Sugar cane (Saccharum officinarum) is one of the most important industrial crops in Mexico with a national annual production of 5.7 million tons of sugar. Sugarcane fields are distributed in seven strategic regions. The state of San Luis Potosí belongs to the Northwest region, which produces 1156265 tons each year (CONADESUCA, 2021). In 2021, in the municipality of Tamasopo, which belongs to the Huasteca Potosina, 653028 tons of sugar were produced (SIAP, 2022).
Sugarcane wilt or pokkah boeng (PB) is a serious disease that affects the stem of sugarcane plants. The initial symptoms appear in young leaves, which develop chlorotic areas at the base (Vishwakarma et al., 2013). If the infection is limited to the leaves, the plant recovers slowly. In advanced and severe stages, the disease penetrates the stem through a growth point causing upper rot, the young leaves die, and reddish asymmetrical necrotic areas appear in the tissue (Jeyakumar and Zhang, 2020). This disease causes losses of 45.2 to 51.2% in crop production and 42.8 to 59.2% in the juice content of the stems (Kumar et al., 2015). The quality of the juice is reduced (Viswanathan, 2020).
Several species of the genus Fusarium such as F. sacchari, F. verticillioides and F. proliferatum (Lin et al., 2014; Zhang and Jeyakumar, 2018) have been identified as causal agents of PB disease (Viswanathan, 2020). They can grow on a wide variety of substrates and have an efficient dissemination mechanism that allows them to spread globally. Fusarium sacchari is the main causative agent of PB in India (Viswanathan et al., 2017). According to pathogenicity studies, once it enters the host it causes severe damage to the plant, but its entry mechanism has not been studied in detail (Viswanathan, 2020).
The roots of sugarcane plants become infected in the early stages of crop growth. However, the way in which F. sacchari spreads from the roots to the stem is still unknown. Environmental and soil factors probably play a vital role in the development and severity of the disease (Viswanathan, 2020). Species of the genus Fusarium inactivate toxic defense substances produced by the host and produce their own toxins, such as enniatins and fusaric acid, which increase their virulence (Agrios, 2005). The present work evaluated the in vitro sensitivity of Fusarium sacchari isolated from sugarcane with PB symptoms to five fungicides from different chemical groups. The mean effective concentration (EC50) of each fungicide was determined.
Using zigzag sampling, 1 m tall sugarcane plants in maturation stage with wilting symptoms were collected between February and April 2019 in nine sugarcane fields (four plants per field) located in Tamasopo (22° 09’ 41.0” N 99° 19’ 30.4” S) in the Huasteca Potosina region. The 36 samples obtained were transported in paper bags to the Biotechnology Laboratory of the Faculty of Agronomy of the Autonomous University of Nuevo León for analysis. Tissue fragments with necrosis symptoms were disinfected with NaClO (2%) for three minutes, rinsed three times with sterile distilled water and dried on sterile absorbent paper. The tissue was placed in Petri dishes with potato dextrose agar culture medium (PDA-Difco®), which were then incubated at 25±1 °C in the dark for seven days. Two representative isolates (HP1 and HP2) were selected, transferred to PDA culture medium and incubated at 25±1 °C in the dark until spores were formed. Conidia suspensions at a concentration of 1x103 were prepared with sterile distilled water and streaked on Petri dishes with PDA culture medium. Isolates from the germinated conidia were purified using the monosporic culture technique.
The monosporic isolates of HP1 and HP2 were morphologically characterized by the color and pigmentation of the colonies. The conidia, phialides and hyphae of Fusarium in temporary mounts of lactophenol were characterized using a compound microscope at 40 and 100X (Leslie and Summerell, 2006).
The pathogenicity of the isolates was determined by inoculating a suspension of conidia (1×106 mL-1) in stems of sugarcane plants (varieties My 55-14 and Mex 79-431) at harvest stage. The phytopathogen was re-isolated on PDA culture medium from the necrotic tissue of the inoculated stems. For the molecular identification of both isolates, DNA was extracted following the protocol of Cenis (1992), and the ITS (internal transcribed spacer) (White et al., 1990) and TEF (translation elongation factor) regions (O ‘Donnell et al., 1998; Carbone and Kohn, 1999) were amplified and sequenced for comparison with GenBank sequences and subsequent deposit therein.
The monosporic isolate of HP1 was used to determine the sensitivity of F. sacchari to five fungicides from different chemical groups. Concentrations of 1, 10, 100 and 1000 µg mL-1 of a.i. were evaluated using the poisoned plate technique (Dahal and Shrestha, 2018) under a completely randomized experimental design with five replications. Five Petri dishes with PDA culture medium without fungicide (0 µg mL-1) were used as control. The fungicides evaluated, and the chemical groups to which they belong, were: azoxystrobin (strobilurin, Bankit®, Syngenta), difenoconazole (thiazoles, Score®, Syngenta), hymexazol (isoxazoles, Tachigaren®, Summit Agro), cyprodinil (anilino-pyrimidines, Switch®, Syngenta), and thiabendazole (benzimidazoles, Tecto 60®).
Mycelium discs (7 mm in diameter) of the isolate HP1 were placed in Petri dishes with modified culture media and control medium for incubation in the dark for seven days at 25 ± 1 °C. During this time, the control treatment filled the dish. Mycelial growth was measured in two perpendicular directions from the center of the colony using a 0.01 mm precision digital caliper (Truper®). The percentage of inhibition of radial growth was determined (Dahal and Shrestha, 2018) and a two-way analysis of variance was performed on the results using a linear model, with the linearized values of percentage of inhibition as the dependent variable. The comparison of means was performed by the Tukey test (α=0.05). The EC50 for each fungicide was determined by linear regression of the logarithm of fungicide concentration and the Probit value of growth inhibition (Finney, 1952). All analyzes were performed using the statistical program R version 4.0.1 (R Core Team, 2020).
The morphological characteristics of the colonies, conidia and conidiophores of the MHP1 and MHP2 isolates corresponded to F. sacchari, according to Leslie and Summerell (2006). The colonies presented abundant, aerial, pale white mycelium, which turned purple 14 days after sowing. Elongated macroconidia with curved apical cell and poorly developed basal cell with three septa were observed, as well as abundant microconidia in false heads on monophialides or polyphialides, ovoid and single cell. The sequences obtained in GenBank ON924470 and ON924471 (ITS) showed a similarity of 100% with the sequence of F. sacchari with access number MT882327.1, while the sequence ON932086 of (TEF) showed a similarity of 99% with the sequence of F. sacchari with access number MT010988.1.
The response of F. sacchari to the fungicides and concentrations evaluated showed significant differences (Figure 1). The analysis of variance showed highly significant differences (p≤0.01) between fungicides and concentrations. The multiple comparison of means of the percentage of growth inhibition formed 12 groups (Table 1). The highest percentage of inhibition was obtained with difenoconazole 1000 µg mL-1 (93.5%) and thiabendazole 100 µg mL-1 (92.1%), 1000 µg mL-1 (91.4%) and 10 µg mL-1 (89.8%). The rest of the treatments showed percentages of inhibition that ranged from 75.8 to 0%. Various in vitro control studies of Fusarium species reported that these fungicides had significant growth inhibition effects. Alburqueque and Gusqui (2018) obtained 100% inhibition in F. oxysporum isolated from tomato (Solanum lycopersicum) and treated with azoxystrobin, carbendazim, copper phosphite and thiabendazole. Dahal and Shrestha (2018) also reported 100% inhibition in F. oxysporum treated with carbendazim at 150 and 200 ppm. Madhavi and Bhattiprolu (2011) obtained 92 and 91% inhibition in F. solani isolated from chili (Capsicum annuum) with the fungicides carbendazim and benomyl, respectively.
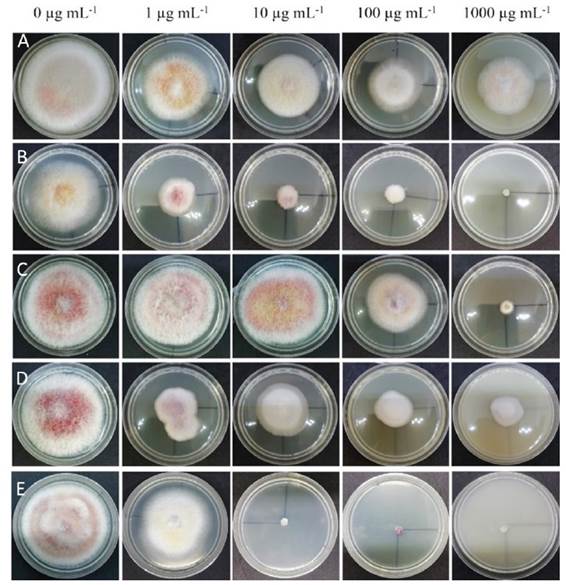
Figure 1 Growth of Fusarium sacchari after seven days of exposure to concentrations of 0, 1, 10, 100 and 1000 µg mL-1 of azoxystrobin A), difenoconazole B), hymexazol C), cyprodinil D), and thiabendazole E).
Table 1 Mean comparison by Tukey (0.05) of the percentage of inhibition of mycelial growth of F. sacchari by the effect of five fungicides at four concentrations.
| Tratamiento | Inhibición (%) | Tratamiento | Inhibición (%) |
|---|---|---|---|
| Difenoconazol 1000 µg mL-1 | 93.5 Az | Cyprodinil 1 µg mL-1 | 47.3 E |
| Tiabendazol 100 µg mL-1 | 92.1 A | Azoxystrobin 100 µg mL-1 | 31.1 F |
| Tiabendazol 1000 µg mL-1 | 91.4 A | Hymexazol 100 µg mL-1 | 28.3 FG |
| Tiabendazol 10 µg mL-1 | 89.8 A | Cyprodinil 10 µg mL-1 | 27.4 FG |
| Hymexazol 1000 µg mL-1 | 75.8 B | Azoxystrobin 1000 µg mL-1 | 26.0 FG |
| Difenoconazol 100 µg mL-1 | 75.1 B | Azoxystrobin 10 µg mL-1 | 18.7 GH |
| Difenoconazol 10 µg mL-1 | 74.7 B | Azoxystrobin 1 µg mL-1 | 13.7 H |
| Cyprodinil 1000 µg mL-1 | 61.8 C | Tiabendazol 1 µg mL-1 | 8.0 HI |
| Cyprodinil 100 µg mL-1 | 60.1 CD | Hymexazol 1 µg mL-1 | 0.3 I |
| Difenoconazol 1 µg mL-1 | 49.3 DE | Hymexazol 10 µg mL-1 | 0.0 I |
zPercentages with the same letter are statistically equal, p≤0.01. /
The results of the present study showed that the fungicides with code G1 (difenoconazole), whose mode of action is inhibition of the biosynthesis of sterols, with point of action in the inhibition of demethylation (FRAC, 2020), and the fungicides with code B1 (thiabendazole), whose mode of action is the inhibition of motor and cytoskeleton proteins, with point of action in the assembly of β-tubulins in mitosis (FRAC, 2020), were the ones that showed the highest inhibition percentage of mycelial growth of F. sacchari.
Regarding the EC50, the lowest values corresponded to difenoconazole with 1.5 µg mL-1 (standard error 2.1) and thiabendazole with 7.2 µg mL-1 (standard error 1.3). Cyprodinil had a value of 36.7 µg mL-1 (standard error 2.2) and hymexazol a value of 371.0 µg mL-1 (standard error 1.3), but the highest value corresponded to azoxystrobin, with 18420 µg mL-1 (standard error 5.2). F. sacchari showed growth with all the concentrations of azoxystrobin. Similar results were obtained by Gutierrez et al. (2006), who reported that there were no significant differences in inhibition percentage and that isolates of Fusarium spp. obtained from tomato fruits showed low sensitivity to different doses of azoxystrobin (3.3, 9.9 and 27.7 µL L-1 from Quadris, Syngenta).
The type of crop and disease caused by a phytopathogen against which a fungicide is applied influences the ability of the latter to inhibit growth. Avonazi et al. (2014) obtained an average EC50 of 0.2 mg L-1 in an assay with F. graminearum isolated from wheat (Triticum aestivum) exposed to azoxystrobin. This was the most effective fungicide in inhibiting spore germination. In the present study, hymexazol, whose mode of action (A3) plays a role in the metabolism of nucleic acids, affecting DNA/RNA synthesis, and cyprodinil, whose mode of action (D1) affects amino acid and protein synthesis by inhibiting methionine biosynthesis. (FRAC, 2020), showed low percentages of inhibition of the mycelial growth of F. sacchari at all the concentrations evaluated, with efficacy values of less than 76%.
Thiabendazole showed an inhibition range of 7.9 to 91.4% and an EC50 of 7.2 µg mL-1. These results demonstrate the antifungal effect of thiabendazole derived from its direct effect on mitosis, preventing the development of the mycelium (Melgarejo and Abella, 2011). Thiabendazole inhibited the mycelial growth of F. sacchari at a concentration of 10 µg mL-1. This result coincides with that of Kawchuk et al. (1994), who performed an in vitro study of isolates of Fusarium spp. obtained from potato tubers with dry rot symptoms and reported that mycelial growth was inhibited with an EC50 of 10 mg L-1. Gachango et al. (2012) obtained an EC50 of 3.7 mg L-1 with isolates of Fusarium spp. also obtained from potato tubers with dry rot. Difenoconazole had an inhibition range of 49.3 to 93.5% with an EC50 of 1.5 µg mL-1. These results coincide with those reported by Gachango et al. (2012), who reported 50% mycelial inhibition with an EC50 of 1.8 mg L-1. Anderson et al. (2020) conducted a sensitivity study of F. graminearum isolated from wheat (Triticum aestivum) to fungicides of the triazole group. They obtained an EC50 lower than 2 µg mL-1 for both tebuconazole and ketoconazole, which confirmed that the fungicides of the chemical group Triazole are effective in decreasing mycelial growth in Fusarium species.
The results obtained in the present study coincide with those of previous works where it was shown that fungicides from various chemical groups such as carbendazim (benzimidazoles, B1), thiophanate methyl (thiophanates, B1), copper oxychloride (inorganic, M01) and mancozeb (dithio carbamates, M03) can be used to control the PB disease (Vishwakarma et al., 2013; Jeyakumar and Zhang, 2020; TNAU, 2020). The first two fungicides have a mode of action similar to thiabendazole with respect to the inhibition of motor and cytoskeleton proteins. The other two fungicides have multisite activity (FRAC, 2020).
F. sacchari has been reported as the causal agent of PB disease in different parts of the world (Lin et al., 2014; Viswanathan et al., 2017; Bao et al. 2020; Paul et al. 2022). In the present study, it was isolated from sugarcane plants found in sugarcane fields of the Huasteca Potosina region. Although the PB disease has not had an economic impact in this region yet, it is a phytosanitary threat. Therefore, it is recommended that future assays include pathogen isolates from different production fields.











 texto en
texto en 


