Climate change threatens the health and survival of urban trees, as well as the diverse benefits that they provide the inhabitants of cities. By the year 2050, most species will be at risk due to the changes projected for temperature and rainfall. Along with the abiotic stress caused by global warming, urban trees face increasing emerging diseases, particularly those caused by fungal pathogens and oomycetes (Rafiqi et al., 2022). Although they are not classified as trees, palms play an important role in the biodiversity of different urban areas or in forestry; however, the majority of studies on the pathologies have been carried out on species of agronomic interest, such as the date palm (Phoenix dactylifera), leaving an area of study to determine what takes place in ornamental species found in urban areas. In this sense, the genus Phoenix (Phoeniceae: Coryphoideae) comprises species that display a wide and diverse geographic distribution (Barrow, 1998). They live in very diverse habitats which range from coastal areas to areas 2000 masl. The former includes P. canariensis, P. reclinata, P. roebeleni and P. sylvestris. P. dactylifera is the only one of agronomic interest. Phoenix canariensis is endemic to the Canary Islands, off the coast of western Africa (Rivera et al., 2013). In their natural habitat, they grow to about 18 to 20 m in height, unless affected by pests or diseases, the plant can live up to 200 and 300 years. Completely mature palms can weight up to 10 tons (Barrow, 1998). This palm has diverse uses: as an ornamental plant, it is enormously valuable, since it is probably the most widely used palm in gardening worldwide, in comparison with other resistant ornamental palm species, such as the Californian fan palm (Washingtonia filifera) and the Mexican fan palm (W. robusta). Phoenix canariensis is distributed worldwide in temperate and warm areas (Spennemann, 2018). In Mexico City, this plant species is enormously important, since it is a part of its landscape identity; there are an estimated 15 thousand palms distributed in the different boroughs (SEDEMA, 2022). However, since 2011 there have been records of palm mortality (SEDEMA, 2021), supposed due to biotic factors, mostly caused by fungi, without having determined the causal agent, therefore the problem persists, and is on the rise, with 500 dead palms on record to date (SEDEMA, 2022). Due to this, the aim of this work was to determine the fungi related to symptoms of decline and death of canary palms in Mexico City, in order to propose the best management strategies.
Nine sites with palms with symptoms of decline and death were sampled: Avenida Paseo de las Palmas (APP) with nine palms, Diagonal San Antonio (DSA) with four, Campestre Churubusco Golf Club (CGC) with four, El Papalote Museo del Niño (PMN) with seven, La Glorieta de la Palma (LGP) with six, Ferrería (FER) with seven, Sara corner with Saúl (SES) with seven, Anselmo de la Portilla (ADP) with six and 535 Avenue corner with Talismán (AET) with six, for a total of 56 palms in Mexico City.
Using directed visual sampling, from February to August of 2022, 10 asymptomatic palms were selected, along with 46 with symptoms of chlorosis, necrosis and death, which were classified based on the proportion of living crown (Prcv), using a scale of modified health visual evaluation of the protocols by Blair et al. (2019ab) and Bond (2012), being (a) a healthy or asymptomatic palm with a Pcrv ˃ 6; Initial, between 5 and 6; Intermediate = 4; and advanced, ≤ 3.
A sample of trunk, petiole, rachis and leaflet was taken from every palm, for a total of 224 samples analyzed. They were separately placed in airtight polyethylene bags and transported in ice chests for processing in the Forest Pathology lab in the Plant Health program of the Colegio de Postgraduados, Campus Montecillo.
For the isolation of fungi, each sample was washed with a 0.5 % sodium hypochlorite solution and dried with sterile paper towels. They were cut in 5 mm2 pieces from the margin between healthy and diseased tissue, then disinfested with a 3 % sodium hypochlorite solution for 2 min, rinsed three times with distilled water for 1 min and dried at room temperature on sterilized paper towels. They were then plated in Petri dishes with water-agar (AA) medium and checked every 24 hours for mycelial growth. Once the mycelium developed, pieces of culture medium were taken and aseptically transferred onto potato-dextrose-agar (PDA) medium and incubated at 22 °C with a 12 h photoperiod until structures developed. Monoconidial cultures were obtained, which were preserved in tubes with PDA covered with sterile mineral oil at 15 °C for their later study. Likewise, humid chambers were performed to promote the sporulation of conidiomata on the surface of diseased plant tissue. The fungal cultures that developed were placed in Petri dishes with culture medium for their cultural and morphometric characterizations using the literature (Barr et al., 1989; Ligoxigakis et al., 2013; Rangel et al., 2021; Troncoso y Tiznado, 2014).
The isolation frequency of the main fungi was estimated by calculating the percentage of isolates of each fungus in all the samples collected (number of isolates/n, where n = 144 total of isolates). Likewise, the rate of occurrence of each species of fungus was calculated as the frequency of occurrence of a species in the nine sampling sites. In the present investigation, 144 fungal isolates were obtained from the trunk, petiole, rachis and leaflets of 56 palms (Phoenix canariensis) sampled in Mexico City with symptoms of decline and death. Eleven fungal species were identified by morphology per damage category (Table 1).
Table 1 Fungal species isolated from palms with decline and death in Mexico City by damage category.
| Categoría de daño | Especies de hongos aisladas | |||
|---|---|---|---|---|
| Peciolo | Raquis | Foliolo | Tronco | |
| Aparentemente sana | Alternaria alternata | Alternaria alternata | ||
| Lasiodiplodia sp. | Lasiodiplodia sp. | Alternaria alternata | ||
| Nalanthamala vermoesenii | Nalanthamala vermoesenii | Lasiodiplodia sp. | ||
| Neopestalotiopsis sp. | Phoma glomerata | Neopestalotiopsis sp. | ||
| Phomopsis sp. | Phomopsis sp. Trichoderma sp. | Phoma glomerata | ||
| Trichoderma sp. | Phomopsis sp. | |||
| Inicial | Nalanthamala vermoesenii | Alternaria alternata | ||
| Neopestalotiopsis sp. | Nalanthamala vermoesenii | Alternaria alternata | Alternaria alternata | |
| Penicillium sp. | Phoma glomerata | Neopestalotiopsis sp. | Fusarium sp. | |
| Phomopsis sp. | Phomopsis sp. | Phoma glomerata | Phoma glomerata | |
| Intermedia | Alternaria alternata | |||
| Cladosporium sp. | Lasiodiplodia sp. | Alternaria alternata | Alternaria alternata | |
| Lasiodiplodia sp. | Nalanthamala vermoesenii | Lasiodiplodia sp. | Phoma glomerata | |
| Nalanthamala vermoesenii | Phoma glomerata | Phoma glomerata | Trichoderma sp. | |
| Neopestalotiopsis sp. | Serenomyces sp. | |||
| Avanzada | Lasiodiplodia sp. | Alternaria alternata | Alternaria alternata | |
| Nalanthamala vermoesenii | Fusarium sp. | Lasiodiplodia sp. | Alternaria alternata | |
| Phomopsis sp. | Lasiodiplodia sp. | Penicillium sp. | Phoma glomerata | |
| Serenomyces sp. | Penicillium sp. | Phoma glomerata | ||
From the 11 fungal species, four represented 81.9 % of the species isolated from the trunk, petiole, rachis and leaflet, these were considered as the most abundant in the nine sites in which the investigation was carried out (Figure 1), namely: Alternaria alternata, with the highest prevalence (34.7 %), followed by Phoma glomerata, Nalanthamala vermoesenii and Lasiodiplodia sp., with 22.9 %, 13.9 % and 10.4 %, respectively. It is worth pointing out that these four species form well-defined groups, that is, those that attack the petiole and rachis, mainly N. vermoesenii and Lasiodiplodia sp., although Neopestalotiopsis sp. and Phomopsis sp. were also found, along with those that attack the trunk and leaflets, such as A. alternata and P. glomerata.

Figure 1 Frequency of fungal species isolated from the trunk, petiole, rachis and leaflet of Phoenix canariensis in nine sampling sites in Mexico City.
On the other hand, Penicillium sp., Fusarium sp., and Serenomyces sp. displayed a moderate frequency of 2.77, 2.08 and 1.38 %, respectively. Trichoderma sp. and Cladosporium sp. had a low frequency, with percentages of 1.38-0.69.
The rate of occurrence of each fungal species in each sampling site was variable (Figure 2). According to the relative frequency, SES and AET housed the greatest diversity of fungal species (six species), followed by APP, DSA and PMN (five species); CGC, LGP and ADP (four species), and finally, FER (three species).
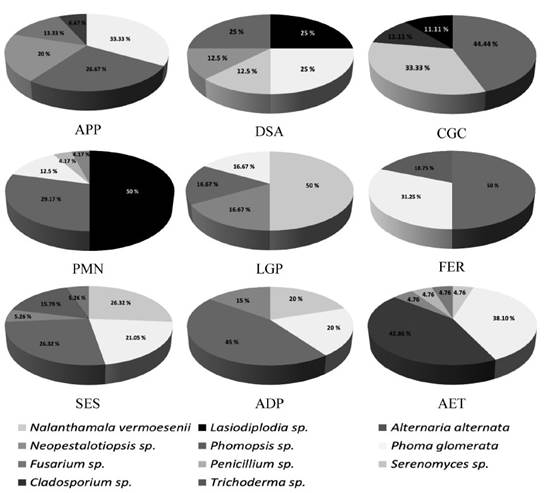
Figure 2 Species of fungi isolated from the trunk, petiole, rachis and leaflets in each sampling site and the percentage of each fungal species in regard to the total species obtained for each site. APP: Avenida Paseo de las Palmas; DSA: Diagonal San Antonio; CGC: Campestre Churubusco Golf Club; PMN: El Papalote Museo del Niño; LGP: La Glorieta de la Palma; FER: Ferrería; SES: Sara, corner with Saúl; ADP: Anselmo de la Portilla; AET: 535 Avenue, corner with Talismán.
Alternaria alternata presented a higher occurrence, since it was present in all nine sites, followed by Phoma glomerata, found in eight sites; these fungi were isolated mainly from the leaflets. On the other hand, out of the fungi found in petioles and rachis, N. vermoesenii had the greatest occurrence, since it was found in seven of the nine sites, followed by Neopestalotiopsis sp. (five sites) and Lasiodiplodia sp. (three sites).
Based in the frequency and the number of isolates obtained, Nalanthamala vermoesenii, Lasiodiplodia sp., Neopestalotiopsis sp., Alternaria alternata, Phoma glomerata and Serenomyces sp. were determined as the fungi with the greatest importance in terms of decline and death of palms in Mexico City, although it is worth pointing out that palms displayed one or more fungi, yet they were recognized based on the type of symptom described and the isolate of the fungus. Due to this, the following symptoms are described:
Nalanthamala vermoesenii. The symptoms were the death of leaves, which progresses from the youngest to the oldest; leaves become dark brown. One of the main characteristics was that the leaves remain attached to the palm, but they precipitate towards the trunk, taking the shape of a skirt. The fronds display necrotic lesions, defined by a moist margin, which covers from the petiole to the apex of the frond (Figure 3 a). The internal symptoms display a rot in the bud with an unpleasant odor, whereas the petiole and rachis in their initial stages present a dark brown decoloring, as in the case of the bud, and in advanced stages (death of fronds) the decoloring is opaquer. The sign that indicates the presence of pink rot are the abundant salmon- or pink-colored spore masses on the infected tissues (Figure 3 b). In some cases, pink rot causes external symptoms that can be distinguished by the lateral decoloring of the petiole and rachis.

Figure 3 Symptoms and signs of the six main fungi isolated from the petioles, rachis and leaflets of Phoenix canariensis. a and b) Nalanthamala vermoesenii; c and d) Lasiodiplodia sp.; e and f) Neopestalotiopsis sp.; g, h and i) Alternaria alternata; j, k and l) Phoma glomerata; m, n and o) Serenomyces sp.
Lasiodiplodia sp. The symptoms were the death of fronds, which progresses from the older to the younger leaves, all of which acquire a pale green to light brown color. The leaves remain attached to the palm, maintaining its natural structure, unlike the palms infected with N. vermoesenii. In initial to intermediate symptoms, leaflets present dieback, a symptom known as blight (Figure 3 c) and the transition zone (area between healthy and diseased tissue) presents an olive green to light brown color; in initial stages they presented dark brown areas on the sides (Figure 3 d), whereas in later stages, the color reaches the inner internal tissues, and finally, when the fronds die, they become black. Occasionally, the external part displays light brown stripes that stretch from the petiole to the rachis of the frond.
Neopestalotiopsis sp. The symptoms were observed mainly in the petiole. The external part displays a dark brown color, mainly at the sides of the petiole (Figure 3 e). Regarding internal symptoms, a light brown color appears with a dark brown to black margin (transition zone) (Figure 3 f). As the symptoms progress, the entire internal tissue is affected, and black acervuli develop in the diseased tissue.
Alternaria alternata. The symptoms were dieback of leaflets (from the tip to the base of the leaflet). In advanced stages, the symptoms appear as blight, covering the entire leaf, leaflets become light brown (Figure 3 g), in the transition between the healthy and diseased tissue, a darker color appears (Figure 3 h e i), and in this zone over the tissue is where the conidia, characteristic of this pathogen, appear.
Phoma glomerata. Symptoms were mainly observed in leaflets and, to a lesser extent, in the rachis. In the leaflets, light brown circular spots surrounded by a dark brown margin (Figure 3 j), whereas in later stages, the lesions grew in size and coalesced, forming longer spots (Figure 3 k). In the rachis, circular spots develop, similar in color to the case of the leaflets (Figure 3 l), and in the lesions, light brown pycnidia are developed.
Serenomyces sp. Symptoms were mainly observed in the petiole and rachis. The begin with slightly sunken, moist-looking spots, bright brown un the center and circumscribed by maroon margins. In later stages, the spots coalesce and form maroon lines, either in the middle part or on the sides of the petiole and rachis (Figure 3 m and n), and on the diseased tissue in a moist chamber, dark brown to black, semi-sunken perithecia develop, with a long neck through which the light brown ascospores are expelled (Figure 3 o).
Little investigation has been carried out on phytopathogenic fungi that affect the Canary Island date palm (P. canariensis), despite being a very important ornamental species. Worldwide, this palm species has presented diseases such as pink palm rot, cause by the fungus Nalanthamala vermoesenii (syn. Penicillium vermoesenii; Gliocladium vermoesenii), which is widely distributed in temperate, Mediterranean and subtropical climates in Spain, Egypt, Australia, Belgium, Congo, Czech Republic, India, Japan, New Zealand, Russia, South Africa, the United Kingdom, United States, Greece (Ligoxigakis et al., 2013; Mohamed et al., 2016).
Likewise, three species of Fusarium have been reported: F. oxysporum f. sp. canariensis, causal agent of wilting by Fusarium, present in France, Italy, Japan, United States, Argentina, Australia, Canary Islands, Greece and Spain (Elena, 2004; Palmucci, 2005; Hernández et al., 2010). Fusarium proliferatum, the causal agent of wilting by Fusarium, syndrome of sudden decline, present in the Canary Islands and Spain (Hernández et al., 2010). F. oxysporum f. sp. palmarum, causal agent of wilting by Fusarium, lethal death, present mainly in the United States, in the state of Florida (Elliott et al., 2010; Elliott, 2011).
In Mexico, this investigation is the first study focused on the identification of the associated agents with the decline and death of P. canariensis palms in Mexico City. The study reveals 11 fungal species via the sampling and diagnosis, as the agents related to the trunk, petiole, rachis and leaflets. Alternaria alternata and Phoma glomerata were mainly isolated from diseased leaflets, and the former coincides with reports by Maitlo et al. (2014), except for the fact that these authors isolated them from Phoenix dactylifera. In the case of Phoma, other species have been isolated from leaflets such as Phoma sp. (Abdullah et al., 2010) and Phoma ucladium (Maitlo et al., 2014), as well as in P. dactylifera. It is worth pointing out that the present investigation also isolated A. alternata and P. glomerata from the trunk, which has not been reported by any other author to date. Regarding the fungi that were most frequently isolated from petiole and rachis, that is, N. vermoesenii, Lasiodiplodia sp., Neopestalotiopsis sp., and Phomopsis sp., in the case of N. vermoesenii, this coincides with reports by Mohamed et al. (2016), Ligoxigakis et al. (2013), who also isolated this species of petiole and rachis in P. canariensis. In regard to Lasiodiplodia sp., our results only coincide with reports by Santos et al. (2020), who isolated L. theobromae and L. pseudotheobromae from rachis (among other tissues) from Cocos nucifera. These and other species of this fungus have been isolated from P. dactylifera such as L. hormozganensis and L. theobromae from roots (Al-Hammadi et al., 2019); L. theobromae from P. dactylifera and P. hanceana from unspecified tissue (Farr and Rossman, 2020), from leaves of Cocos nucifera (Ramjegathesh et al., 2019) and the pod of the leaf of P. roebelenii. In Germany L. brasiliensis, L. euphorbicola, L. lodoiceae and L. mexicanensis were found in leaves from Mexico of the palm species Chamaedorea elegans, C. metallica, C. seifrizii, Dypsis lutescens and Lodoicea maldivica (Douanla and Scharnhorst, 2021). Neopestalotiopsis sp. has been reported in oil palm with the species N. saprofitica (Ismail et al., 2017).
Out of the least frequently isolated species, it is worth mentioning the presence of Fusarium in rachis and trunk, unlike reports by Vergara et al. (2023), where the most frequently isolated fungi were F. incarnatum, F. verticillioides and F. solani, and in which the latter was pointed out as the cause of the regressive death of Phoenix canariensis in urban areas of Querétaro, Mexico. This fungus is considered one of the most devastating for date palm in the United Arab Emirates (Alwahshi et al., 2019), and it causes different diseases called sudden decline and wilting syndrome from Fusarium, attributed to Fusarium oxysporum, F. moniliforme, F. proliferatum and F. solani; vascular fusariosis or Bayoud’s disease, caused by F. oxysporum f. sp. albedinis, found in northern Africa, and which caused de death of around two thirds of the date palm plants (Tantaoui et al., 1996); wilting by Fusarium, caused by Fusarium oxysporum f. sp. canariensis, is the main cause of disease in P. canariensis and other palm species in the Canary Islands (Hernández et al., 2010). In addition, during this investigation, the identification and in vitro planting of Serenomyces sp., isolated from the petiole and rachis of P. canariensis, becomes important, which coincides with reports by Elliott and Jardin (2006) and Elliott and Jardin (2014), who also isolated this fungus from the petiole and rachis of the same palm species, except in Florida, United States, where it causes the disease known as chlorosis and necrosis of the petiole and rachis. Based on the most frequent fungi, Nalanthamala vermoesenii and Lasiodiplodia sp. were related to the decline and death of palms in Mexico City, and less frequently, Neopestalotiopsis sp., Serenomyces sp., Alternaria alternata and Phoma glomerata.











 texto en
texto en 


