Forests are natural assets with high environmental value due to their contribution to ecosystems and society. They provide raw materials such as wood and products for food medicine, and fuel. They also provide ecosystem services such as soil erosion protection, oxygen generation, carbon sequestration, regulation of global climatic conditions and biodiversity preservation (FAO, 2011; FAO, 2018). Furthermore, 75% of the freshwater available on the planet comes from hydrographic basins associated with forests (Springgay, 2019). In addition, agroforestry activities provide recreational and tourist activities that can be turned into a source of income (Balvanera, 2012; Brown and Verschuuren, 2018).
An estimated 1000 million people throughout the world depend on forest ecosystems for their subsistence as a direct or indirect source of income. Forests provide more than 580 000 million dollars a year of labor income, as well as 8000 million dollars of non-timber forest products (FAO, 2018; FAO and UNEP, 2020). However, the intensive and unsustainable use of forests, which is associated with fires, intensive agriculture, overgrazing, atmospheric pollution, and the presence of pests and diseases, has caused them to decline (Ken et al., 2020).
In Mexico, despite the efforts made by the forestry sector, only a low proportion of the forested area is subjected to diagnosis and timely detection of pests and diseases. Pesticides, which are applied indiscriminately to keep the phytosanitary conditions below the economic threshold, are another great cause of adverse effects. Most of these products are highly toxic chemical formulations that should be used under pest incidence monitoring and integrated forest systems to avoid affecting the balance of ecosystems. The use of new technologies, such as biological control agents, that are safer and more effective than traditional methods of pest control should be more widely promoted. Until now, these technologies have been little used by the forestry sector (Villacide and Corley, 2012; Flores-Villegas et al., 2019). The present work is a review of the current knowledge on the phytopathogenic agents associated with the main forestry diseases and the biological control agents that have been used to control them.
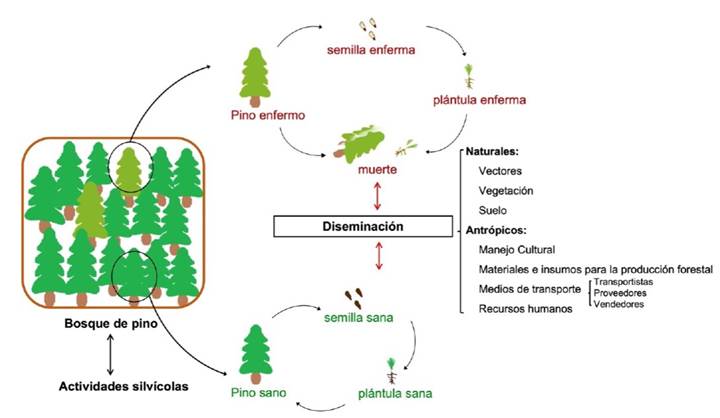
Diseases of Pinus spp. Pine Forest diseases are alterations of forest health associated with the interaction between pathogens, susceptible trees, and a favorable environment. Fungal diseases are one of the main causes of the decrease in forest biomass in Mexico (Gutiérrez-Flores et al., 2020). The presence of diseased Pinus spp. trees favors the dissemination of pathogens in the ecosystem through natural and anthropic factors (Figure 1). Phytopathogenic fungi attack different parts of pine trees, causing various diseases (Belén et al., 2011). In Pinus patula, for example, fungal infection causes the needles to fall. The damage starts from the needles and spreads to the branches, stems and sometimes even the roots, causing the tree to die and the forest population to decrease (Figure 2). This disease is mainly associated with pathogens such as Alternaria alternata and Meria laricis, but there are also reports of its association with the genera Annulohypoxylon, Botryosphaeria, Curvularia, Daldinia, Diplodia, Lophodermium, and Myrmaecium in different pine species such as P. arizonica, P. cembroides, P. patula, and P. pseudostrobus from different places such throughput the world as Canada, China, USA, and Mexico, among others (Table 1) (Guo et al., 2008; Cram et al., 2012; Marmolejo-Monciváis, 2018; Gutiérrez-Flores et al., 2020). The availability of nutrients in the soil also contributes to needle drop (Gutiérrez-Flores et al., 2020).

Figure 2 Symptoms associated with phytopathogenic fungi. The symptoms observed in Pinus patula trees are foliage ye llowing, needle drop, yellow lesions and central wood necrosis.
Diplodia sapinea is an opportunistic pathogen with a worldwide distribution that mainly affects P. patula and P. sylvestris. It has been associated with dieback, a disease in which diseased needles initially turn yellow, then red, brown or gray, and branch deformation results in needle death, with cankers on stems, branches and buds (Ospina et al., 2011; Cram et al., 2012; Gutiérrez-Flores et al., 2020; Larsson et al., 2021). Other pathogens such as Fusarium circinatum cause pine pitch canker, in which the needles wither and change from yellow to reddish until dry, and the shoots are defoliated, frequently causing crown dieback and eventually leading to the death of the tree. This pathogen is widely distributed in various countries, including Mexico. Therefore, it affects a wide variety of pine species (Cram et al., 2012; Carrasco et al., 2016; Martínez-Álvarez et al., 2016; Flores-Pacheco, 2017; Iturritxa et al., 2017; García-Díaz et al., 2019; Yu and Luo, 2020). The genus Lophodermium is present in forests of Mexico, Europe and Asia. It has been associated with the species Pinus ayacahuite, P. patula, Pinus montezumae, and P. sylvestris. Infected needles develop brown spots with yellow margins, ending with tissue death (Cibrián et al., 2007; Reignoux et al., 2014; Behnke‐Borowczyk et al., 2018; Gutiérrez-Flores et al., 2020; Sheller et al., 2020). Other fungal genera that have been reported to affect Pinus spp. are Botrytis, Ceratocystis, Cylindrocarpon, Cronartium, Curvularia, Daldinia, Dothistroma, Mycosphaerella, Lecanosticta, Phytophthora, Pythium, and Rhizoctonia (Figure 3). These fungi trigger different pathologies such as gray mold, regressive death, blue spot, blackfoot, blight, etc. (Table 1) (Ospina et al., 2011; Cram et al., 2012; Moreno-Rico et al., 2015; van der Nest et al., 2019; Gutiérrez-Flores et al., 2020; Oskay et al., 2020; Raitelaityté et al., 2020).
Table 1 Fungi reported national and internationally that are associated with the main diseases present in Pinus spp.
| Patógeno fúngico | Enfermedad | Hospedante | Distribución | Referencia |
|---|---|---|---|---|
| Alternaria alternata | Caída de acículas | Pinus sp. | China | |
| P. arizonica | Guo et al., 2008 | |||
| P. cembroides | México | Marmolejo-Monciváis, 2018 | ||
| P. patula | Gutiérrez-Flores et al., 2020 | |||
| P. pseudostrobus | ||||
| Annulohypoxylon stygium | Caída de acículas | P. patula | México | Gutiérrez-Flores et al., 2020 |
| Botrytis cinerea | Moho Gris | P. massoniana | Suecia | Capieau et al., 2004 |
| Botryosphaeria dothidea | Caída de acículas | Pinus sp. | México | Marsberg et al. 2017 |
| Muerte regresiva | P. patula | Sudáfrica | Gutiérrez-Flores et al., 2020 | |
| Ceratocystis sp. | Mancha azul en trozas | P. patula | Colombia | Guerra et al., 2004 |
| P. radiata | Osorio, 2007 | |||
| P. tropicalis | Ospina et al., 2011 | |||
| Cronartium quercuum | Roya de agallas | Pinus sp. | EUA | Cram et al., 2012 |
| Cronartium quercuum | Oxidación fusiforme | Pinus sp. | EUA | Cram et al., 2012 |
| f. sp. fusiforme | ||||
| Curvularia sp.C. lunata | Caída de acículas | P. patula | México | Gutiérrez-Flores et al., 2020 |
| C. pseudobrachyspora | ||||
| C. spicifera | ||||
| C. trifolii | ||||
| Cylindrocarpon sp. | Pie negro | Pinus sp. | EUA | Cram et al., 2012 |
| Daldinia sp. | Caída de acículas Chancrosis | P. patula | España | Stadler et al. 2014 |
| D. eschscholtzii | Sanz-Ros et al. 2015 | |||
| D. fissa | P. sylvestris | México | Gutiérrez-Flores et al., 2020 | |
| D. petriniae | ||||
| Diplodia sapinea | Caída de acículas | Pinus sp. | Colombia | Ospina et al., 2011 |
| P. patula | EUA | Cram et al., 2012 | ||
| Muerte descendente | P. sylvestris | México | Gutiérrez-Flores et al., 2020 | |
| Suecia | Larsson et al., 2021 | |||
| Dothistroma sp. | Banda roja | Pinus sp. | Cibrían, 2007 | |
| P. contorta | México | Cram et al., 2012 | ||
| P. ponderosa | Suiza | Queloz et al., 2014 | ||
| P. nigra | Alenezi et al., 2015 | |||
| P. radiata | ||||
| Fusarium sp. | Fusariosis vascular | Pinus sp. | Colombia | Ospina et al., 2011 |
| F. oxysporum | P. patula | México | Robles-Yerena et al., 2016 | |
| F. solani | P. pseudostrobus | |||
| F. circinatum | Chancro resinoso | Pinus sp. | Chile | Cram et al., 2012 |
| del pino | P. greggii | China | Carrasco et al., 2016 | |
| P. massoniana | EUA | Martínez-Álvarez et al., 2016 | ||
| P. nigra | España | Flores-Pacheco, 2017 | ||
| P. pinaster | México | Iturritxa et al.,2017 | ||
| P. pinea | García-Díaz et al.,2019 | |||
| P. radiata | Yu y Luo, 2020 | |||
| P. sylvestris | ||||
| P. tadea | ||||
| Lecanosticta acicola | Banda marrón | Pinus sp. | Guatemala | van der Nest et al., 2019 |
| P. mugo | México | Oskay et al.,2020 | ||
| P. sylvestris | Polonia | Raitelaityté et al.,2020 | ||
| Turquía | ||||
| Lophodermium sp. | Caída de acículas | P. ayacahuite | Escocia | Reignoux et al. 2014 |
| L. indianum | Chancrosis | P. montezumae | México | Behnke‐Borowczyk et al., 2018 |
| L. seditiosum | P. patula | Polonia | Gutiérrez-Flores et al., 2020 | |
| P. sylvestris | Rusia | Sheller et al.,2020 | ||
| Meria laricis | Caída de acículas | Pinus sp. | Cánada | Cram et al., 2012 |
| EUA | ||||
| Mycosphaerella sp. | Banda roja de la acícula | Pinus sp. | Colombia | Ospina et al., 2011 |
| P. patula | EUA | Cram et al., 2012 | ||
| Myrmaecium rubricosum | Caída de acículas | P. patula | México | Gutiérrez-Flores et al., 2020 |
| Passalora sequoiae | Moho de la hoja | Pinus sp. | EUA | Cram et al., 2012 |
| Pestalotiopsis funerea | Tizón del follaje | Pinus sp. | EUA | Cram et al., 2012 |
| Phoma eupyrena | Phomosis | Pinus sp. | EUA | Cram et al., 2012 |
| Phomopsis juniperovora | Phomosis | Pinus sp. | EUA | Cram et al., 2012 |
| P. lokoyae | ||||
| Phythophthora cinnamomi | Muerte de raíces | P. radiata | Chile | Ahumada et al., 2013 |
| Phytophthora sp. | Volcamiento o pudrición de la raíz | Pinus sp. | Colombia | Ospina et al., 2011 |
| Pythium sp. | P. patula | EUA | Cram et al., 2012 | |
| Rhizoctonia sp. | ||||
| Ophiostoma sp. | Mancha azul de la madera | P. leiophylla | México | Moreno-Rico et al., 2015 |
| O. pulvinisporum | P. teocote | |||
| O. pluriannulatum |
The phytopathogenic fungi associated with Pinus spp. are difficult to identify, due to the relationship between their life cycles, the phenological development of the host, and the presence of environmental factors that makes them behave as saprophytes and mutualists (Gutiérrez-Flores et al., 2020). The great diversity of fungal agents that cause foliar loss and death in Pinus spp. throughout the world has elicited concern and interest among the scientific community and society in general (Gutiérrez-Flores et al., 2020; Larsson et al., 2021). This phytosanitary situation has driven the search for ways to manage and control diseases of fungal origin using synthetic molecules and biological agents (Adusei-Fosu and Rolando, 2018; Kang et al., 2019).
Importance of pesticides. Pesticides are anthropogenic substances or mixtures of substances with broad-spectrum activity and environmental persistence that are intended to prevent, destroy or control crop pests (Narváez-Valderrama et al., 2012; del Puerto-Rodríguez et al., 2014). The dispersion of pesticide chemicals among plants depends on the physicochemical properties of pesticides, their formulation and presentation, climatic conditions, geological characteristics, forms of application and transport processes (Ramírez and Lacasaña, 2001). Pesticides can be classified according to their use as insecticides, fungicides, herbicides, bactericides, etc.; They are also classified based on their chemical composition into organochlorines, organophosphates, carbamates, pyrethroids, bipyridyl compounds, inorganic salts, etc (Ferrer, 2003; Serra et al., 2020). The excessive and irrational use of these chemical products is an important problem not only because their residual persistence in the environment and the resistance that pest organisms can generate against them, but also because they can get into human bodies through drinking water, air, soil, and indirectly, through the biological chain of food. This can cause several health problems, including genetic mutations, hormonal alterations and endocrine modifications (Tobón-Marulanda et al., 2010; Ortíz et al., 2014; Bravo et al., 2020).
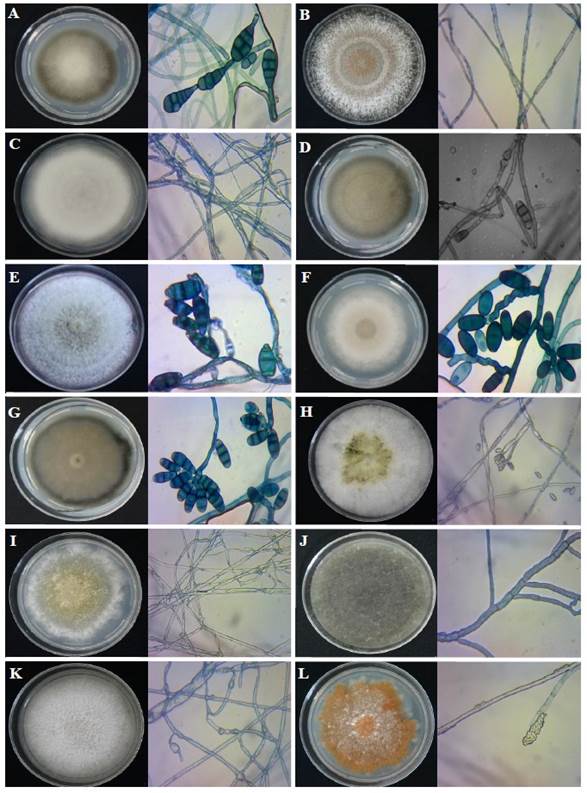
Figure 3 Most frequent fungi associated with Pinus patula de Tetela de Ocampo, Puebla, México. A. Alternaria alternata M1AAR1; B. Annulohypoxylon stygium T1R1; C. Botryosphaeria dothidea M7MtPpA-CH3 (2); D. Curvularia lunata M1MtPpA-AA; E. C. pseudobrachyspora M41MtPpA-CHR1; F. C. spicifera M21MtPpA-AAR2; G. C. trifolii M2MtPpA-AA; H. Daldinia eschscholtzii T5R2; I. Daldinia sp. M3MtPpA-CHR2 (2); J. Diplodia sapinea M3MtPpA-CH; K. Lophodermium indianum M1MtPpA-PDAR1; and L. Myrmaecium rubricosum M1MtPpA-PDA ((Modified by Gutiérrez-Flores et al., 2020).
Fungal control by chemical agents. Fungicides have been widely used to control fungi and are classified according to their characteristics, including their chemical or biological origin. Chemical fungicides have been applied as foliar sprays to control phytopathogenic fungi in forest species. In Mexico, fungicides used in forestry have also been used in agriculture. These include Azoxystrobin, Chlorothalonil, Fosetil-Al, Calcium Phosphite, Copper Phosphite, Potassium Phosphite, Mancozeb, Metalaxyl-Chlorothalonil, and Propiconazole (Table 2). These function as residual or contact protectants that control or prevent the spread of fungi to healthy hosts when applied correctly (Adusei-Fosu and Rolando, 2018). Table 2 lists some fungicides with systemic action, Azoxystrobin (strobilurins), Fosetil-Al (organophosphate), Metalaxil (acylalanines), and Propiconazole (triazoles), as well as fungicides with protective and healing activity, Chlorothalonil (chloronitriles), and Mancozeb (dithiocarbamates), that must be applied before the fungal infection to prevent the development of new lesions (DEAQ, 2022). It is important to know which fungi affect forest trees because there are many tree diseases that are natural (old age) and cyclical ecological phenomena, and most of them are untreatable with fungicides (Adusei-Fosu and Roland, 2018).
Table 2 Main chemical fungicides recommended for forestry use in Mexico.
| Ingrediente activo: concentración (%) | Actividadz | Grupo químico | Intervalo entre aplicaciones (días) | Dosis para uso forestal en 100 L |
|---|---|---|---|---|
| Azoxystrobin: (23) | Sistémico, protector y curativo | Estrobilurinas | 7-14 | 0.20-0.40 L |
| Clorotalonil: (52-54) | Protector y curativo | Cloronitrilos | 7-10 | 1.75-2.6 L |
| Fosetil-Al: (80) | Sistémico | Organofosforados | 10-14 | 200-300 g |
| Fosfito de calcio: (25) | Inductor de defensa, y aporta nutrientes | Oxisal | 7-14 | 0.25 L |
| Fosfito de cobre: (40) | Inductor de defensa, y aporta nutrientes | Oxisal | 20-25 | 0.25 L |
| Fosfito potásico: (70) | Inductor de defensa, y aporta nutrientes | Oxisal | 7-10 | 0.250 L |
| Mancozeb: (30-80) | Protector no sistémico | Ditiocarbamatos | 7-10 | 250-300 g |
| Metalaxil: (9), Clorotalonil: (72) | Sistémico, protector y curativo | Acilalaninas, Cloronitrilos | 10-14 | 400-1000 g |
| Propiconazol: (26) | Sistémico, protector y curativo | Triazoles | 7-10 | 0.5 L |
zDictionary of Agrochemical Specialties (DEAQ, 2022); Federal Commission for the Protection against Sanitary Risks.
Fungicides are classified as highly dangerous since they can cause harmful effects in humans such as contact dermatitis, chronic skin diseases, visual disturbances, and pulmonary edema, sometimes with lethal consequences. This is often the result of not complying with the criteria established by the United Nations Organization (UN) for the use and commercialization of fungicides (Speck-Planche et al., 2012; FAO and WHO, 2016). Some fungicides authorized for use in Mexico by the Federal Commission for the Protection against Sanitary Risks (COFEPRIS) include Azoxystrobin, Chlorothalonil, Fosetil-Al, Mancozeb, Metalaxyl-Chlorothalonil, and Propiconazole (COFEPRIS, 2019). However, Chlorothalonil is on the list of extremely dangerous substances for human health (NJ Health, 2017) due to its carcinogenic properties and the fact that it is highly toxic to fauna, posing a serious environmental risk (Reglinski and Dick, 2005; Ortíz et al., 2014).
Unfortunately, there is a lack of information about the characterization and effective use of chemical fungicides in pine trees. Not enough is known about the physiological, biochemical and molecular modes of action of these products, the procedures that should be followed to avoid the development of resistance to chemical molecules in disease-causing agents, their effects on the soil microbiota, the application dose, or the time, place and methods with which to apply them (Adusei-Fosu and Rolando, 2018).
Fungal control by biological agents. Biological fungicides, also known as biofungicides (Heydari and Pessarakli, 2010; Liu et al., 2021) or biocontrol agents, are formulations developed from bacteria or fungi that are designed to control and/or eradicate pathogenic fungi. These products have been used as an alternative method to reduce the damage caused by pathogenic fungi while generating little or no environmental contamination and without generating resistance in the targeted fungi (Guédez et al., 2008; Heydari and Pessarakli, 2010).
The biological control of fungi and also involve the participation of an antagonist organism, an agent or a combination of biological agents that can interfere with the physiological processes of pathogenic parasites (Legrand et al., 2017). The efficient use of biological control agents affects various interactions between the biological control agent, the pathogen, the host, and the environment, among others. It can be effective in reducing the incidence of pathogenic fungi among forest populations (Figure 4).

Figure 4 Interactions that play a role in biological control (Adaptado de Mora-Aguilera et al., 2017).
The interest in biological control has increased in recent decades due to its advantages over traditional fungal control methods (Table 3), mainly due to the indiscriminate use of chemical products, which has led pathogens to develop resistance (Gepp et al., 2012; Yang et al., 2019). The increasing commitment to environmental conservation has promoted the development of sustainable and comprehensive methods to reduce the excessive use of agrochemicals. Biological control agents have shown to be effective in reducing the inoculum load of the pathogen. Antagonist organisms are involved in different mechanisms that may act together against pathogens, such as competition for space and nutrients, parasitism, production of secondary metabolites (antibiotics, lytic enzymes and volatile compounds), and induced systemic resistance in plants (Hernández-Lauzardo et al., 2007; Martínez et al., 2013).
Table 3 Advantages and disadvantages of the use of biological control agents for fungal ma nagement.
| Ventajas | Desventajas |
|---|---|
| Mayor especificidad | Ignorancia sobre el método |
| Baja resistencia de las plagas al control biológico | Falta de apoyo económico |
| Sustitución parcial o total a los plaguicidas sintéticos de sustancias químicas | Falta de personal especializado |
| Relación coste/beneficio mayor | Antagonistas susceptibles a plaguicidas |
| Evita plagas secundarias | No provee una supresión inmediata |
| Escasas intoxicaciones | Poca investigación toxicológica |
| Amigable con el ambiente | Sobrevivencia y adaptabilidad |
Source: Guédez et al. (2008); Holmes et al. (2016)
Some microorganisms secrete secondary metabolites that aid in biological control. These compounds are not directly involved in the growth, development or reproduction of pathogens, but can interfere indirectly with their growth and/or activities. An example of this metabolic action is the production of antibiotics. Some microorganisms produce and secrete one or more antibiotic compounds (Liu et al., 2021). The production of lytic enzymes that can hydrolyze a wide variety of polymeric compounds, such as chitin, proteins, cellulose, hemicellulose and DNA, is associated with the control of pathogens (Vargas-Hoyos and Gilchrist-Ramelli, 2015).
Another biocontrol mechanism is mycoparasitism, in which the antagonist acts as a mycoparasite, with the pathogen as the host (Steyaert et al., 2003). An example of mycoparasitism (attack of a fungus by another fungus) is the genus Trichoderma, which is widely used as biological control agent (Alfiky and Weisskopf, 2021) against a variety of phytopathogenic fungi.
Pathogenic fungi can also be managed through induced systemic resistance (ISR), a promising non-chemical strategy for effective disease management (Meena et al., 2020). It is an infection-activated plant response that is enhanced by plant growth-promoting rhizobacteria (PGPR) such as Azospirillum, Azotobacter, Gluconacetobacter, Pseudomonas, and Bacillus. These bacteria represent a biological alternative for pathogen control and ecosystem conservation (Choi et al., 2014; Guo et al., 2015; Moreno-Reséndez et al., 2018). However, the systemic resistance response can be limited by stress conditions affecting the plant such as water deficiency, causing alterations in the signals associated with the containment of the pathogen (Arango-Velez et al., 2016). In conifers, the systemic resistance response works through lignification induced by the inoculation of pathogens, as is the case with the fungi Sphaeropsis sapinea and Diplodia scrobiculata in Pinus nigra (Bonello and Blodgett, 2003; Blodgett et al., 2007).
Nutrient competition is another mechanism that can be used to control pathogenic fungi. It is a proven biocontrol method when the growth of antagonist organisms causes the depletion of nutrients and/or the invasion of the space available for pathogens, thereby reducing their growth rate and incidence (Guerrero-Prieto et al., 2011; van Lenteren et al., 2018). An example of this mechanism is the bacterium Serratia marcensces strain PWN146, isolated from Pinus pinaster specimens with wilting symptoms. The genome of this bacterium contains genes associated with the production of siderophores, heavy metal transporters that are involved in the sequestration and transport of iron. The genome also contains genes related to the biosynthesis of antibiotics, such as the igrB gene (gramicidin), tycC (tyrocidine), ppsDE (plipastatin), and srfAD (surfactin), enzymes that degrade the cell wall (Chitinase Class I) and a toxin with insecticide activity (cytolytic delta-endotoxin cyt1Aa type-1Aa) (Vicente et al., 2016).
The biological control of fungi is a method widely used in the agricultural sector. It has also been proposed for the management of forest diseases as an alternative to the use of chemical agents that can help conserve and recover forests that have been lost or degraded by disease. Table 4 shows some examples of biological control agents against fungi affecting pine trees. The most widely studied biofungicides are species belonging to Trichoderma and Bacillus.
Table 4 Use of biological control agents for the control of fungi that cause diseases in Pinus spp.
| Agente de Biocontrol | Patógeno | Planta | Aplicación | Referencia |
|---|---|---|---|---|
| Hongos | ||||
| a. Trichoderma | ||||
| T. harzianum | Fusarium circinatum | P. greggii | Invernadero | García-Díaz et al., 2019 |
| T. koningiopsis | Fusarium oxysporum | P. massoniana | In vitro | Yu y Luo, 2020 |
| Invernadero | ||||
| T. harzianum | Botrytis cinerea | P. sylvestris | In vitro | Capieau et al., 2004 |
| T. polysporum | Vivero | |||
| T. virens, T. atrobrunneum | Armillaria spp. | Robles y abetos | In vitro | Chen et al., 2019 |
| Campo | ||||
| b. Otro | ||||
| Chaetomium, Alternaria | F. circinatum | P. nigra | Martínez-Álvarez et al., 2016 | |
| P. pinaster | In vitro | |||
| P. pinea | Campo | |||
| P. radiata | ||||
| P. sylvestris | ||||
| Bacterias | ||||
| a. Aneurinibacillus | ||||
| Aneurinibacillus migulanus | Dothistroma septosporum | P. contorta | In vitro | Alenezi et al., 2015 |
| b. Bacillus | ||||
| B. pumilus | Sphaeropsis sapinea | P. massoniana | In vitro | Dai et al., 2021 |
| Invernadero | ||||
| B. simplex | Heterobasidion annosum | P. radiata | In vitro | Mesanza et al., 2016 |
| Armillaria mellea | Invernadero | |||
| F. circinatum | P. radiata | In vitro | Iturritxa et al., 2017 | |
| Invernadero | ||||
| B. subtilis | Fusarium sambucinum | P. elliottii | In vitro | Maciel et al., 2014 |
| Invernadero | ||||
| F. circinatum | P. taeda | In vitro | Soria et al., 2012 | |
Trichoderma. Biofungicides based on the genus Trichoderma are considered highly effective. They work by producing enzymes (chitinases, b-1,3 glucanases and proteases), antibiotics (6-pentyl-a-pyrone), volatile compounds, siderophores, and acid indole-3-acetic acid. They also promote plant growth (Michel-Aceves et al., 2004; Infante et al., 2009; Rios-Velasco et al., 2016; Ruiz-Cisneros et al., 2018; Illa et al., 2020; Yu and Luo, 2020). Another mechanism associated with Trichoderma involves chemotropism induced by the existence of a chemical gradient of amino acids and/or sugars, recognition (physical interaction by specific binding to the host surface), penetration and finally, mycoparasitism of the host cell wall by the action of lytic enzymes. (Steyaert et al., 2003; Alfiky and Weisskopf, 2021). Some studies show that strains of Trichoderma sp. isolated from forest plants have the ability to biocontrol pathogenic fungi of the genus Armillaria, which causes root rot (Chen et al., 2019). Commercial products such as Binab® TF.WP, formulated with strains of Trichoderma harzianum and T. polysporum, have been used as fungicides and have been as effective as the chemical fungicide (tolylfluanine 50% p/p) recommended against Botrytis cinerea. They have also reduced needle damage in P. sylvestris by up to 94% in growth chambers and by 57% under nursery conditions (Capieau et al., 2004). T. harzianum (PHC®) reduces the incidence of the disease caused by F. circinatum in P. greggii seedlings by up to 22% (García-Díaz et al., 2019). T. koningiopsis inhibits the growth of F. oxysporum (in vitro) by up to 78.6% and decreases the incidence of the disease by 50% in P. massoniana while promoting seedling growth (Yu and Luo, 2020).
Bacillus. It is the most exploited bacterial genus for the production of biofungicides due to its versatility in terms of biological control mechanisms. Members of this genus can produce volatile compounds, antibiotics (bacillomycin, iturins, phenycins, fengycins, subtilins, surfactins), lytic enzymes (chitinases and b-1,3-glucanases), siderophores (Bacillibactin), and toxins (d-endotoxins). They also have the ability to promote plant growth (Villarreal-Delgado et al., 2017; Jiménez-Delgadillo et al., 2018; Kang et al., 2019; Ocegueda-Reyes et al., 2020; Dai et al., 2021). Some studies have shown that these bacteria can induce a systemic resistance response in plants by eliciting molecules (lipopeptides, phytohormones, and volatile compounds). They also activate the synthesis of salicylic acid, ethylene, jasmonic acid and abscisic acid, which regulate the defense system of different crop plants (Villarreal-Delgado et al., 2017; Vinod and Sabah, 2018). B. subtilis has demonstrated its ability to control fungi, inhibiting up to 18.7% of the in vitro growth of F. sambucinum, and providing greater vigor and growth to P. elliottii seedlings (Maciel et al., 2014). The secondary metabolites produced by B. subtilis represent an efficient fungicide alternative. They inhibit 50% of the growth of F. circinatum isolated from P. taeda (Soria et al., 2012). Biocontrol methods have managed to reduce the high incidence and severity of F. circinatum infection in P. radiata and P. muricata (Gordon et al., 2006). In addition, B. simplex is capable of biocontrolling Heterobasidion annosum and Armillaria mellea in vitro. It also reduces by 55% the infection incidence of H. annosum and by 46.9% the infection incidence of A. mellea in P. radiata seedlings (Mesanza et al., 2016). B. simplex also has antagonistic activity against F. circinatum (17%) in vitro and reduces P. radiata lesions by up to 22% in two-year-old trees grown in greenhouses (Iturritxa et al., 2017). B. pumilus isolated from P. massoniana can biocontrol 85% of the damage caused by Sphaeropsis sapinea in vitro and 90% under greenhouse conditions due to the production of secondary metabolites that damage the mycelium and delay spore germination (Dai et al., 2021).
Conclusions
Pine forests are ecosystems of great importance due to the ecological, economic, and social benefits they provide. The global forest biomass has been reduced by several factors such as excessive logging and the conversion of forests into agricultural or residential areas. In addition, forests face biotic problems such as fungal diseases that spread through natural and anthropogenic factors. Therefore, it is important to have a comprehensive understanding of the various fungi that have been reported to infect pine species (Alternaria, Botrytis, Ceratocystis, Cylindrocarpon, Cronartium, Curvularia, Daldinia, Diplodia, Dothistroma, Fusarium, Meria, Mycosphaerella, Lecanosticta, Lophodermium, Phytophthora, Pythium, and Rhizoctonia, among others). It is also important to know the different factors involved in plant fungal infections, such as the host, environmental conditions, management, and anthropogenic influences.
In Mexico, the forestry sector has made excessive use of chemical products to control fungi; however, these products are registered for agricultural and non-forestry use. Given the recent interest in reducing the use of agrochemicals, the use of biological control agents is promoted as a friendly alternative for the recovery of forests with no negative ecological impact. These products can be implemented in urban areas since they pose be no danger to people or animals that interact with forests.
The information documented in the present work shows that Trichoderma sp. and Bacillus sp. are the most studied biofungicides associated with Pinus spp. The use of these agents helps maintain and conserve forest resources. Further research is needed to promote the use, management and conservation of forests with biological methods that allow to control phytopathogenic species.











 texto en
texto en