Members of the family Geminiviridae have circular, single-stranded DNA (ssDNA) genomes encapsidated in twinned icosahedral particles; they form the second-largest family of plant viruses. The genus Begomoviridae comprises viruses with monopartite (one ~2.9-kb DNA) and/or mostly bipartite genomes (two ~2.6-kb DNAs, referred as DNA-A and DNA-B) (Brown et al., 1995; Fauquet and Stanley, 2005; Jones, 2003). Begomoviruses includes more than 440 species, members of which infect dicot plants and are transmitted by whiteflies of theBemisia tabacicryptic species complex (Fiallo-Olive et al., 2021). In particular, devastating diseases to cultivated plants caused by begomoviruses have been documented through Central America, Mexico, the Caribbean Basin, the Southern United States of America, and South America, yield losses range from 50 to 90% of total production. (Ala-Poikela et al., 2005; Brown et al., 2005; Morales et al., 2005; Rausch et al., 2005; Torres-Pacheco et al., 1996). Previous reports have confirmed the incidence of begomoviruses in a range of solanaceous crop plants and native flora from various parts of Mexico (Ascencio-Ibañez et al., 1999; Garrido-Ramirez and Gilbertson, 1998; Gregorio-Jorge et al., 2010; Hernandez-Zepeda et al., 2007a; Hernandez-Zepeda et al., 2007b; Hernandez-Zepeda et al., 2007c; Torres-Herrera et al., 2019; Torres-Pacheco et al., 1996). In particular, PepGMV was reported in cultivated and wild pepper, in different regions of Mexico, as Baja California, Sinaloa, Sonora, the Comarca Lagunera, Campeche, Yucatan, San Luis Potosi, Michoacan, among others (Hernandez-Espinal et al., 2018; Morales-Aguilar et al., 2019; Rodelo-Urrego et al., 2015; Rodriguez-Negrete et al., 2019).
Capsicum annuum (family: Solanaceae), a high-value crop for its non-pungent (sweet pepper) and pungent (chili syn. hot pepper) fruits, is the foremost pepper species cultivated worldwide (Bosland and Votova, 2000; Park et al., 2021). This species, together with other pungent, valuable species, originating and domesticated in the tropical Americas-such as C. chinense (chili Habanero), C. pubescens (chili Manzano), and C. frutescens (chili Tabasco or Picopaloma)-are broadly cultivated and consumed in the diet throughout Mexico (Cazares-Sánchez et al., 2005). Persistent problems affecting these crops include a range of viruses and their vectors. Of these, the viral disease that accounts for much of the yield losses is attributed to begomoviruses and the use of susceptible cultivated pepper genotypes (Anaya-Lopez et al., 2003; Torres-Pacheco et al., 1996). Pepper diversity is an essential resource for genetic improvement and crop management; in combination with natural weeds, they are host reservoirs of viruses, which, together with the whitefly-vector, might support pathogen-diversifying events that should be investigated. In the states of the Yucatan Peninsula of Mexico (YPM), variations in C. annuum var. annuum landraces (such as those the Mayan named Ya´ax iik, X´kat iik, Dulce, and the wild grown C. annuum var. aviculare, named Maax iik) in response to changing biotic and/or abiotic stresses are significant. These landraces, which are primarily grown in home gardens, the Milpa system (associated with other crops), or harvested in situ, are utilized for local consumption and as a source for rural family income (Cazares-Sanchez et al., 2005). Despite their importance, information regarding the distribution and disease incidence by different pathogens, such as begomoviruses, is limited. In addition, the Habanero chili, an essential crop for both local use and export, is cultivated in extensive cropping areas; however, increased use of agrochemicals has not diminished losses on fruit harvest; rather, whitefly infestation and viral diseases exceeded 90% during the dry season (March to May of each year, farmer, and author observations). The severity of these problems has impacted Habanero field cultivation; traditional field production has moved toward alternative cropping systems, such as mulching, tunneling and greenhouses. Thus, as a first step towards the development of broad control strategies, pathogens common to cultivated, wild relatives, and weed species, as well as their vectors, must be recognized and genetically characterized. The objective of this study was to characterize the partial identity, the genetic diversity and the phylogenetic relationship of the begomoviruses infecting different peppers, including the cultivated C. chinense, various C. a. annuum landraces and C. frutescens and the wild grown C. annuum var. aviculare (Maax iik), as well as some uncultivated plants grown as weeds from different locations in the state of Yucatan and Campeche. Genetic and phylogenetic analyses based on nucleotide sequences of the core Coat Protein gene (Cp) region (CPR) were used to explore begomovirus genetic diversity and resolve the possible relationship with other known bipartite begomovirus.
Materials and methods
Plant material and source of viruses
A total of 151 symptomatic plant samples were collected, including cultivated Capsicum chinense Habanero-type (45) and Scotch Bonet-type (12), C. annuum var. annuum landraces regionally identify as Dulce (18), X´kat iik (12), and Ya´ax iik (8) plants of C. annumm. var. aviculare locally called Maax iik (43), and C. frutescens (8) Tabasco-type plants that grow wild or in-home gardens. Additionally, we collected representative weeds from different families: Callicarpa spp., (Verbenaceae) (1), Croton malvaviscifolius (Euphorbiaceae) (1), Dicliptera sexangularis (Acanthaceae) (1), Herissantia crispa (Malvaceae) (1), and Nicotiana tabacum (Solanaceae) (1). Plant material was collected in locations within the states of Yucatan (YS) and Campeche (CS) in the YPM (Table 1, Figure 1). The weed leaf samples were gathered from wild grown plants in non-cropping sites and from pepper cultivated areas during a severe whitefly infestation seasons (February-May and July to the beginning of October of 2017). The samples were stored -20 ºC in the molecular virology laboratory of CICY, to serve as a source of viral DNA. Total genomic DNA from the leaves of pepper and weed plants was extracted according to the methods of Dellaporta et al. (1983) and Echevarria-Machado et al. (2005), respectively. Leaf samples were cleaned with sterile water before the DNA extraction in order to eliminate the possible contamination of whiteflies.
Table 1 Locations where the biological material was collected within the Yucatan (YS), and Campeche (CS) states in Mexico.
| State | Region/ zone | District |
|---|---|---|
| Yucatan | Metropolitan influence zone | Conkal (10)z, Cholul (8), Chicxulub Pueblo (8), Dzitya (5), Hunucma (6), Hunxectaman (6), Merida (6), Uman (8) |
| North-central coastal | Dzidzantun (6) | |
| East coastal zone | Espita (10) | |
| Western coastal zone | Halacho (6) | |
| South central zone | Cuzama (8) | |
| Southern zone | Catmis (6), Peto (10), Polinkin (6), Tixmehuac (6), Tekax (10) | |
| Southwestern zone | Timucuy (8), Subincancab (8) | |
| Campeche | Region of Yaxchii | Hopelchen (10) |
zNumbers between parentheses represent the number of plants collected by locality
Polymerase chain reaction (PCR) amplification, cloning, sequencing, and sequence analysis
The primer pair prAV324/AC889 was used for PCR amplification of the begomoviruses diagnostic ~576 bp core coat protein (core Cp) gene fragment (Brown et al., 2005), following the protocol recommended by the authors. Positive virus-specific PCR products of ~576 bp were purified with a QIAEX II kit (QIAGEN) and cloned into pGEM T-Easy vector (Promega, Madison, WI, USA) following the manufacturer’s instructions. The nucleotide sequences were determined with an ABI 377 sequencer (Davis Sequencing Co., CA, USA). Most clones were sequenced in forward and reverse directions. To enhance the possibility of detecting multiple begomoviral genotypes due to mixed infections in field samples, three to five PCR products were cloned and their nucleotide sequences analyzed. The nucleotide sequences (120) from randomly selected cloned samples (52) were edited manually to remove degeneracy imposed by the primers (EditSeq, DNASTAR version 5.08, Madison, WI, USA), resulting in a 533 nt sequence of the core Cp, and partial identification of Begomovirus species was done by comparing with sequences available in the NCBI-GenBank database (http://www.ncbi.nlm.nih.gov) by BLASTn (Altschul et al., 1997).
Sequence data analysis
For genetic and phylogenetic analysis, data of 25 selected core Cp partial begomoviruses sequences identified in the present study were used, 23 of which were isolated from species and landraces of Capsicum and two from different weed species. In addition, 36 reference standard sequences were obtained by cropping the core Cp of the full-length begomoviral genome from the GenBank database (Table 2), and included in the phylogenetic analysis. Multiple sequence alignment of the taxa was prepared with the CLUSTAL W in MegAlign (DNASTAR version 5.08, Madison, WI). Genetic distance matrix, diversity and pattern of nucleotide substitutions (total, synonymous and non-synonymous) analyses were complete with 23 PepGMV CP selected nucleotide sequences (Table 2). Selected PepGMV sequences included three samples of each Capsicum species and one sequence of each weed plant; the CP genomic region (CPR) comprised position 147-679 according to GenBank sequence AF077025. Molecular evolutionary testing was conducted using the Kimura 2-parameter and Kumar methods, Bootstrap analysis with 1000 replicates, with MEGA version 11 (Tamura et al., 2021). Codon-based Z-test analysis averaging over all sequence pairs was performed to test for the selective force operating on CP sequences, using the Kumar method in MEGA 11. All positions containing gaps and missing data were eliminated (complete deletion option). There were a total of 177 positions in the final dataset. To reconstruct a phylogenetic tree, multiple aligned sequences were analysed by multiple parsimony using MEGA 11 with 1000 iterations per search. The strict consensus tree was constructed using the standard settings of MEGA 11, and statistical support for each major clade was calculated using Bootstrap with 1000 iterations per search. The tree was rooted using the CP sequence edited from the core CP sequence of the Maize streak virus (MSV-CP) of the genus Mastrevirus, family Geminiviridae (Table 2).
Table 2 Geminivirus used in the nucleotide alignment and phylogenetic analyses and the respective virus acronym and GenBank accession number.
| Geminivirus | Acronym | GB Acc No |
|---|---|---|
| African cassava mosaic virus-[Nigeria] | ACMV-[NG] | X17095 |
| Abutilon mosaic virus | AbMV | X15983 |
| Bean calico mosaic virus | BCaMV | AF11018 |
| Bean golden mosaic virus-[Brazil] | BGMV-[BR] | M88686 |
| Bean golden yellow mosaic virus-[Mexico] | BGYMV-[MX] | AF17355 |
| Bean golden yellow mosaic virus-[Guatemala] | BGYMV-[GT] | M91604 |
| Cabbage leaf curl virus | CaLCuV | U65529 |
| Chino del tomate virus | CdTV | AF22666 |
| Corchorus yellow vein virus | CYVV-[Hoa] | AY72790 |
| Cotton leaf curl Gezira virus | CLCuGV | AY03600 |
| Cucurbit leaf crumple virus-[Arizona] | CuLCrV-[Ari] | AF25620 |
| Euphorbia mosaic virus-[Yucatan Peninsula] | EuMV-[YP] | DQ31893 |
| Euphorbia mosaic virusz | H6-EuM-YP (C.chinense cv. Habanero)z | EU15585 |
| Maize streak virus | MSV-CP | NC00134 |
| Melon chlorotic leaf curl virus-[Guatemala] | MCLCuV-[GT] | AF32549 |
| Pepper golden mosaic virus-[Chiapas] | PepGMV-[Chi] | AF07702 |
| Pepper golden mosaic virus-[Costa Rica] | PepGMV-[CR] | AF14922 |
| Pepper golden mosaic virus-[Distorter] | PepGMV-[D] | AY92851 |
| Pepper golden mosaic virus-[Mo] | PepGMV-[Mo] | AY92851 |
| Pepper golden mosaic virus-[Serrano] | PepGMV-[Ser] | AY92851 |
| Pepper golden mosaic virus-[Tamaulipas] | PepGMV-[Tam] | U57457 |
| Pepper golden mosaic virus-[Nicaragua] | PepGMV-[Ni] | AJ842140 |
| Pepper golden mosaic virusz | 1-3-PepGMV (D. sexangularis)z | EU155830 |
| 3-PepGMV (C. annuum cv Sweet pepper)z | EU155831 | |
| 43-PepGMV (C. annuum)z | EU155834 | |
| 45-PepGMV C.annuum cv Cubano)z | EU155835 | |
| 120-PepGMV (C. chinense cv Habaneroz | EU155836 | |
| 122-PepGMV (C.chinense cv Habaneroz | EU155838 | |
| 138-PepGMV(C.annuum cv Sweet pepper)z | EU155839 | |
| 144-PepGMV (C.annuum cv Xkaticz | EU155841 | |
| 151-PepGMV (C.annuum cv Sweet pepper)z | EU155843 | |
| 164-PepGMV (C.annuum cv Xkaticz | EU155844 | |
| 240-2-PepGMV (C.annuum cv Maax)z | EU155846 | |
| 250-2-PepGMV (H. crispa)z | EU155847 | |
| R3-PepGMV (C.annuum)z | EU155853 | |
| R6-PepGMV (C. frutescens) z | EU155854 | |
| R10-PepGMV (C.chinense cv Habaneroz | EU155855 | |
| R13-PepGMV C.chinense cv Scotch bonnetz | EU155856 | |
| R15-PepGMV (C.annuum cv Maax)z | EU155858 | |
| R16-PepGMV (C.chinense cv Habaneroz | EU155859 | |
| R12-PepGMV (C. annuum cv Maax)z | ||
| Pepper huasteco yellow vein virus | PHYVV | X70418 |
| Pepper huasteco yellow vein virusz | 40-PHYVV (C. annuum)z | EU155833 |
| 120- PHYVV (C. chinense cv Habanero)z | EU155837 | |
| 183- PHYVV (C. chinense cv Habanero)z | EU155845 | |
| Rhynchosia golden mosaic virus | RhGMV | AF239671 |
| Rhynchosia golden mosaic virus-[Chiapas] | RhGMV-[Chi] | AF408199 |
| Sida golden mosaic Costa Rica virus | SiGMCRV | X99550 |
| Sida golden mosaic virus-[Florida] | SiGMV-[Flo] | AF049336 |
| Sida golden mosaic Honduras virus | SiGMHV | Y11097 |
| Squash leaf curl virus | SLCV | M38183 |
| Tomato golden mosaic virus-Yellow vein | TGMV-YV | K02029 |
| Tomato mild yellow leaf curl Aragua virus | TMYLCAV | AY927277 |
| Tomato mottle virus-[Florida] | ToMoV-[Flo] | L14460 |
| Tomato severe leaf curl virus-[Guatemala] | ToSLCV-[GT] | AF130415 |
| Tomato severe leaf curl virusz | 144- Tomato severe leaf curl virus Guz | EU155842 |
| Tomato yellow leaf curl virus-Mild | TYLCV-Mld | X76319 |
| Tomato yellow leaf curl virus-[Dominican Republic] | TYLCV-[DR] | AF024715 |
| Tomato yellow leaf curl virusz | 138-TYLCV DR (C. annuum cv Sweet pepper)z | EU155840 |
zBegomovirus clones isolated from plants collected in the states of Campeche and Yucatan.
Results
Search for viral symptoms in cultivated and field plants
In a disease survey during high whitefly infestation seasons at locations of the YS and CS (Figure 1), virus-like symptoms were collected on diverse cultivated and non-cultivated pepper and weed plants. All of sampled cultivated pepper plants, namely C. chinense, C. a. var. annuum, landrace Dulce, X´kat iik and Ya´ax iik, C. frutescens, and wild grown C. a. var. aviculare Maax iik, showed symptoms of golden mosaics, distorted leaves and stunting growth. Symptoms, clearly distinguished in C. frutescens, were often moderately perceived in one or two branches of Maax iik, rarely affecting the whole plant (Figure 2A). On the other hand, landrace X´kat iik (Figure 2B), Chile Dulce (Figure 2C and D) and Habanero chili (Figure 2E and F) plants showed noticeable foliar symptoms of varied severity, including yellowing with downward curly leaves, golden mosaics, and stunting growth. Comparable symptoms were recorded for weed species (images not shown). Nevertheless, in the majority of cases, chili plants showing small curled leaves were stunted and bushy in appearance because of reduced length internodes (Figure 2D).

Figure 2 Symptoms observed in wild-grown (A) and cultivated (B, C, D, E and F) plants sampled at Catmis (A), Muna (B), Merida (C), Cuzama (D), and Tekax (E and F) infected with Begomovirus (PCR+). (A) Capsicum annuum var. aviculare (Maax iik) plant showing branches with golden mosaic and distorted leaves, and stunting growth; (B) C. annuum var. annuum (X´kat iik) with severe golden mosaic, and distorted leaves; (C and D) C. annuum var. annuum (Chile Dulce) with severe golden mosaics, distorted leaves, and stunting growth; (E and F) C. chinense (Habanero type) with golden mosaics, and with downward curly leaves and severe stunting growth.
Begomoviruses preliminary identification
Of the total collected plant samples (151), including 146 peppers and 5 weeds, 90.1% were PCR positive using the core CP primer (Table 3). That is, 89.7% of the peppers (131), including plant samples of all the pepper species and all the weed (5) plant samples were positive to begomoviruses. Sequencing of amplicons, on average two to three per sample of plants (52), uncovered the likely incidence of single and mixed begomovirus infections. Of these, virus species determined in 120 sequenced clones were provisionally identified based on the comparison of nucleotide identity (>89% nt) with viral core CP sequences of well-studied begomoviruses (Table 3). Single infections with Pepper golden mosaic virus PepGMV predominated, representing 82.5% of the sequenced clones (99) detected in materials of the different Capsicum species and in the weed species Callicarpa spp., C. malvaviscifolius, D. sexangularis, H. crispa, and N. tabacum (Table 3). This was followed by Pepper huasteco yellow vein virus PHYVV (5 %) found in two C. chinense plants, one clone isolated from Habanero and the other from Scotch Bonnet type, plus the Tomato severe leaf curl virus ToSLCV (4.2%) found in the Habanero and Dulce plants. Interestingly, four sequenced clones (3.3%) from a single Habanero chili plant (sample H6 EuMV-YP[C. chinense H]) displayed high identity (98-99%) to the Euphorbia mosaic virus-[Yucatan Peninsula] EuMV-YP isolate described from Euphorbia heterophylla (Hernandez-Zepeda et al., 2007b). Possible mixed infections were confirmed by bi-directionally searching of three to five clones of each partial begomoviral species isolated from each plant. These included a mixture of PepGMV-PHYVV in the Habanero chili (sample 120, C. chinense H), the PepGMV-TYLCV in the Dulce pepper (sample 138, C. annuum D) and the PepGMV-ToSLCV in a X´kat iik plant (sample 144, C. annuum, Table 3).
Table 3 Provisional sequence identification of Begomovirus (identity >89% nt) found in samples of cultivated*, semi-cultivated☼, wild † grown pepper plants and weed (w) plants collected in the state of Yucatan. Viruses revealed by PCR with universal primers for the core Cp. Number of +PCR positives clones over total sampled plants per species and/or landrace type. In parenthesis number of positive sequenced clones, underlined are the possible mixed virus infections found.
| Host species | No. +PCR / total samples | Virus species identity >89% nt (No. of sequenced clones to core Cp) |
|---|---|---|
| Capsicum chinense (habanero)z | 53/57 | (35) PepGMV, (6) PHYVV, (2) ToSLCV, (4) EuMV-YP, 1PepGMV:1PHYVV |
| C. annuum var. Annuum (dulce)* | 16/18 | (12) PepGMV, (3) ToSLCV, 1PepGMV:1TYLCV. |
| C. a. var. Annuum (X´kat iik)* | 11/12 | (9) PepGMV, 1PepGMV:1ToSLCV. |
| C. a. var. Annuum (Ya´ax iik ) ☼ | 8/8 | (7) PepGMV |
| C. frutescens (Pico de paloma) ☼ | 6/8 | (5) PepGMV |
| C. a. var. aviculare (Maax iik)† | 37/43 | (18) PepGMV |
| Callicarpa spp (w) | 1/1 | (3) PepGMV |
| Croton malvaviscifolius (w) | 1/1 | (3) PepGMV |
| Nicotiana tabacum (w) | 1/1 | (3) PepGMV |
| Dicliptera sexangularis(w) | 1/1 | (2) PepGMV |
| Herissantia crispa (w) | 1/1 | (2) PepGMV |
Genetic variation of PepGMV isolates based on nucleotide distance analysis from partial DNA-A begomovirus sequences
Genetic distance analysis based on pairwise analysis of 23 chosen core CP nucleotide sequences (Table 4) indicated that PepGMV isolates were closely related strains sharing from 95 to 99% nucleotide sequence identity; percentage nt identity was calculated as [1-genetic distance] x100 (Brown et al., 2005). The average nt distance of CPR estimated for the number of base substitutions per site over all 23 sequence pairs was equal to 0.027 (±0.004 SE), with a calculated transitional to transversional distance per site ratio (R) equal to 0.641(± 0.21 SE). In general, the estimated average codon-based divergence of all sequence pairs showed a low value of nonsynonymous (dN) substitutions to synonymous (dS) substitutions, dN = 0.003 < dS = 0.130, and a low dN / dS ratio of 0.023 (Table 4), respectively.
Phylogenetic analysis based on partial DNA-A begomovirus sequences
The phylogenetic analysis examined the probable relationship between begomovirus-infected pepper and those of weed species collected in the YS and CS relative to their corresponding sequences from a database (Gen-Bank). The analysis incorporated the nt sequences of the Coat protein gene (CP) core region from 61 taxa. The matrix was constructed with 23 nt sequences isolated from Capsicum spp., two from plants grown as weeds (Table 3), and 36 from Gen-Bank sequences (Table 2). The evolutionary history was inferred using the Maximum Parsimony method. The most parsimonious tree with length =3191 was show (Figure 3). The consistency index is 0.376378 (0.309704), the retention index is 0.634911 (0.634911) and the composite index is 0.238966 (0.196634) for all sites and parsimony-informative sites (in parentheses). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) were show next to the branches. The MP tree was obtained using the Subtree-Pruning-Regrafting (SPR) algorithm with search level 1 in which the initial trees were obtained by the random addition of sequences (10 replicates). There were a total of 566 positions in the final dataset. Evolutionary analyses were conducted in MEGA11. The cluster of Eastern Hemisphere begomoviruses with a 99% Bootstrap supported value includes the Tomato yellow leaf curl virus, of which clone 138-TYLCV-[C. annuum D], isolated from a single plant with a likely mix infection (Table 3, Figure 3), showed high identity (99%) to the Dominican Republic isolate of TYLCV. Interestingly, a divergent clade containing three clones of PepGMV isolates from different C. annuum cultivars was observed close to the MSV-CP, the Mastrevirus that was selected as root of the phylogenetic tree, with 89% Bootstrap supported value. The Western Hemisphere cluster divides into two subclades (63% support node). One group holds the Tomato severe leaf curl virus clone 144-ToSLCV-[C.annuum X]), isolated from a X´kaat ik plant with a probable mixed infection (Table 3, Figure 3). This is related to the ToSLCV-[GT] reference isolate (82% Bootstrap), shares common nodes with other begomoviruses, and is grouped with species of Sida golden mosaic virus (SiGMV), Tomato mottle virus (ToMoV), Chino del tomate virus (CdTV), and Abutilon mosaic virus (AbMV). The second group, with two branches, contains the pepper isolate of the Euphorbia mosaic virus isolate, H6 EuMV-YP (C. chinense H) with its sister species, the EuMV-YP reference isolate (99% Bootstrap) and the Tomato mild yellow leaf curl Aragua virus (TMYLCAV), a relation supported by a 77% value (Figure 3). The former groups are assembled with a begomovirus species that received no Bootstrap support, including the SLCV clade. The other relationships inferred for the collections with no Bootstrap support are subdivided into two subclades. One subclade comprising the Pepper huasteco yellow vein virus (PHYVV) cluster is grouped with three isolates from C. chinense, clones 120 and 183 of PHYVV- from Habanero and the 40-PHYVV isolate from the Maax iik plant, with 88 to 99 % Bootstrap values. The second subclade is composed of collections of the Pepper golden mosaic virus PepGMV from the most of the pepper and weed isolates set with well-known begomovirus reference isolates forming a major PepGMV clade supported by Bootstrap (83%). This PepGMV clade visibly split into three subclades (Bootstrap 89%) (Figure 3). Two clones of PepGMV, R3 and 164 were grouped in a separate clade with TYLCV-DR, with 89%% of Bootstrap support. A future analysis with the complete sequence of the A Component of both clones should be necessary, in order to corroborate this phylogenetic relationship.
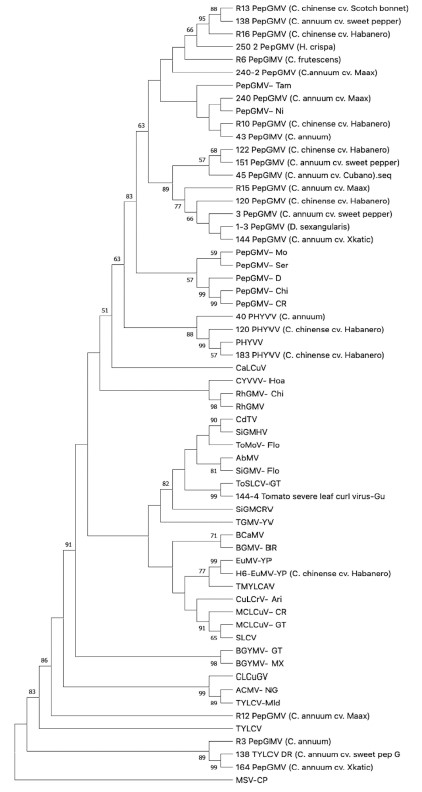
Figure 3 Cp sequence-inferred phylogenetic relationships of begomoviruses identified in field-grown and cultivated pepper plants from Yucatan and Campeche, Mexico; the analysis also includes the Cp sequence of selected, well-studied begomoviruses which are available in GenBank. Presented is the single most parsimonious tree; numbers placed at nodes represent Bootstrap values over than 50%, lower values are not shown (1000 iterations).
Discussion
Plant viruses have enormous potential for genetic variation and rapid evolution; although mutation, reassortment, and recombination are known as mechanisms that generate variation, less is known about the selection pressures that operate and drive their evolution (Font et al., 2007; Garcia-Arenal et al., 2003; Seal et al., 2006). Evidence is provided here that YPM infections by members of the genus Begomovirus are common in cultivated peppers, as well as non-cultivated weed plants. Nearly all plants in fields with cultivated peppers, landrace Dulce, X´kat ikk, Ya´ax ikk, Pico de Paloma, and Habanero chili analyzed during this work showed severe symptoms. In fact, most of the collected tested samples (90%) were PCR begomovirus-positive (PCR-BG-+), in which the conserved core Coat Protein region of the AV1 gene was used for the provisional identification of begomoviruses. This partial sequence provides provisional virus identification, while for definite classification, complete DNA-A and DNA-B particle sequences are required (Brown et al., 2001; Fauquet et al., 2003). Actually, Central America, México, and Brazil appear to be important Western Hemisphere centers of begomovirus diversity (Ala-Poikela et al., 2005; Fernandes et al., 2006; Hernandez-Zepeda et al., 2007a; Rodriguez-Negrete et al., 2019). Actually, more than 440 begomovirus species are reported, and among these a large number are emerging pathogens of pepper, bean (Phaseolus vulgaris), cotton (Gossypium sp.), cucurbits, okra (Abelmoschus esculentus), papaya (Carica papaya), tomato (Solanum lycopersicum), and weed plants (ICTV, 2022). In a previous study, from 119 symptomatic plants belonging to 16 different species, only 58% of the YPM samples were positive to begomovirus. In these samples, 13 distinct begomovirus species were identified, including the PepGMV isolated from C. annuum (Hernandez-Zepeda et al., 2007c). In contrast, almost 90% of the analyzed samples in this work were positive to PepGMV. In both cases, all the collected plants showed viral symptoms. As reported before, one possibility is that some symptomatic plants collected here were infected with other viruses that produce similar symptom (Hernandez-Zepeda et al., 2007c). Another report showed that geminivirus infection in tomato, with similar symptoms to those caused by geminivirus was induced by Potyviridae and Tobamoviridae (Polston and Anderson, 1997). It is notable that C. anuumm var. aviculare Maax iik plants examined here did show mild symptoms present in even one branch of the plant and rarely in two. Yet 86% of the collected material of Maax iik was PCR-BG-+. Symptom remission or host recovery has been associated with specific resistance of pepper plants to PepGMV, and silencing mechanisms in the recovery process have been suggested (Carrillo-Trip et al., 2007). This is important, as evidence from the literature suggests that the sources of resistance to PHYVV and PepGMV are to be found within wild relatives of C. annuum and some accessions of C. chinense (Anaya-Lopez et al., 2003; Hernandez-Espinal et al., 2018; Hernandez-Verdugo et al., 2001).
Based on the Cp sequences, the five partially identified species of begomovirus associated with cultivated pepper plants in the YPM are in accordance with those described by other authors (Anaya-Lopez et al., 2003; Godinez-Hernandez et al., 2001; Hernandez-Espinal et al., 2018; Hernandez-Zepeda et al., 2007b; Morales-Aguilar et al., 2019). The unusual finding of EuMV-YP infecting a Habanero chili plant, reported here, is remarkable given that this viral species commonly lives in the neotropical weed Euphorbia heterophylla (Ala-Poikela et al., 2005; Gregorio-Jorge et al., 2010; Hernandez-Zepeda et al., 2007a; Hernandez-Zepeda et al., 2007c). In Mexico, this virus was reported in the YPM and in Jalisco (Gregorio-Jorge et al., 2010; Hernandez-Zepeda et al., 2007a). In Cuba, it was reported that the EuMV infects tobacco plants collected in the field (Fiallo-Olive, 2010). Although experimental data have confirmed that P. vulgaris, S. lycopersicum, Nicotiana benthamiana, C. annuum, C. chinense, Cnidoscolus chayamansa, Jatropha curcas, Ricinus comunis, Arabidopsis thaliana, and Nicotiana tabacum plants are hosts of EuMV-YP (Hernandez-Zepeda et al., 2007b; Villanueva-Alonzo et al., 2013), additional field studies are needed to establish the extent of host range and distribution of EuMV-YP in Mexico and other countries of Central and South America.
In the present study, PepGMV was the most widespread begomoviral species found to infect cultivated C. chinense, C annuum landrace Dulce and X´kat iik, the home-garden grown C. frutescens, and wild grown Maax ikk, as well as collected weed plants in the YPM. Most weed samples should be analyzed in order to look for PepGMV infection in the YPM. This species has also been reported in Central America, infecting peppers in Costa Rica and Honduras (Lotrakul et al., 2000; Morales et al., 2005), and tomato, pepper, and cucurbits in Nicaragua (Ala-Poikela et al., 2005). In addition, tested samples of Habanero chili showed a single infection with PHYVV and ToSLCV, consistent with other studies reported for other parts of Mexico and Nicaragua (Ala-Poikela et al., 2005; Hernandez-Espinal et al., 2018; Morales-Aguilar et al., 2019). Moreover, the finding of an isolate of TYLCV reported here in a possible mixture with PepGMV infecting a single plant of C. annuum Dulce is supported by earlier reports of the presence of TYLCV in tomato in Yucatan since 1999 Ascencio-Ibañez et al., 1999). This strain of TYLCV shares 99% identity with the Dominican Republic strain (Ascencio-Ibañez et al., 1999), a strain that is considered a component of the Eastern North American and Caribbean clade (ENAC) of TYLCV (Duffy and Holmes, 2007). The other possible mixed infections, PepGMV-PHYVV, PepGMV-TYLCV and PepGMV-ToSLCV, frequently occur in association with only cultivated peppers, such as Habanero chili and C. annuum var. annuum, landrace Dulce and X´kat ikk, in farming areas where striking whitefly infestations were recorded (Escobedo-Garcia Medrano, unpublished data). It is remarkable that PepGMV and PHYVV mixed infections indeed occur naturally in many horticultural crops in México, where the distribution of the two viruses often overlaps (Hernandez-Espinal et al., 2018; Morales-Aguilar et al., 2019). Symptom expression in PepGMV-PHYVV interactions seems to be host-dependent (Mendez-Lozano et al., 2003). Future research on PepGMV-PHYVV, PepGMV-TYLCV, and PepGMV-ToSLCV interactions should address the biological significance of each mixture, how their respective genomes interact with the host cell genome during each of these mixed infections, and what is their relationship to the vector biotype involved in their transmission.
The results shown here concerning C. annuum var. aviculare, Maax iik, and the weed, e.g., D. sexangularis, and H. crispa are relevant because they demonstrate that native hosts could be reservoirs of genetic diversity for PepGMV-associated viruses. This may be important for the understanding of epidemic dynamics and evolution of the viruses under the prevailing conditions in YPM. Support for this comes from this fact: although Maax ikk plants showed mild virus symptoms when collected, the majority that tested BG positive by PCR (86.05%) turned out to be infected by the partially identified PepGMV. It is interesting to note that the genetic evidence provided here concerning a low genetic diversity within PepGMV (particularly the low nucleotide substitutions of dN/dS ratio in the CP-core gene region (CPR) analyzed) strongly suggests that a negative selective pressure (dN/dS) is operating on the gene region analyzed. This is comparable to that reported for TYLCV (Font et al., 2007).
Conclusions
In this work, almost 90% of the sampled plants were positive to begomovirus, including the partially identified species of PepGMV, PHYVV, ToSLCV, TYLCV and EuMV-YP. Single infections with PepGMV predominated, representing 82.5% of the sequenced clones. Mixed infection including the PepGMV-PHYVV the PepGMV-TYLCV and the PepGMV-ToSLCV were observed in different plant species. These partial sequences provide provisional virus identification isolates in the YPM, while for definite classification and phylogenetic analyzes, complete DNA-A and DNA-B component sequences will be required.











 texto en
texto en 



