 2020). Moreover, processes such as the overexpression of enzymes like deacetylases, which facilitate the conversion of chitin to chitosan, are also implicated (Kappel et al., 2020). Furthermore, it’s worth noting that the expression of one or more chitin synthase genes can be intricately tied to the developmental stage of the fungus (Roncero et al., 2003; Kappel et al., 2020). For instance, F. oxysporum mutants with single chitin synthase gene disruptions (1, 2, or 7) exhibited no discernible variations in colony morphology or chitin production when compared to the wild-type strain (Martín-Udíroz et al., 2004). Conversely, the elimination of the CHS5 gene in F. oxysporum strains led to swollen hyphae, demonstrating heightened sensitivity to H2O2 (Roncero et al., 2003). This indicates that the loss of activity in a single chitin synthase gene can result in significant structural alterations. However, the impact of such loss on virulence or fungal development can vary across interactions. For instance, when an F. oxysporum strain with a lethal CHS5 gene mutation infected tomato plants, it exhibited reduced efficiency in colonizing the tomato vascular system and slower growth (Madrid et al., 2003). Conversely, the analysis of lethal mutant strains of the CHS1 and CHS2 genes of F. oxysporum didn’t yield effects on chitin content or sporulation, and viable mutants of the CHS3 gene couldn’t be obtained (Martín-Udíroz et al., 2004). In another vein, Cheng et al. (2015) demonstrated the expression of CHS3 constructs that silenced gene expression in F. graminearum during its interaction with wheat plants, resulting in a remarkable 61% reduction in infected spikes.
The disruption of the CHS4 gene didn’t induce phenotypic changes in B. cinerea. In other species such as Aspergillus sp. and Neurospora crassa, where CHS4 has been identified as an enzyme with overlapping function, its removal didn’t result in conspicuous morphological shifts in the fungal wall. However, it did lead to a reduction in wall chitin concentration (Din et al., 1996). A divergent scenario is seen in Saccharomyces cerevisiae, where the CHS4 gene’s product accounts for 90% of the cell wall chitin production and is also intertwined with the modulation of the sexual cycle (Morcx et al., 2013). Similarly, silencing the CHS4 gene in Penicillium chrysogenum diminished its expression by 91%, subsequently causing reduced colony size and a decreased count of conidia (Liu et al., 2013). In contrast, for fungal species such as F. oxysporum, isolating CHS4 proved challenging. Only through in silico analysis using F. graminearum data could the existence of one or more CHS4 genes in F. oxysporum be ascertained, along with its connection to CHS7 (Martín-Udíroz et al., 2004). Thus, the application of VIGS technology for the analysis of multiple or challenging-to-isolate genes obviates the need for complete elimination. This approach allows for the study of essential genes, mutation of which proves lethal (Mascia et al., 2014). In this study, transient silencing of the Fusarium sp. CHS4 gene was achieved using VIGS. This transient silencing permits the examination of its plausible role in the infection process in N. benthamiana, and it also proposes the potential utilization of EuMV-YP-based VIGS vectors for the analysis of other genes within this gene family.
In both the E-CHS4 and O-CHS4 treatments, noticeable alleviation of symptoms was observed in Fusarium sp.-infected plants, irrespective of the timing of VIGS EuMV-YPΔCHS4 vector inoculation. Prior studies by Villanueva-Alonzo et al. (2013) and Luna-Rivero et al. (2016) showcased the systemic and uniform movement of the pEuMV-YP:ΔAV1 vector during infection. This trait facilitates the evaluation of gene fragment replication. Thus, the observed silencing of the Fusarium sp. CHS4 gene could be attributed to the translocation of RNAi from the infection site in N. benthamiana to encompass the entire plant and fungal cells. This systemic movement advantage characterizes VIGS-mediated silencing as distinct from other RNAi strategies in plants, where RNAi can traverse the organism systematically from the site of inoculation (Mascia et al., 2014).
A plethora of studies underscore the bidirectional exchange of RNAs and RNAi across diverse kingdoms during plant-pathogen interactions. This process empowers pathogens to curtail plant defenses while concurrently furnishing plants with up to 60% disease resistance (Mapuranga et al., 2023). The bidirectional nature of this exchange engenders evidence of such phenomena. For instance, Zhang et al. (2016) noted that when isolating the fungus Verticillium dhaliae from cotton plants, the obtained microRNA sequences primarily aligned with cotton microRNA sequences rather than those of the fungus. This observation implies that these RNAs were transported from cotton to the fungus during the infection process.
Likewise, evidence of the capacity of B. cinerea fungal cells to absorb plant exosomes emerged via in vitro exposure of the fungus to TET8-GFP-labeled exosomes from A. thaliana. The uptake of exosomes by fungal cells was confirmed as they were found inside the fungal cells two hours after exposure, persisting until cellular lysis with Triton X-100 (Cai et al., 2018). Additionally, studies have indicated that spraying dsRNA onto barley leaves impeded F. graminearum infection by approximately 50% through constructs targeting AGO and DCL genes. Notably, these genes contribute not only to silencing but also to mycotoxin production and disease progression in F. graminearum. This observation highlights fungal pathogens’ ability to absorb environmental RNA (Werner et al., 2020).
The achieved results underscore that VIGS technology facilitates gene analysis in plant-fungus interactions without necessitating protocols for fungal transformation or generation of transgenic plants, unlike methodologies such as HIGS (Hua et al., 2018). Moreover, it obviates the reliance on obtaining resistant cultivars and mitigates the risk of instability inherent to dsRNA-based SIGS methodologies (Hua et al., 2018).
Conclusions
The VIGS EuMV-YPΔCHS4 vector, derived from EuMV-YP, exhibited the capacity to partially suppress the CHS4 gene amid the Fusarium sp. infection in N. benthamiana plants. The principal consequence of silencing, brought about by the VIGS EuMV-YPΔCHS4 vector, lay in the alleviation of symptoms. These included diminished leaf drop, reduced lesion size on the stem, and a curbed loss of turgor in the plants. The assessment of this VIGS vector within the context of plant-pathogen interaction illuminates its potential in deciphering gene significance in fungal viability and pathogenicity.











 texto en
texto en 


