Black Sigatoka (BS) is a disease caused by the fungus Pseudocercospora fijiensis. It is present in plantain and banana plantations around the world and can cause the total loss of the crop (Marín et al., 2003). The reduction of the photosynthetic area is the reason why P. fijiensis is so harmful (Hidalgo et al., 2006).
Conventional control of BS is based on cultural practices and the application of chemical fungicides. The first consists, primarily, in periodically cutting the parts of the leaves with presence of advanced lesions of the disease, finally removing the whole leaf. The second is carried out through the alternate application of protective and systemic fungicides, which can also be applied together in a mixture (Orozco-Santos et al., 2008). These fungicides, although effective, contaminate the environment (Geissen et al., 2010) and are used with increasing frequency (Martínez and Guzmán, 2010). For the latter reason, the control of BS represents between 35-45% of the production cost of plantains and bananas (Garrido-Ramírez et al., 2011).
An alternative method of BS control is the use of biological products, including antagonistic microorganisms and their metabolites, as well as plant extracts (Okigbo and Emoghene, 2003). In the laboratory, extracts from Commelina diffusa (canutillo), Momordica charantia (bitter melon), Pavonia spp. (tiger flower), Plenax sp., Piper hispidum (cofalillo), Pelargonium peltatum (ivy geranium), Sida rhombifolia (arrowleaf sida), Syzygium aromaticum (clove), and Topobea discolor (amarraboyo) inhibited the germination of spores and the colony growth of P. fijiensis (Mosquera et al., 2009; Riveros and Arciniegas, 2003).
Under shade house conditions, Vargas et al. (2009) evaluated the ethanolic extracts of leaves of Heliotropium indicum (Indian heliotrope), Lippia origanoides (Mexican oregano), Ricinus communis (castor bean), and their combinations, in three-month-old “Harton” plantain plants infected with P. fijiensis. They found that the lowest weighted average incidence of the infection, and the highest position of the youngest diseased leaf were obtained with H. indicum + R. communis, while the highest total number of leaves was obtained with the treatment with H. indicum + L. origanoides + R. communis. In three-months-old “Gran Enano” banana clone inoculated with conidia of P. fijiensis, Morales et al. (2011) found that spraying every 15 days the aqueous extracts of cundeamor (Motorbike charantia), sage (Salvia officinalis), and lemon grass (Cymbopogon citratus) yielded a similar response to treatment with Mancozeb.
Likewise, the combination of alcoholic extracts of clove (S. aromaticum) and garlic (Allium sativum), in concentrations of 36 µg mL-1 and 150 µg mL-1, respectively, reduced the colony growth of P. fijiensis by up to 41% (Adriano-Anaya et al., 2018). Therefore, the present work aimed to evaluate the incidence and severity of Black Sigatoka in ‵Macho’ plantain plants sprayed with a mixture of alcoholic extract of clove and garlic, under field conditions.
The work was carried out at the Estancia Agroecológica “AYOL” in Tapachula, in the state of Chiapas (14° 49’ 44.7” N 92° 17’ 48.5” W; 76 masl; warm humid climate with abundant rains in summer), between September 2018 and May 2019. The plants used were three months old ‵Macho′ plantain sword type suckers (1.0-1.2 m high) planted in 100 m2 plots (20 plants per row and three rows) at distances of 1.5 m between plants and 2.0 m between rows. All plants were fertilized bimonthly with compost (25 kg plant-1) and weekly with biol (4 L plant-1). The non-useful shoots (suckers) were removed every two months and the accompanying flora was subjected to mechanical pruning every 28 days. When required, the plants were hydrated with well water.
To evaluate the effect of the mixture of garlic (150 µg mL-1) and clove (36 µg mL-1) extracts, three treatments (plots) were established according to the frequency of application: in Treatment 1 (T1) the mixture was applied every 7 days; in Treatment 2 (T2) the mixture was applied every 14 days; in Treatment 3 (T3), or control, only an alcohol solution in water was applied. All solutions used commercial soap as adherent. All treatments were applied (5 L manual sprinkler, Truper) between 07:30 and 08:30 am on the top and underside of the leaves up to the dew point.
The garlic extract was prepared by crushing 45 g of garlic material, which was subsequently subjected to reflux extraction (six cycles) with 200 mL of ethanol (60%) at a temperature of 80 °C in a Soxhlet equipment. The extract was stored in an opaque glass container and kept at room temperature until further use (for a maximum of one month). The clove extract was prepared by grinding 100 g of the material, which was subsequently homogenized in 1 L of ethanol (96%) contained in an opaque container. The homogenate was kept at room temperature for 28 days, during which it was manually shaken every third day. At the end of the extraction time, the homogenate was filtered (Wathman no. 1) and kept at room temperature until further use (for a maximum of two months). The concentration of total soluble solids of each extract was determined by evaporation (70 °C) of 10 mL in a thermo-balance (Oahus BL-MB23).
Every 14 days, and before applying the mixture of extracts, the total number of leaves (TL), the weighted average incidence of BS (IBS), the weighted average severity of BS (SBS), the youngest leaf free of BS symptoms (LY0), and the time to flowering were determined in all plants. In leaves with BS symptoms, the degree (G) of severity was determined using the Stover scale modified by Gauhl (1989). Leaves that showed a degree of severity of 6 (G6) were removed from the plant. The IBS was calculated as the quotient between the leaves with BS symptoms divided by the total number of leaves [IBS = (LWBS) (TL)-1]. The SBS was calculated by adding the multiplication values between the number leaves (Ln) and the degree of severity of the disease (G0-6) divided by 100 [SBS = ∑ [(G0L0) + (G1H1) + … (G6H6)] (100)-1]. All the data were subjected to analysis of variance. Significant differences were determined using Duncan’s test at p> 0.05. The statistical analyses were performed with the software InfoStat Professional Ver. 2018. The results were described and discussed considering that banana plants do not produce leaves after flowering.
Figure 1 shows the flowering dynamics of the ‘Macho’ banana plantain plants used in the different treatments under study. As can be seen, the T1 plants started flowering 140 days after the start of the study (DASS). The T2 plants started at 182 days. No T3 plant produced flowers during the study period. The maximum number of flowering plants (~80%) in T1 and T2 was reached, respectively, at 196 and 224 DASS. The observed induction of flowering in plants (Figure 1) sprayed with clove and garlic extracts may have been related to the components of said extracts, since none of the control treatment plants flowered during the study time. The flowering results suggested that it was necessary to reach a critical concentration to induce flowering since the plants sprayed every 14 days were “delayed” 42 days compared to the plants sprayed every 7 days. The induction of flowering through the spraying of plant extracts is a phenomenon that hadn’t been reported before in studies using plant extracts to control BS (Vargas et al., 2009; Thangavelu et al., 2013; Kumakech et al., 2017). The possible reason could be that in previous studies the extracts were sprayed less frequently than in the present work.
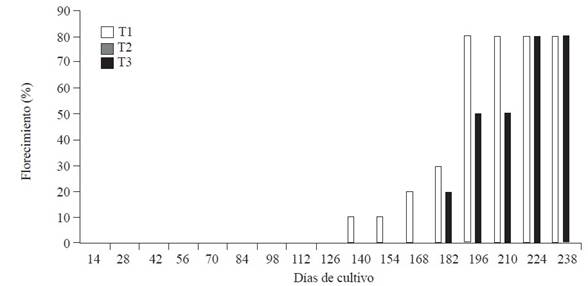
Figure 1 Flowering dynamics of “Macho” plantain plants subjected to Black Sigatoka control treatments with a mixture of clove and garlic extracts. T1: mixture applied every 7 days. T2: mixture applied every 14 days. T3: control plants.
During the entire time of the study, the plants of T1 and T2 had, compared to T3, a greater amount of TL, a situation that is repeated when comparing the TL before the beginning of flowering and after the beginning of said process (Table 1). The differences found were statistically significant (Duncan a=0.05; EE =0.17, 0.15 and 0.12 for T1, T2 and T3, respectively). The significant differences between the treatments could be associated with the damage caused by the disease, since the leaves with G6 were always removed. Therefore, the more leaves on the plants, the greater health of the plantation.
Table 1 Average total leaves in “Macho” plantain plants grown with control of Black Sigatoka based on a mixture of alcoholic extract of garlic and cloves applied every 7 days (1) and 14 days (2). Control plants (3).
| Tratamiento | Todo el tiempo | 126 días después de iniciado el trabajo | Después de iniciado el florecimiento |
|---|---|---|---|
| 1 | 9.25 A | 8.01 AB | 10.63 A |
| 2 | 8.78 B | 8.30 A | 9.34 B |
| 3 | 8.54 C | 7.75 B | 8.54 C |
In plants with application of the extract mixture every 7 days (T1) and every 14 days, the IBS (Table 2) was 20.5 and 13.0%, respectively. These values were lower compared to the plants where said extracts were not applied (T3). The differences found were statistically significant (Duncan a=0.05; EE = 0.01, 0.01 and 0.01 for T1, T2 and T3). A decrease in IBS has been rarely reported in field studies. However, Kumakech et al. (2017) found a 34.8% decrease in the number of lesions in banana vitroplants of the Mpologoma, Mbwazirume and Kibuzi varieties sprayed with aqueous extract of Azadirachta indica.
Table 2 Weighted average of the incidence of Black Sigatoka in “Macho” plantain plants treated with a mixture of alcoholic extract of garlic and cloves applied every 7 days (1) and 14 days (2). Control plants (3).
| Tratamiento | Todo el tiempo | 126 días después de iniciado el trabajo | Después de iniciado el florecimiento |
|---|---|---|---|
| 1 | 0.35 C | 0.36 C | 0.35 C |
| 2 | 0.39 B | 0.40 B | 0.40 B |
| 3 | 0.44 A | 0.46 A | 0.44 A |
The SBS in T3 plants was 25.3 and 11.6% higher compared to plants with an application frequency of 7 and 14 days, respectively (Table 3). The differences found were statistically significant (Duncan a=0.05; EE = 0.02, 0.03 and 0.03 for T1, T2 and T3). The observed decrease in SSN was lower than that reported by Deshmukh et al. (2018), who found average decrease values of 72.8% using aqueous extracts of Datura ferox (toloache), Azadirachta indica (neem), Parthenium hyster (asthma herb), and Allium sativum (garlic). It was also lower than the value reported by Thangavelu et al. (2013), who found a 55% decrease using an aqueous extract of zimmu leaves (Allium cepa x Allium sativum). Both of those studies were carried out in “Gran Enano” clone banana plants. However, those studies were conducted under shade house conditions, which limited contact with conidia of P. fijiensis. Likewise, the results of SSN obtained in the present work, with the application of the garlic and clove extracts (0.45 - 0.98), were better than those reported by Vargas et al. (2009), who used ethanolic extracts from dry leaves of Ricinus communis (castor bean), Heliotropium indicum (Indian heliotrope), and Lippia origanoides (mexican oregano) on Musa bananas AAB cv. Harton, with values between 1.84 and 3.15.
Table 3 Weighted average of the severity of Black Sigatoka in “Macho” plantain plants treated with a mixture of alcoholic extract of garlic and cloves applied every 7 days (1) and 14 days (2). Control plants (3).
| Tratamiento | Todo el tiempo | 126 días después de iniciado el trabajo | Después de iniciado el florecimiento |
|---|---|---|---|
| 1 | 0.71 C | 0.74 C | 0.67 C |
| 2 | 0.84 B | 0.85 B | 0.78 B |
| 3 | 0.95 A | 0.97 A | 0.95 A |
Table 4 shows the values of the LY0. During the entire time of the study, the T1 plants had, on average, 1.57 healthier leaves than the control plants (T3). The BS symptoms were observable in older leaves (lower leaves). The number of leaves free of BS symptoms in plants to which the mixture of garlic and clove extracts had been applied increased, compared to control plants, after the start of flowering. The differences found were statistically significant (Duncan a=0.05; EE = 0.15, 0.14 and 0.11 for T1, T2 and T3).
Table 4 Average of the youngest leaf free of symptoms of Black Sigatoka in “Macho” plantain plants treated with a mixture of alcoholic extract of garlic and cloves applied every 7 days (1), 14 days (2) and control plants (3).
| Tratamiento | Todo el tiempo | 126 días después de iniciado el trabajo | Después de iniciado el florecimiento |
|---|---|---|---|
| 1 | 7.16 A | 6.29 A | 8.00 A |
| 2 | 6.38 B | 5.86 B | 7.33 B |
| 3 | 5.59 C | 5.16 C | 5.70 C |
In the control plants, the LY0 was similar to that reported by Torrado-Jaime and Castaño-Zapata (2018) for the plantain and banana varieties Dominico Harton, Africa, FHIA 20, and FHIA 21. The LY0 of control plants was lower than that reported by Thangavelu et al. (2013) for the ‘Gran Enano’ banana clone (youngest diseased leaf 7.6). However, in the plants to which the mixture of extracts was applied every 7 days and every 14 days (Table 4), the LY0 was better than that reported by Vargas et al. (2009), who reported the youngest leaf without BS symptoms between 1.42 and 3.75 when spraying ethanolic extracts from leaves of R. communis, H. indicum and L. origanoides on banana plants (Musa AAB cv. Hartón). However, the HMJ0 was lower than that reported by Thangavelu et al. (2013) for ‵Gran Enano′ banana clone sprayed with a zimmu extract.
In conclusion, the application, every 7 or 14 days, of a combination of alcoholic extracts of clove and garlic (36 and 150 µg mL-1) was able to control BS and induce flowering in “Macho” banana plantain plants, under field conditions.











 texto en
texto en 


