Avocado (Persea americana) has an enormous economic importance in Mexico due to the income it produces, making it one of the main agricultural products for export (SIAP, 2022); however, pests and diseases cause important losses in this crop (Urrea and Cardona, 2020). One of the diseases that affects fruit quality is avocado scab, which damages the epicarp of the avocado fruits and manifests itself as corklike lesions (Everett et al., 2011). The layers of corklike tissue are formed as histological defense structures for plants when attacked by a pathogen (Cruz et al., 2006).
Elsinoe perseae is the fungus that, since 1934, has been related to the symptomatology caused by avocado scab disease (Everett et al., 2011; Fan et al., 2017); however, there is a confusion regarding the damages caused by this pathogen and those caused by other biotic factors such as a high incidence of thrips and mites, and abiotic factors such as mechanical damages, damages caused by wind, etc. (SENASICA, 2018).
Jenkins reported that avocado scab is caused by E. persea and described the symptoms as dark, round or irregular spotty lesions than, when they join, can cover the avocado fruit partially or entirely, giving it a brown, corklike aspect (Everett et al., 2011; Fan et al., 2017). On the other hand, Morales (2017) described a similar symptom which he called purple spot, or purple spot, which displays round, initially purple lesions that become darker as they grow, and when the coalesce, look like scabs. However, other authors, such as Djeugap et al. (2015), Martínez-Hernández et al. (2017) and Becerra and Morales (2019), did not associate E. perseae to the symptom of the avocado scab, and rather related other pathogens such as Colletotrichum sp., Alternaria sp., Pestalotiopsis sp., Nigrospora sp. and Curvularia sp.
Due to this confusion, the aim of this study was to morphologically and molecularly identify the pathogens related to the avocado scab symptom, taking samples of fruits with a corklike aspect that are commonly known in the field as “typical scab” and “purple spot”. Pathogenicity tests were also carried out to reveal the causal agent of this disease.
Materials and methods
Gathering sites. Avocado fruits with symptoms of corklike aspects, which are commonly known in the field as “typical scab” and “purple spot”, were gathered from different orchard in Michoacán (Figure 1). After gathering, the fruits were placed in brown paper bags (26 x 12.5 x 6.5 cm) to avoid humidity and the development of saprophytic fungi.
To gather the avocado fruits, the agroecological areas were divided according to altitude (meters above sea level, or m.a.s.l.) in which, according to Anguiano et al. (2007) three types of climates stand out for the favorable development of this crop and to express its greatest productive potential: humid (1,600 to 1,800 m.a.s.l.), semi-warm subhumid (1,200 to 1,600 m.a.s.l.) and temperate subhumid (1,900 to 2,300 m.a.s.l.) (Table 1).
Isolation of the fungi. The avocado fruits gathered from the field were washed with tap water and later rinsed with distilled water to eliminate pollutants. Fresh lesions were taken from the epicarp of the fruit in 5 × 5 mm fragments. For the disinfection of fruits, the method proposed by Zhi Li et al. (2008) was used, with slight modifications. The fragments of the epicarp of avocado fruits were placed in a 6% sodium hypochlorite solution for 90 s, then rinsed with sterile distilled water. They were then washed for a second time with 70% ethanol for 60 s, and rinsed for the last time with sterile distilled water. The tissue was placed in sterilized paper towels to absorb humidity. Once the fragments of the plant material were disinfected, they were planted equidistantly in Petri dishes with a PDA culture medium, then the dishes were sealed with parafilm, incubated at 28 °C with a humidity of 80% for 72 h. After this time, the Petri dishes were inspected for fungal growth.
Table 1 Gathering sites of avocado fruit with symptoms of “typical scab” and “purple spot”.
| Áreas agroecológicas (m.s.n.m.) | Sitios de colecta | Coordenadas | Altura m.s.n.m |
|---|---|---|---|
| Semicálido subhúmedo (1,200 y 1,600) | Cerro colorado | 19°31’69”N, 100°46’58”O | 1,243 |
| Ordeñitas | 19°23’50”N, 102°18’94”O | 1,060 | |
| Patuan | 19°39’16”N, 101°91’50”O | 1,260 | |
| Zumpimito | 19°37’29”N, 100°55’4”O | 1,600 | |
| Ziracuaretiro | 19°43’57”N, 101°92’93”O | 1,380 | |
| Taretan | 19°33’38”N, 101°918’33”O | 1,130 | |
| Mesa de Cazares | 19°37’75”N, 101°85’39”O | 1,560 | |
| San Ángel Zurumucapio | 19°44’77”N, 101°88’86”O | 1,600 | |
| Pareo | 19°33’13”N, 102°45’08”O | 1,430 | |
| Jicalan | 19°38’33”N, 102°07’61”O | 1,600 | |
| Húmedo (entre 1,600 y 1,800) | Zacandaro | 19°35’94”N, 102°18’58”O | 1,700 |
| Cutzato | 19°36’63”N, 102°13’47”O | 1,700 | |
| Toreo el alto | 19°46’00”N, 102°00’30”O | 1,800 | |
| La Basilia | 19°46’25”N, 102°06’50”O | 1,860 | |
| San Andrés Coru | 19°46’75”N, 101°94’50”O | 1,700 | |
| San Juan Nuevo | 19°41’66”N, 102°12’86”O | 1,880 | |
| Templado subhúmedo (entre 1,900 y 2,300) | Ario de Rosales | 19°20’72” N, 101°70’80”O | 1,910 |
| Canacuas | 19°64’38” N, 102°04’83”O | 2,200 | |
| Tingambato | 19°50’19” N, 101°85’25”O | 1,980 | |
| Zirahuen | 19°54167”N, 101°73’19”O | 2,090 | |
| Tancitaro | 19°33’75”N, 102°36’30”O | 2,080 | |
| El Durazno | 19°08’25”N, 101°66’00”O | 2,080 | |
| Lagunillas | 19°56’25”N, 101°41’58”O | 2,100 | |
| Cheran | 19°68’66”N,101°95’47”O | 2,380 | |
| Salvador Escalante | 19°40’64”N,101°64’00”O | 2,239 |
Morphological identification. To identify the fungal isolations, the taxonomical keys by Barnett and Hunter (2006) were used. To identify E. perseae, the strain ATCC 11190 of this species was taken as a reference, which is found in the microbial collection in Miami, Florida, U.S.A. and which was provided by the Technology and Design Research and Assistance Center for the State of Jalisco (Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. - CIATEJ), Zapopan, Jalisco, Mexico.
Molecular identification. The purified isolations taken from the fungus were taken to the CIATEJ for the extraction of DNA by breaking the cell wall and membrane. The buffer solution was CTAB (hexadecyltrimethylammonium bromide) to promote cell lysis. Chloroform-isoamyl alcohol was used (in a 24:1 ratio) as an organic solvent and centrifuged at 5,000 rpm for 10 min to promote the denaturation and separation of proteins. The DNA was then precipitated and its quality was verified by electrophoresis in a 1.0% agarose gel.
The concentration of the genomic DNA was quantified with a Nanodrop and it was adjusted to 20 ng mL-1. DNA amplification was carried out with a PCR, using primers ITS-5/ITS-4, ITS1/ITS4, EF-1α F/ EF-1α R and Sph1F/Sph1R. The amplifications of the PCR products were verified by electrophoresis with a 5μL gel sample in a 1.5% agarose gel. The PCR products were purified following the instructions by the manufacturer (Wizard® SV Gel and PCR Clean-Up System, Promega®). The amplified fragments were sent to the Macrogen lab (Seoul, South Korea) for sequencing.
The bidirectional sequences were aligned with the Geneious prime v. 2021.0.3 (Kearse et al., 2012) and MAFFT v.7.475 (Katoh y Standley, 2013) programs, they were verified in BioEdit (Hall, 1999) in order to obtain a consensus sequence and the sequence obtained was assembled and compared with those available in the National Center for Biotechnological Information (NCBI), using the BLAST tool for highly similar sequences.
For the identification with BLASTn, the percentage of coverage and similarity was considered; values of ≥80% of coverage and a range of 97 to 100% of similarity of the sequence was considered, that is, a divergence of up to 3% of the sequence to assign a species (Raja and Oberlies, 2017).
Phylogenetic analysis. A multilocus phylogenetic analysis was carried out for the isolations identified as E. perseae, using the sequences obtained with the primers ITS5/ITS4 or ITS1/ITS4, SPh1 F/SPh1 R (LSU) and EF-1α F/ EF-1α R. For this analysis, sequences from species of the genus Elsinoe, previously deposited in the GenBank® data base were used. In the case of the isolations obtained from “typical scab”, an analysis was carried out based on the sequencing of primers ITS4/ITS5.
Once the consensus sequences were obtained, they were compiled in a FASTA format. Later, the sequences were aligned using the MAFFT v.7.475 program (Katoh and Standley, 2013). The sequences from LSU, EF-1α and ITS, obtained from strains identified as E. perseae were linked in the MESQUITE v.3.6 program (Maddison and Maddison, 2016). The phylogenetic analysis was based on the Bayesian inference (BI) algorithm and previously, the evolutionary model was determined using the JModeltest 2.1.7 program (Nylander, 2004).
For the phylogenetic analysis of the strains identified as Colletotrichum gloeosporioides, the sequence obtained with primers ITS5/ITS4 was used, as well as sequences from homologous species and others belonging to the same genus obtained from the GenBank. For the location of the isolations, the program MrBayes 3.2.7 (Ronquist et al., 2012) was used, with a Jukes Cantor (JC) evolutionary model obtained in the program JModeltest 2.1.7 (Nylander, 2004). Ten thousand generations were considered and samples were taken from trees every 1,000 repetitions. It was executed under the Markov Monte Carlo (MCMC) model and the analysis stopped once an average was obtained, along with the frequency partition standard deviation (se) between chains, below 0.01 (se = 0.0099). Twenty-two phylogenetic trees were obtained, out of which 18 were sampled, and 25% of the trees were discarded. The remaining phylogenetic trees were combined to calculate the posterior probability and obtain a consensus tree (Holder and Lewis, 2003). C. boninense, strain 126 was used as an external group to the species, since it belongs to another complex of the same genus.
A multilocus phylogenetic analysis was carried out for the species E. perseae in order to provide greater reliability and support to the identification of the isolations of this fungus. The sequences obtained in this investigation were considered, along with homologous sequences and those from other species of the same genus that cause similar damages to other plant species. The fungus Myriangium hispanicum, which belongs to the family Myringiaceae, was used as an external group. The sequences were obtained from the GenBank database, according to Fan et al. (2017). Once the sequences were obtained, a FASTA file was created. The sequences were aligned with the program MAFFT v.7.475 (Katoh and Standley, 2013) for each gene. The genes were linked with the program Geneious prime v. 2021.0.3 (Kearse et al., 2012) and MESQUITE v.3.6 (Maddison and Maddison, 2016). The evolutionary model was obtained using the software JModeltest 2.1.7 (Nylander, 2004). The phylogenetic analysis was carried out using the BI algorithm with the program MrBayes v. 3.2.7 (Ronquist et al., 2012). A total of 575,000 generations were considered and tree samples were taken every 1,000 repetitions. It was executed under the MCMC model and the analysis stopped once an average and a standard deviation of frequency partition between chains below 0.01 (de = 0.0097) were obtained. A total of 1,152 phylogenetic trees were obtained, out of which 864 were sampled; 25% of the trees sampled were discarded. The rest of the trees were combined to calculate the posterior probability and obtain a consensus tree (Holder and Lewis, 2003).
Pathogenicity tests. In order to evaluate pathogenicity, 25 avocado plants from nurseries were used (creole, Hass, Flor de María, Méndez), approximately 18 months after being transplanted. The avocado fruits were inoculated when their size was 6-9 cm in length by 4-6 cm in width (stage of the filling of the fruit). The isolations selected for pathogenicity tests were those that had a higher percentage of growth of every symptom sampled. Leaves, branches and fruits from each plant of every variety were inoculated.
Two types of inoculation were carried out: direct and indirect. The former consisted in cutting a lesion in the epicarp of the fruit and in the young stems with a number 0 entomological pin. Mycelium discs were then placed (≈ 1 cm in diameter) in the isolated fungi on the lesion, and covered with cotton dampened with sterile distilled water and “kleen pack” plastic. The indirect method consisted of performing small lesions on the fruits and young branches, followed by the spraying of a suspension with conidia, spores and fragments of mycelia at a concentration of 1 x 106 propagules mL-1, prepared following Gilchrist Saavedra et al. (2005). Finally, the plants were covered with a plastic bag, as if in a humid chamber, for three days. The incidence of the symptom was evaluated in the different varieties.
The inoculated fruits were observed weekly for three months to detect the presence of the symptoms. Next, the fungi were reisolated to verify the identity of the pathogen.
Results and discussion
Morphological identification. Out of the plant tissues planted with symptoms of “typical scab”, 16 different isolations were obtained, eight of which were identified as possible phytopathogenic fungi, which correspond to the genera Colletotrichum, Pestalotiopsis, Fusarium, Curvularia, Alternaria, Leptosphaerulina, Cladosporium and Epicoccum. Out of these, the genus Colletotrichum was the most frequent (90%), followed by Pestalotiopsis (40%). The rest of the fungi did not surpass 3%. These data coincide with works by Djeugap et al. (2015), Alfaro et al. (2017) and Becerra (2019), who reported the presence of species of the genus Colletotrichum more frequently in isolations of the avocado scab symptom.
Based on the studies carried out by Hernández and González (2010) and Pérez et al. (2016), the remaining eight species were discarded due to their lower frequency of culture development (presence in a maximum of three dishes planted), as well as to the identification as pollutant fungi, for the case of the genera Aspergillus, Penicillium and Trichoderma.
In the morphological identification of the strain with the highest growth frequency, the development of white cultures with a gray center and a cottonlike texture was observed, with a production of orange conidial masses in the center. This culture was characterized for its rapid growth (it covered the 15 cm Petri dish in 8 days). In its microscopic characteristics, it displayed a septated mycelium, unicellular conidia elongated to the point of being almost cylindrical, with a slight narrowing in the center, giving it an appearance similar to a septum, and with rounded edges (Barnett and Hunter, 1998). These characteristics coincide with those described for the genus Colletotrichum, belonging to the phylum Ascomycota, class Hypocreomycetidae and order Glomerellales (Réblová et al., 2011) (Figure 2).
The second isolation with the highest growth percentage, Pestalotiopsis sp., developed white septated mycelium cultures with a brush-shaped growth. In addition, the development of black fructiferous bodies that presented multicellular spindle-shaped conidia (3 to 5 cells) with three apical flagella and one basal one (Barnett and Hunter, 2006). These characteristics coincided with those described for the genus that belongs to the phylum Ascomycota, class Sordariomycetes and order Xylariales (Réblová et al., 2011) (Figure 2).

Figure 2 A) Isolations of the genus Colletotrichum sp. that show the cottonlike growth with a high content of formation of orange to pink waxy propagules in the center. B) Formation of hyaline, ovoidal or oblong conidia, with a shortened shape and an even, 25- 30 μm, circular ending. C) Pestalotiopsis sp. cultures and a development of bright black and irregularly shaped cirrus. d) Conidia with septa and three flagella.
“Purple spot” symptom. In the plant tissue with symptoms of “purple spot”, the cultures that were obtained most frequently were those that grew in viscous and cerebriform stroma, with a slow growth of 0.35 mm per day, elevations in the center, a great variability of shapes and light orange and light yellow colors, which became darker with age, turning reddish browns, some of which were plushy-looking, with aerial mycelia and humid in the center, with micro and macro conidia from 3 to 14 microns and tuberculate mycelium; these were identified morphologically as Elsinoe sp., since these characteristics coincide with those presented by Hyde et al. (2013) as characteristics of the genus Elsinoe. Colletotrichum sp. and Alternaria sp. were also identified with occasional growth (1-2%) (Figure 3).
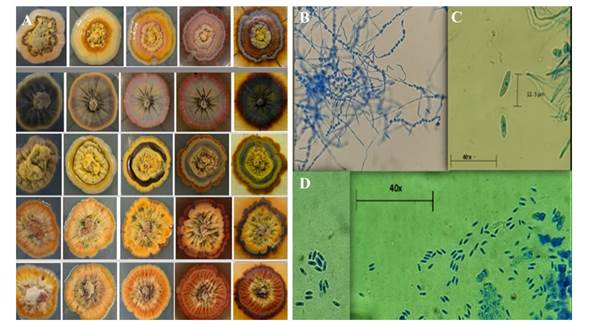
Figure 3 A) Variability in color and shape of the growth of E. perseae isolations. B) tuberculate mycelium. C) and D) conidia measuring 3-14 micrometer
Molecular identification. With the amplification of primers ITS5/ITS4, C. gloeosporioides was identified; however, for Pestalotiopsis sp., the sequencing results were not conclusive (Table 2). On the other hand, the sequencing of genes LSU, EF-1α and ITS helped identify E. perseae (Table 3). The nucleotide sequences identified were deposited in the GenBank data base (Table 4).
Table 2 Molecular identification of the strains isolated from the symptom of “typical scab” by the amplification of primers ITS5/ITS4.
| Aislamiento | Identificación morfológica | Secuencia homóloga del Gene Bank | Especie | Identidad (%) |
|---|---|---|---|---|
| C7 | Colletotrichum sp. | MK758005.1 | C. gloeosporioides | 100 |
| C9 | Pestalotiopsis sp. | N/A | N/A | N/A |
Table 3 Molecular identification of the fungus isolated from the symptom of “purple spot” by sequencing the genes LSU, EF-1α and ITS.
| Aislamiento | Identificación morfológica | Secuencia homóloga del GenBank® | Especie | Identidad (%) |
|---|---|---|---|---|
| C1 | E. perseae | MH855586.1 | N/A | N/A |
| C4 | E. perseae | MH855586.1 | E. perseae | 99.69 |
| H3 | E. perseae | MH855586.1 | E. perseae | 99.67 |
| H4 | E. perseae | MH855586.1 | E. perseae | 99.51 |
| H6 | E. perseae | MH855586.1 | E. perseae | 97.46 |
| EF-1α | ||||
| C1 | E. perseae | KX886902.1 | E. perseae | 100 |
| C4 | E. perseae | KX886902.1 | E. perseae | 99.71 |
| H3 | E. perseae | KX886902.1 | E. perseae | 100 |
| H4 | E. perseae | KX886902.1 | E. perseae | 100 |
| H6 | E. perseae | KX886902.1 | E. perseae | 100 |
| LSU | ||||
| C1 | E. perseae | NG_063977.1 | E. perseae | 99.72 |
| C4 | E. perseae | NG_063977.1 | E. perseae | 99.86 |
| H3 | E. perseae | NG_063977.1 | E. perseae | 99.17 |
| H4 | E. perseae | NG_063977.1 | E. perseae | 99.72 |
| H6 | E. perseae | NG_063977.1 | E. perseae | 99.86 |
Table 4 Sequences selected for the phylogenetic analysis of E. perseae.
| Especie | Código del aislamiento | Número de acceso al Genbank | ||
|---|---|---|---|---|
| ITS | LSU | EF-1α | ||
| C. gloeosporioides | C7 | MZ314618 | ||
| E. perseae | C1 | N/A | MZ310447 | MZ319391 |
| E. perseae | C4 | MZ310437 | MZ310448 | MZ319392 |
| E. perseae | H3 | MZ310438 | MZ310449 | MZ319393 |
| E. perseae | H4 | MZ310439 | MZ310450 | MZ319394 |
| E. perseae | H6 | MZ310440 | MZ310451 | MZ319395 |
Phylogenetic analysis of the isolations obtained from avocado fruits with symptoms of “typical scab”. The phylogenetic tree grouped six clades, along with the external group, out of which two group the entire species of C. gloeosporioides, in which the strain identified in this study is found. This verifies the identity of the isolations obtained in this study as C. gloeosporioides (Figure 4).
Phylogenetic analysis of the strains isolated from avocado fruits with symptoms of “purple spot”. The Bayesian phylogenetic tree grouped more than 14 clades, along with the external group. The sequences obtained from the strains morphologically identified as E. perseae were grouped with the strains deposited in the GenBank with the same species, which corroborates the identity of the strains isolated from the “purple spot symptom” as E. perseae and it supports the monophyletic origin of this species (Figure 5).
Pathogenicty tests. It was inoculated with Pestalotiopsis sp. and C. gloeosporioides to evaluate the “typical scab” symptom; and with an isolation identified as E. perseae to evaluate the “purple spot” symptom.

Figure 4 Bayesian phylogenetic tree for the complex Colletotrichum gloeosporioides, reconstructed using primers ITS5/ITS4. Red text is the isolation obtained in this work. The bar indicates substitution per site. Nodes exemplify the posterior probability (PP). C. boninense, isolation 126, which belongs to another complex of the genus, was used as an external group.
Pestalotiopsis sp. The symptom caused by the fungus identified as Pestalotiopsis sp. was dry necrosis, which moves up the stem until the base of the branch and caused the mummification of the fruits in plants of the varieties Hass and Flor de María. The least severe symptoms were found in creole plants and the variety Flor de María, causing a partial necrosis with vertical cracks; these symptoms coincide with reports by Tamayo (2007), such as the drying of branches and leaf spots.
Authors such as Morales et al. (2009) have isolated Pestalotiopsis versicolor from avocado fruits with symptoms of anthracnose and Pestalotiopsis sp. from fruits with avocado scab symptoms. However, despite the inoculated strain of Pestalotiopsis sp. having reproduced a corky bark, the typical scab symptom observed in the field was not reproduced, therefore Pestalotiopsis sp. as the causal agent of typical scab was discarded.

Figure 5 Bayesian phylogenetic tree for species of the genus Elsinoe, reconstructed using assembled sequences of primers ITS1/ITS4, ITS5/ITS4, EF-1 α F/EF-1 α R, Sph1 F/Sph1 R. Red text indicates the isolations obtained in this work. The bar indicates substitution per site. Nodes exemplify the posterior probability (PP). Myriangium hispanicum, aislamiento CBS 227.59, which belongs to another family of the same genus, was used as an external group.
Pestalotiopsis sp. species have been reported as pathogens of some varieties of avocado and other fruit trees such as mango, apple, banana and grape. However, the exact damage this fungus may cause in avocado fruits was unknown (Zhang et al., 2003; Djeugap et al., 2015). The pathogenicity tests carried out in this study showed that Pestalotiopsis sp. may cause a total dry necrosis of the fruit, to the extent of leaving it mummified, and in branches it causes necrosis with corky cracks. These symptoms are different to those reported for typical scab.
Colletotrichum gloeosporioides. The diseases caused by Colletotrichum sp. include anthracnose, rotting of fruits and flowers, leaf spot, and other types of rot. It affects a wide range of crops, including avocado (Freeman et al., 1998; Silva and Ávila, 2011). Because it is a fungus of rapid growth, it can enter as a secondary pathogen by using lesions caused by other fungi such as E. perseae. Zentmyer (1984) described that Colletotrichum sp. is incapable of entering the fruit directly and it is generally established in the lesions of the fruit caused by Cercospora sp. or E. perseae. Therefore, Colletotrichum sp. may be found as an opportunist fungus.
The pathogenicity tests for the strain identified as C. gloeosporioides reproduced symptoms similar to the avocado scab, which proves that other pathogens can stimulate the production of layers of cork, as described by Cruz et al. (2006). Plants inoculated with the strain identified as Colletotrichum sp. displayed an initial symptom of necrotic spots that formed corky cracks during growth, similar to those described by Everett et al. (2011) and Fan et al. (2017), caused by E. perseae. In stems, symptoms of necrosis were found with longitudinal cracks, similar to those reported by Pestalotiopsis sp., whereas in soft branches and the peduncle, the symptom was similar to the one described by Everett et al. (2011) as avocado scab.
Elsinoe perseae. The isolation morphologically described as E. perseae was obtained from fruits with the symptom of “purple spot”. The initial symptoms were reproduced, which are commonly found in the field, similar to those reported by Morales (2017), and at the same time, the initial lesions coincide with reports by Jenkins in 1934 as avocado scab (Everett et al., 2011; Fan et al., 2017), therefore it is synonymous with purple spot described by Morales (2017). This describes that these common names are synonymous and they are related to the same causal agent. Small circular lesions or lesions with irregular purple to violet edges were observed. It was evaluated for two months and at the end of the evaluation, the lesions reached a maximum size of 5 mm. The symptoms produced on the fruits were reisolated and the isolations obtained coincided morphologically with the inoculated strains (Figure 6).
Everett et al. (2011) and Fan et al. (2017) relacted Elsinoe perseae as the causal agent of the avocado scab. Another situation is the erroneous identification of the symptom of this disease in the field, because in the sampling of this work, other pathogens, pests and mechanical damages were proven to cause corky trunk in the pericarp of the fruit as a defense mechanism (Cruz, 2006). Therefore, the characterization and identification of the symptom is crucial for the adequate integrated management of this disease.
Conclusions
Sixteen different isolations were obtained, of the genera Colletotrichum, Pestalotiopsis, Fusarium, Curvularia, Alternaria, Leptosphaerulina, Cladosporium and Epicoccum associated with the symptom “typical scab”

Figure 6 Symptoms in fruits inoculated with A) Elsinoe perseae, B) Pestalotiopsis sp. and D) Colletotrichum gloeosporioides.
Colletotrichum gloeosporioides did not reproduce symptoms identical to those of “typical scab” in the pathogenicity tests, since the corky bark produced penetrated the mesocarp. Meanwhile, Pestalotiopsis sp. Reproduced descending anthracnose and mummification of the fruits.
Twenty-five isolates of the purple blotch symptom were obtained and corresponded to the genus Elsinoe
The fungus identified as E. perseae initially reproduced irregular brown to violet spots that formed a corky bark limited to the epicarp when they joined; these symptoms are related to the disease known as “purple spot”.
The presence of E. perseae in Michoacán has been corroborated.











 texto en
texto en 



