Alternaria alternata and Fusarium solani are phytopathogenic fungi that cause great economic losses to agricultural producers and reduce food availability (Pérez-Rodríguez et al., 2019; Martínez-de la Parte et al., 2021). Alternaria alternata is characterized by causing an accelerated infection (Mata-Santoyo et al., 2018). With a temperature between 28 and 30 °C and relative humidity greater than 80%, the infection can begin or worsen in approximately 4 hours (Castrillo et al., 2021). The spores of A. alternata are dispersed mainly by wind or rain splash, and these spores can overwinter among crop residues until conditions are favorable for germination (Abbas et al., 2021). Fusarium solani mainly attacks plants in the base of the stem and root areas (Martínez-Martínez et al., 2020), causing wilting, yellowing of the leaves and growth delay (Martínez-Solórzano et al., 2020). Under conditions of high humidity and temperatures over 20 °C in the soil, a severe attack of F. solani can make the plants brittle. This pathogen survives in the soil among crop debris and live plants, and agricultural machinery can play an important role in its spread (Reyes-Tena et al., 2019). These phytopathogenic fungi are controlled mainly through the use of synthetic chemical fungicides, which, although effective, can lead fungi to generate resistance to their active compounds, which would pose new risks to human health and the environment (Abdel-Monaim et al., 2011; Mantecon, 2015). This has driven the search for more environmentally friendly alternatives such as antifungal compounds of plant origin.
Plants synthesize phytochemicals, such as phytoanticipins and phytoalexins, that play an important role on plant growth and reproduction, attracting pollinators and protecting against predators. They also contribute to the morphological and sensory characteristics of plants and plant by-products (Takshak and Agrawal, 2019; Singh and Chandrawat, 2017). The phytochemical group that has been most studied is that of phenolic compounds (Andrade-Andrade et al., 2018), which can inhibit the germination of spores and the growth of fungi (Rodríguez-Castro et al., 2020; Joaquín-Ramos et al., 2020; Oufensou et al., 2020). These compounds are synthesized through the shikimate, polyketide and mevalonate pathways, and are characterized by the presence of one or more hydroxyl groups (-OH) attached to a 6-carbon aromatic ring (Gutiérrez-Grijalva et al., 2016). Polyphenols derived from phenylalanine or benzoic acid have one or more phenol groups (Mutha et al., 2021). One of the characteristics of these compounds is their antioxidant activity or ability to counteract free radicals by chelating metal ions that are involved in their production. In addition, these compounds have ability to donate a hydrogen anion (an unpaired electron) and relocate it within the aromatic structure (García et al., 2019). Among polyphenols, flavonoids comprise some of the most important compounds. They are subdivided into Flavonols, Flavones, Catechins, Flavanones, Anthocyanidins, and Isoflavones (Mutha et al., 2021).
The biological activity (antimicrobial, antiviral, anticancer, etc.) attributed to polyphenols has given rise to a large number of studies focused on extracting them from various plant sources (Zhang et al., 2022). However, the biological potential of polyphenols is limited by their low solubility and stability against environmental factors such as light, high temperatures (>50 °C) and changes in pH (Costa et al., 2021). Generally, the extraction of these compounds is carried out through conventional techniques such as Soxhlet, percolation, maceration, infusions, etc. These techniques are associated with a high consumption of organic solvents, some of which are unfriendly to the environment, which limits the industrial extraction of those compounds. Furthermore, conventional techniques require long extraction times, involving high energy consumption (Soto-García et al., 2016) as well as considerable time and effort (Chemat et al., 2019). In recent decades, alternative techniques have been proposed for the extraction of polyphenols, including the use of microwaves, by which electromagnetic radiation is used to heat vegetal material, which increases the permeability of cell membranes (Bocker and Silva, 2022). Another alternative technique is ultrasound which, through cavitation, increases the mass transfer and speed of chemical reactions, promoting cell lysis and the separation of cell debris by the cohesive forces of solvent molecules (Chemat et al., 2017). The extraction of polyphenols has been carried out by ultrasound or microwave (Chemat et al., 2019), either one or the other (Chemat et al., 2017), or sequentially (Valdés et al., 2021). However, there are few studies where these processes have been used simultaneously for the extraction of polyphenols. The present study had the following objectives: 1. To extract polyphenols from the leaves of Creosote bush (Larrea tridentata), Tarbush (Flourensia cernua) and Soursop (Annona muricata) through the simultaneous use of ultrasound and microwaves, with different m/v ratios and water and ethanol as solvents, and 2. To determine how much these polyphenols inhibit the mycelial growth of Alternaria alternata and Fusarium solani.
Materials and methods
Plant material. Leaves of soursop (Annona muricata), Creosote bush (Larrea tridentata), and Tarbush (Flourensia cernua) were used for the extraction of polyphenols. Soursop leaves from “Frutas Nayarit” were purchased at a store in Saltillo, Coahuila, Mexico, while the leaves of Creosote bush and Tarbush were collected at the Experimental Station of the Antonio Narro Autonomous Agrarian University (UAAAN) in Buenavista, Coahuila, Mexico. Healthy leaves were then selected, discarding those that had damage or malformations, likewise, all contaminants found in the samples were also discarded. The selected leaves were then dehydrated for 3 days at room temperature and for 24 h in a desiccator (NWT-5, Northwest Technology®, Italy). Subsequently, the dry material was ground into powder in a Biobase mill (MD-120) and then sieved using an 850 μm mesh, number 20 (W.S. TAYLER®) (Valdez-Guerrero et al., 2021).
Preparation of extracts and extraction of phytochemicals. The extracts were prepared using distilled water and mixtures of ethanol/distilled water (70/30 and 30/70). Different ratios (m/v) between mass (plant material) and volume ((quantity and concentration of the solvent) were used). Specifically, m/v ratios of 1:8, 1:12 and 1:16 were used, following the method of Valdez-Guerrero et al. (2021) with minor modifications. The five (CA, CB, CC, CD and CE) solutions produced from each plant material under these conditions (Table 1) were placed in a glass reactor (1 L) and ultrasound and microwave processes were simultaneously applied using an Ultrasonic equipment (Microwave Cooperative Workstation XO-SM 400) with the following parameters: Ultrasonic (Power Radio - 20, Ultrasonic on Relay - 10, Ultrasonic off Relay -3, Amplitude off Relay - 25 and Set Time - 20) and Microwave (Power Radio - 800, Display power - 0, Set Temp - 70°C and Holding Time - 5). Subsequently, the extract was filtered to remove the bagasse.
Table 1 Mass/volume ratios (m/v) and ethanol gradients. The conditions used to obtain polyphenols by ultrasound-microwave.
| Factor | Extraction condition | ||||
|---|---|---|---|---|---|
| CAz | CB | CC | CD | CE | |
| Vegetal material | 62.5g | 62.5g | 125g | 125g | 83.33 |
| Solvent | EtOH 70% | distilled H2O | distilled H2O | EtOH 70% | EtOH 30% |
| m/v ratio | 1:16 | 1:16 | 1:8 | 1:8 | 1:12 |
z In the rest of the document these solutions will be referred to as the group of polyphenols
Purification and fractionation of polyphenols. The method of Valdez-Guerrero et al. (2021) was used for the chromatographic separation of the extracts. The extracts were first filtered using Whatman No. 41 paper, then separated by ion exchange column chromatography, using 200g of amberlite (Amberlite XAD16N) as stationary phase and water and 96% ethanol as mobile phase. The sample was then decanted in the column, with Amberlite® (XAD 16N) resin (previously activated with methanol for 10 minutes) as stationary phase, and water (to remove water-soluble compounds) and ethanol (to recover the polyphenolic content) as eluents. The column was protected from light due to the photosensitivity of the compounds under study. The aqueous fraction was discarded when its color became clear. The ethanolic fraction was collected in an amber container. Once the ethanolic fraction was obtained, it was distributed into heat-resistant glass plates, which were left to dry at room temperature (25-30 °C) in the dark for 3 days. The dry extract was collected from the plates in an amber-colored bottle for later analysis. The chemical compounds found in each dry extract were identified as polyphenols associated with one of the five (CA, CB, CC, CD and CE) solutions of each plant material (Table 1).
Determination of hydrolysable polyphenols. Powder extracts were diluted in water to a volume of 400 µL. After adding 400 µL of Folin-Ciocalteu reagent, the solution was left to stand for 5 min. Afterward, 400 µL of 0.01 M Na2CO3 were added and the solution was allowed to react for 5 min. Then, 2.5 mL of distilled water were added and the solution was read at 790 nm in a UV-Vis Spectrophotometer ( Biomate) (Gómez-Martínez et al., 2020).
Determination of condensed polyphenols. 500 µL of sample were added to a tube, followed by 3 mL of 10% HCl-butanol. The resultant solution was stirred well. Subsequently, 100 µL of the ferric reagent were added to the solution, stirring the mixture vigorously. The tubes were transferred to a water bath and boiled for one hour, making sure that the tubes were perfectly closed. Afterward, the samples were allowed to cool to room temperature (25-30 °C) and were then read at 460 nm in a UV-Vis Spectrophotometer (Biomate) (Gómez-Martínez et al., 2020).
Identification of polyphenols. First, 10 mg of each powder extract were weighed and dissolved in 1 mL of methanol, sonicated for 5 min at room temperature and filtered through 0.45 µm membranes. The samples were then placed in vials and put in an HPLC equipment (Varian Prostar 330 with UV-visible diode array detector) coupled to a mass detector (Varian 500-Ms). The chromatographic analysis was conducted using a flow rate of 0.2 mL/min, a Denali C18 reversed-phase column (150 mm × 2.1 mm, 3μm, Grace, Albany, OR USA), and a mass detection limit of 100 to 2000 m/z. All experiments were performed in negative-ion mode [M-H]. Nitrogen was used as nebulizer gas and helium as buffer gas. The ion source had the following parameters: spray voltage (5.0 kV), capillary voltage (90.0 V), and temperature (350 °C). The data was collected and processed using the M.S. Workstation software (V.6.9). The samples were first analyzed in full scan mode at a mass-to-charge ratio (m/z) in the range of 50-2000. Tandem mass spectrometry analysis (MS/MS) was performed on a series of selected precursor ions. The obtained compounds were compared using a bioactive compound database (WorkStation database version 2.0, VARIAN, Palo Alto, CA, USA).
Isolation of phytopathogenic fungi. The phytopathogenic fungi Fusarium solani and Alternaria alternata were isolated from diseased plants (yellowing, curling of the leaves, scabs, stem and fruit rot). Five-millimeter pieces of plant material were cut from the diseased plants. The pieces were disinfected with NaClO (2%) for 1 min, then immersed twice in sterile distilled water for 1 min and placed in Petri dishes with PDA medium (DifcoTM), which were incubated at 28±2 °C for 7 days. After the germination of monosporic cultures, fungi with characteristics of A. alternata and F. solani were purified by hyphal tip (Morales et al., 2007). The taxonomic identification of the species was based on observations of the growth of the fungal colonies in the culture medium to determine their pigmentation, shape and growth speed, as well as the formation of sporodochia, the number of septa in the conidia, the presence of chlamydospores, and the shape of conidia (micro and macroconidia). The specific identification was done with a compound microscope (10x, 40x and 100x), observing slides with morphological structures and taxonomic characteristics typical of the species, according to the taxonomic keys of Simmons (2007) for A. alternata, and of Leslie and Summerell (2006) for Fusarium solani.
Preliminary determination of the polyphenol concentration. A preliminary test was carried out to determine the inhibitory effect of the concentration (6000, 8000 and 10000 ppm) of two groups of polyphenols (CD = m/v (1:8) and EtOH-70%, and CE= m/v (1:12) and EtOH-30%) from Larrea tridentata on the mycelial growth of Fusarium solani, compared to the following three controls: 1) without polyphenols (negative); 2) EtOH 70% (positive); 3) fungicide [TectoR 60 at 1% ] (positive). The evaluation was carried out under a completely randomized design with three replications, using 100 x 20 mm Petri dishes with PDA agar (DifcoTM). Five hundred microliters of each concentration of each group of polyphenols (CD and CE) suspended in 70% ethanol were added separately to each dish, including the three control treatments. The polyphenols were spread over the entire surface of the agar with the help of a glass rod adapted to the size of the Petri dish. They were left to stand for 30 min, during which time the polyphenols were absorbed into the agar. A 0.5 cm diameter disk with F. solani mycelium was then placed inside each Petri dish (Berlanga-Padilla et al., 2011). The dishes were sealed and incubated at 25 °C until the mycelium of the negative control completely covered the dish. At that time, the colony diameter of each treatment (9 treatments) was measured. The arrangement of the Petri dishes in the incubator allowed for a completely randomized experimental design. Colony diameter values were converted into inhibition percentages (ICM), proportional to control 1, using the following mathematical expression (Apolonio-Rodríguez et al., 2017):
Where dTa and dt represent the diameter of the mycelial growth of control 1 and of each of the other treatments, respectively.
Based on the results of the preliminary assay, the concentration of 8000 ppm was selected to evaluate the groups of polyphenols (CA, CB, CC, CD and CE) obtained from Tarbush, Creosote bush and Soursop. Sowing and temperature conditions were similar to those used in the preliminary study. All groups of polyphenols were evaluated against F. solani and A. alternata. The controls for Fusarium were: 1) without polyphenols (negative); 2) EtOH 70% (positive); 3) fungicide [TectoR 60 at 1%] (positive). The controls for Alternaria were: 1) without polyphenols (negative); 2) EtOH 70% (positive). The response variable was mycelial growth, converted to inhibition percentage using the formula indicated above (ICM).
Statistical analysis. The data resulting from all the bioassays were analyzed using an analysis of variance. The significance was determined with a probability of p<0.05. When necessary, the means of the treatments were compared using the Tukey test. All statistical analyses were performed using SAS software (version 9.0).
Results and discussion
Preliminary determination of the polyphenol concentration. The analysis of variance showed no differences (P< 0.00001) in mycelial inhibition between the concentrations of each of the two groups of polyphenols (CD and CE) obtained from Creosote bush. However, the inhibition of mycelial growth did show differences between the two groups of polyphenols and between these groups and the negative control (Figure 1) (P<0.00001). The mycelial inhibition was greater in Fusarium solani when using the CD polyphenol group (obtained with a ratio of m/v 1:8 and 70% ethanol). With the CD polyphenol group, the range of mycelial inhibition varied between 88 and 90%. In contrast, the group of polyphenols obtained with an m/v ratio of 1:12 and 30% ethanol only inhibited between 43 and 50% of mycelial growth, while the positive control (Tecto 60® (thiabendazole) at 1% inhibited only 27% of F. solani mycelial growth.

Figure 1 Growth inhibition percentage against Fusarium solani by different concentrations (ppm) of polyphenols from Creosote bush (Larrea tridentata) obtained with different m/v ratios and ethanol concentrations and using ultrasound-microwaves. Groups of CD polyphenols obtained with the m/v ratio of 1:8 and EtOH-70%, and CE polyphenols obtained with m/v ratio of 1:12 and EtOH-30%. Columns with the same letter are not different according to Tukey’s multiple range test.
The results show that the polyphenols obtained from Creosote bush leaves by ultrasound-microwave have biological activity against the fungus F. solani. Four groups were formed based on the percentage of mycelial growth inhibition: a group formed by the negative control (without polyphenols), another formed by the positive control (Tecto 60® (thiabendazole) at 1%, and another two formed by the polyphenolic groups CD and CE. This coincides with what was reported by Peñuelas-Rubio et al. (2017), who mention that the inhibitory effect of fungal growth by extracts of L. tridentata can be attributed to the fact that the phenolic substances present in the extracts react with fungal enzymes, rendering them catalytically inactive. Farag et al. (2011) suggest that extracts with high amounts of polyphenols prevent the development of F. oxysporum due to the reduction of lipid peroxidation levels. In the present study, fungal growth inhibition was observed to be greater with the CD polyphenol group than with the CE group, which could be due to the presence of different polyphenolic compounds in each extract (Oufensou et al., 2020).
Inhibition of Fusarium and Alternaria by polyphenols from Creosote bush, Tarbush and Soursop. In the case of Fusarium, the analysis of variance showed differences in mycelial growth inhibition against F. solani between the two groups of polyphenols (CD and CE) obtained from Creosote bush (L. tridentata) and the controls (negative and positive; tecto-60® and ethanol- 70%) (Figure 2a). It was observed that at 8000 ppm the polyphenols obtained with the 1:8 m/v ratio and 70% ethanol (CD), inhibited mycelial growth by 90%, while the polyphenols obtained with the 1:12 m/v ratio and ethanol 30%, and the positive control (Tecto 60® (thiabendazole) at 1%) only inhibited mycelial growth by 50% and 27%, respectively. The results of this study coincide with those reported by Peñuelas-Rubio et al. (2017) who mention that extracts at 500 ppm and obtained by the Soxhlet system and 70% ethanol, inhibited F. oxysporum by 98%, however, in the present work 8000 ppm of polyphenols were needed to inhibit mycelial growth by 90%, when the polyphenols were extracted with EtOH-70% and 50% when EtOH-30% was used. In this study, polyphenols were extracted in 20 minutes using simultaneously ultrasound-microwave, while extraction using Soxhlet takes at least 36 hours (Sepúlveda-Rincón et al., 2016).

Figure 2 Mycelial growth inhibition against Fusarium solani by different groups of polyphenols from a) Creosote bush (Larrea tridentata), b) Tarbush (Flourensia cernua) and c) Soursop (Annona muricata) obtained by ultrasound-microwave, at a concentration of 8000 ppm, compared to controls (negative and positive; tecto- 60® and ethanol-70%). Polyphenol group CA obtained with m/v ratio of 1:16 and EtOH-70%; polyphenol group CB obtained with m/v ratio of 1:16 and EtOH-0%; polyphenol group CC obtained with m/v ratio of 1:8 and EtOH- 0%; polyphenol group CD obtained with m/v ratio of 1:8 and EtOH-70%; polyphenol group CE obtained with m/v ratio of 1:12 and EtOH-30%. Columns with the same letter within the same graph are not different according to Tukey’s multiple range test.
In the case of Tarbush (F. cernua), the analysis of variance showed significant differences in mycelial growth inhibition against F. solani between the different groups of polyphenols and the negative control (Figure 2b). The polyphenol groups (CA, CC, CD and CE) inhibited a greater proportion of mycelial growth than the positive control (tecto-60®). The groups of polyphenols extracted using ethanol as solvent (CA, CD and CE) had a greater inhibitory effect on mycelial growth, followed by the groups of polyphenols obtained using water (CB and CC). The inhibition efficiency of the polyphenolic groups extracted with ethanol was proportional to the m/v ratio, that is, polyphenols with a higher m/v ratio were associated with greater fungal growth inhibition. The inhibition percentage of the groups of polyphenols extracted with water was similar to that of the positive control (Tecto-60®). The group of polyphenols that had a greater inhibitory effect (60%) was the one obtained with 70% ethanol and an m/v ratio of 1:16 (CA), followed by the groups of polyphenols CE and CD, with 46.5 and 42.8%, respectively. The inhibition percentage of the polyphenols obtained with water (CC and CB) was very similar to the inhibition percentage associated with the positive control (Tecto-60®), 32 and 26%, respectively.
There are few studies on the effect of extracts of F. cernua against F. solani. Most studies have used F. oxysporum. The mycelial inhibition percentage of the polyphenolic groups of F. cernua studied here was greater than that reported by Gamboa-Alvarado et al. (2003), who did not find any inhibitory effect against F. oxysporum with any of the extract concentrations that they studied (4000, 8000, 12000, 16000 and 20000 ppm). This can be explained by the method (Soxhlet extraction) and solvent (methanol) used by Gamboa-Alvarado et al. (2003). They used a 10-day-long extraction method that turned out to be less efficient and effective than ultrasound-microwave technology, which achieves extraction in 20 minutes and with a more controlled and efficient mass transfer. There are reports of complete inhibition of Fusarium growth using extracts from tarbush, however, these results were against F. oxysporum (Peñuelas et al., 2017).
Concerning the polyphenolic groups from soursop (A. muricata), the analysis of variance showed that, at 8000 ppm, there were significant differences among treatments (Figure 2c). The groups of polyphenols CA and CB showed a greater inhibitory effect against fungal growth, even greater than the positive controls (Tecto-60® and 70% ethanol). In addition, the polyphenols obtained with an m/v ratio of 1:16 had a greater inhibitory effect against F. solani, regardless of whether they were obtained with ethanol or water. The CA group of polyphenolic compounds showed the greatest inhibitory effect (43.7%) against the mycelial growth of F. solani. This group of polyphenols was obtained with an m/v ratio of 1:16 and EtOH-70%. The CA group was followed by the groups of polyphenolic compounds CB and CD, with 36 and 27% of inhibition, respectively. The CE and CC polyphenol groups caused a lower percentage of inhibition than the positive control (Tecto-60®), 23 and 21%, respectively. Although soursop polyphenols showed a higher inhibitory effect against F. solani than the positive control (Tecto-60®), the growth of this fungus was not inhibited by more than 50% at a concentration of 8000 ppm. Thus, the fungicidal potential of these polyphenols should be tested at higher concentrations (Figure 2c). It was not possible to find reports about the antifungal properties of polyphenols obtained from soursop against Fusarium solani, so the results obtained in the present study cannot be compared.
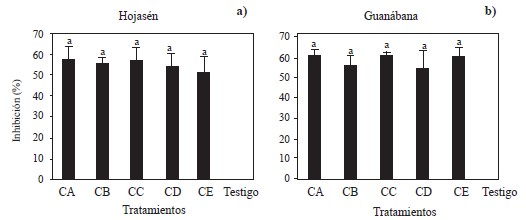
Figure 3 Mycelial inhibition against Alternaria alternata by 5 groups of polyphenolic compounds obtained by ultrasound-microwave from a) Tarbush (Flourensia cernua) and b) Soursop at 8000 ppm. Groups of polyphenols CA obtained with m/v ratio of 1:16 and EtOH-70%; polyphenols CB obtained with m/v ratio of 1:16 and EtOH-0%; polyphenols CC obtained with m/v ratio of 1:8 and EtOH-0%, polyphenols CD obtained with m/v ratio of 1:8 and EtOH-70%; polyphenols CE obtained with m/v ratio of 1:12 and EtOH-30%. Columns with the same letter within the same graph are not different according to Tukey’s multiple range test.
Against Alternaria alternata, the five groups of polyphenols obtained from Tarbush (F. cernua) and Soursop (A. muricata) were evaluated. The analysis of variance showed significant differences between these groups of polyphenols and the negative control (Figure 3a and 3b). The groups of polyphenols obtained from Tarbush and soursop inhibited between 51 and 58% of the mycelial growth of A. alternata, in contrast to the negative control. The results of this assay were lower than those reported by Guerrero et al. (2007), who mentioned that extracts from tarbush leaves inhibited 81.6-82.0% of the mycelial growth of A. alternata. However, these authors used solvents such as methanol: chloroform (1:1) and hexane, respectively, the latter being polar and alkaline in nature, characteristics that make it a very efficient solvent but also highly flammable, harmful to the environment and damaging to the health of those who breathe or are in direct contact with it (Pan et al., 2017). The same occurs with chloroform, whose C-Cl bond is useful for the polarization and synthesis of organic molecules, and with methanol, which has the ability to remove water from solutions, during synthesis or separation processes (Sridhar et al., 2011; Ashurst and Nappe, 2022). These three compounds are outstanding alternatives for extraction, but their harmful effects on human health and the environment are much greater than those of ethanol and water, which are used in the present study. The latter are cheap, efficient in the extraction of compounds and do not pose a threat to human or the environment. Moreover, ethanol can be produced by fermentation, and is highly volatile (Baümler et al., 2016). Guerrero et al. (2007) reported an inhibition percentage of 80.3% at 2000 ppm using ethanol as extraction solvent. The differences between these results and those obtained in the present study can be explained by the method used to obtain the extracts, the number of molecules with fungicidal activity, the affinity of the polyphenols with the solvent used, the polarity and type of solvent used, the temperature of the extraction process, and the forms of recovery (Gómez-Martínez et al., 2020).
All the polyphenolic groups of soursop (A. muricata) obtained by ultrasound-microwave showed an inhibitory effect between 55 and 61% against the mycelial growth of A. alternata, compared to the negative control (Figure 3b). To date, no reports have been found about the antifungal properties of polyphenols obtained from soursop against A. alternata. The in vitro fungal inhibition values observed in the present study are promising (Vitola and Pérez, 2016).
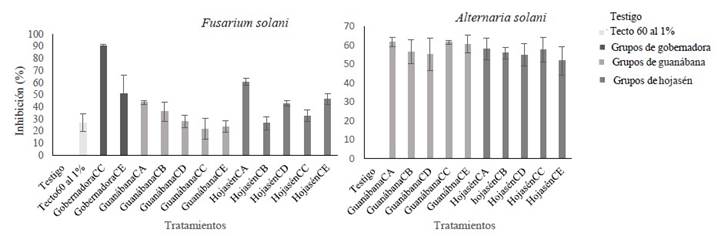
Figure 4 Antifungal activity of various groups of polyphenolic compounds (at 8000 ppm) obtained with different m/v ratios and proportions of ethanol against Fusarium solani and Alternaria alternata. Group of polyphenols CA obtained with m/v ratio of 1:16 and EtOH-70%; polyphenols CB obtained with m/v ratio of 1:16 and EtOH-0%; CC polyphenols obtained with m/v ratio of 1:8 and EtOH-0%; polyphenols CD obtained with m/v ratio of 1:8 and EtOH-70%, polyphenols CE obtained with m/v ratio of 1:12 and EtOH-30%.
In general terms, it can be said that the simultaneous use of ultrasound and microwave was an efficient extraction method to obtain compounds with antifungal properties, at least against the pathogens Alternaria alternata and Fusarium solani. The inhibition percentage of these compounds against the mycelial growth of both pathogenic fungi exceeded 50% at 8000 ppm, compared to the negative control. Sixteen percent of the groups of polyphenols evaluated had an inhibitory effect greater than or equal to 50% against F. solani, while 90.9% of the groups of polyphenols had an inhibitory effect greater than or equal to 50% against A. alternata (Figure 4). This means that, at 8000 ppm, only 3 groups of polyphenols (CD, CE and CA) have polyphenolic compounds with mycelial inhibition capacity (between 50-90%) against F. solani, these being those obtained from L. tridentata and F. cernua with m/v ratio of 1:8 and EtOH-70% as solvent (CD) and 1:12/EtOH-30% (CE) for the first plant species, and 1:16/EtOH-70% (CA) for the latter one. In the three groups of polyphenols (CA, CD, and CE), ethanol was used as solvent at 70, 70 and 30% respectively. The differences in fungal inhibition might be associated with the amount of plant material used. In the case of the Creosote bush, the most efficient polyphenolic groups were those where ethanol was used at a higher concentration (70%) together with the largest amount of plant biomass (125 g L-1). In the case of Tarbush, the inhibitory efficiency was inversely proportional to the plant biomass used; the lower the biomass (62.5 g L-1), the higher the inhibitory effect (Figure 2). The mycelial growth of A. alternata was inhibited by all the groups of polyphenols evaluated, regardless of the m/v ratio and the type of solvent (Figure 4).
The different m/v ratios and solvent concentrations used in the extraction allowed obtaining different amounts of total polyphenols (condensed and water-soluble), expressed in mg per gram of plant material. Figure 5 shows that a m/v ratio of 1:16 and EtOH-70% (CA) made it possible to recover the highest proportion of both types of polyphenols in both plant species. However, the groups of Creosote bush polyphenols that had the greatest inhibitory effect against the mycelial growth of F. solani were those obtained with 1:8/EtOH-70% (CD) and 1:12/EtOH -30% (CE). When Tarbush was used, the greatest inhibitory effect coincided with one of the groups that had the highest amount of polyphenols (CA). All the polyphenol groups had a similar inhibitory effect against the mycelial growth of A. alternata. Therefore, the m/v ratio and the solvent proportion did not affect the mycelial inhibition percentage.
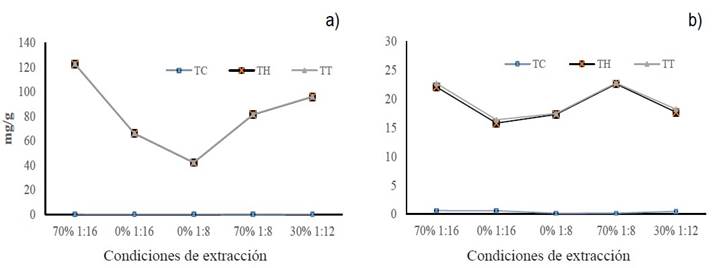
Figure 5 Concentration of total polyphenols (condensed and water-soluble) obtained with different m/v ratios and ethanol proportions; a) Creosote bush polyphenols, b) Tarbush polyphenols.
The inhibitory activity against mycelial growth may be caused by the polyphenolic compounds present in each group rather than by the amount of hydrolysable or condensed polyphenols. The differences in the profile of polyphenolic compounds of each group (Tables 2 and 3) are given by the m/v ratio, the affinity of polyphenols for the solvent used, the polarity and type of solvent used, the temperature during the production process, and the forms of recovery (Gómez-Martínez et al., 2020). A greater number of polyphenolic compounds was observed in the group of CD polyphenols than in the CE group obtained from Creosote bush. Some compounds found in the CD group, such as epirosmanol, have been reported to have an antimicrobial or antioxidant effect. Epirosmanol results from the oxidation of carnosic acid and has a high antioxidant capacity. The CD group also has molecules with not only highly antioxidant activity but also antimicrobial activity such as rosmadiol (Quintana et al., 2019; Ávila et al., 2011). The CD polyphenol group includes apigenin, a compound that has been described as one of the most abundant flavonoids. There is a positive relationship between the consumption of this flavonoid and a decrease in mortality due to stomach cancer. It also has antiviral and antibacterial activity (Álvarez and Orallo, 2003). Catechins have a high antioxidant potential, with the ability to cross-link many proteins, which gives them antimicrobial activity, possibly by damaging microbial cytoplasmic lipids and proteins (Rahardiyan, 2019). The antifungal activity of catechin against phytopathogenic fungi has been mentioned in other studies (Istúriz et al., 2019). It has also been reported that procyanidin C1 can inhibit microbial growth through the inhibition of extracellular enzymes, deprivation of essential microbial substrates, disintegration of the bacterial outer membrane and negatively affecting microbial metabolism (Dasiman et al., 2022). The CA and CB polyphenol groups obtained from Tarbush were compared. In addition to containing apigenin, the CA group also has rosmarinic acid, which can destroy bacterial cells and proteins and inhibit the activity of Na+/K+ -ATP-ase in cells (Zhang et al., 2021). The CA group of polyphenols also contains nepetin, which can decrease the virulence of various pathogens by binding to caseinolytic protein peptidase P (ClpP) and to Ser-22 and Gln-47 amino acids of the CLpP protein. (Shisong et al., 2022). Another CA compound is quercetin, a flavonoid approved by the FDA with antimicrobial capabilities. It damages cell membranes, modifies membrane permeability, inhibits both nucleic acid and protein synthesis, reduces the expression of virulence factors, induces mitochondrial dysfunction, and prevents biofilm formation (Nguyen et al., 2022). The activity of quercetin against phytopathogenic fungi such as Penicillium expansum has been reported elsewhere (Sanzani et al., 2009). The antifungal activity of polyphenolic compounds has been of interest due to their low toxicity and ephemeral persistence in the environment, proving useful applications for organic agriculture (López et al., 2019).
Table 2 Compounds identified in Creosote bush powders according to the mass volume ratio.
| Compuestos | ||||
|---|---|---|---|---|
| T.R (min) | Masa (m/z) | Grupo CD (70% 1:8) | Grupo CE (30% 1:12) | Familia |
| 39.23 | 346.9 | 5- heptadecil resorcinol | Alquilfenoles | |
| 34.09 | 504.8 | Peonidin 3-O-(6-acetil-glucòsido) | Antocianinas | |
| 27.6 | 288.8 | Catequina | Catequina | |
| 33.42 | 304.9 | Epigalocatequina | Catequina | |
| 22.61 | 304.8 | Galocatequina | Catequina | |
| 25.7 | 304.9 | (+) Galocatequina | Catequinas | |
| 35.08 | 302.8 | Taxifolina o dihidroquercetia | Dihidroflavonol | |
| 44.21 | 268.8 | Apigenina | Flavones | |
| 30.45 | 592.9 | Apigenina 6,8- di glucósido | Flavones | |
| 27.97 | 592.8 | Luteolin 7- rutinoside | Flavones | |
| 22.64 | 284.9 | Luteolina | Flavones | |
| 24.2 | 284.9 | Scutellarina | Flavones | |
| 34.37 | 608.9 | Quercetina 3-O glucósido 7-O rhamnoside | Flavonoles | |
| 32.05 | 608.8 | Quercetina 3-O-glucòsido 7-O-rhamnoside | Flavonoles | |
| 35.82 | 770.7 | Quercetina 3-O-glucosyl-ramnosil-galactòsido | Flavonoles | |
| 32.28 | 754.9 | Quercetina 3-O-ramnosil glucósido | Flavonoles | |
| 30.17 | 754.8 | Quercetina 3-O-ramnosil-glucòsido | Flavonoles | |
| 49.52 | 300.9 | NDGA | Lignanos | |
| 51.69 | 301 | NDGA | Lignanos | |
| 28.05 | 542.8 | Ácido 3,4- feruloiquínico | Ácido metoxicinámico | |
| 48.16 | 298.9 | Hispidulina | Methoxiflavones | |
| 48.46 | 298.9 | Hispidulina | Methoxiflavones | |
| 40.95 | 314.8 | Nepetin | Methoxiflavones | |
| 43.03 | 314.8 | Nepetin | Methoxiflavones | |
| 29.46 | 370.8 | Sinensetina | Methoxiflavones | |
| 27.59 | 328.9 | 3,7- Dimetil quercetina | Methoxiflavones | |
| 44.96 | 328.8 | 3,7- Dimetil quercetina | Methoxiflavones | |
| 52.48 | 283 | Metil galadin | Methoxiflavones | |
| 50.31 | 282.9 | Metil galadin | Methoxiflavones | |
| 38.28 | 330.8 | Ácido carnósico | Diterpeno fenólico | |
| 40.39 | 330.8 | Ácido carnósico | Diterpeno fenólico | |
| 42.39 | 344.7 | Epirosmanol | Diterpeno fenólico | |
| 41.58 | 344.8 | Rosmadial | Diterpeno fenólico | |
| 28.74 | 862.7 | Procianidina C1 | Trímeros de protoantocianidinas | |
In bold, similar compounds found in each group obtained with different extraction conditions.
Table 3 Compounds identified according to the mass-volume ratio for powdered senna.
| Compuestos | ||||
|---|---|---|---|---|
| T.R (min) | Masa (m/z) | Grupo CA (70% 1:16) | Grupo CB (0% 1:16) | FAMILIA |
| 242.8 | Dihidroquercetina | Dihidroflavonoles | ||
| 44.19 | 268.7 | Apigenina | Flavones | |
| 28.19 | 592.8 | Apigenina 6,8 glucósido | Flavones | |
| 28.06 | 592.8 | Apigenina 6,8 glucósido | Flavones | |
| 29.9 | 562.8 | Apigenina glucósido- arabinósido | Flavones | |
| 29.92 | 562.8 | Apigenina glucósido-arabinòsido | Flavones | |
| 40.66 | 676.7 | Metoxiflavona | Flavones | |
| 44.97 | 298.7 | Quercetina | Flavonol | |
| 34.74 | 514.7 | Ácido 1,3- dicafeoilquínico | Ácido hidroxicinámico | |
| 35.79 | 514.7 | Àcido 1,3- dicafeoilquìnico | Ácido hidroxicinámico | |
| 18.51 | 352.8 | Ácido 1- cafeoilquínico | Ácido hidroxicinámico | |
| 24.16 | 352.8 | Ácido 1- cafeoilquìnico | Ácido hidroxicinámico | |
| 24.66 | 352.7 | Ácido 3- cafeoilquínico | Ácido hidroxicinámico | |
| 25 | 352.8 | Ácido 3-cafeoilquìnico | Ácido hidroxicinámico | |
| 47.11 | 358.7 | Ácido rosmarínico | Ácido hidroxicinámico | |
| 26.88 | 310.8 | Cafeoil ácido tartárico | Ácido hidroxicinámico | |
| 26.56 | 310.8 | Cafeoil ácido tartárico | Ácido hidroxicinámico | |
| 35.74 | 514.7 | Ácido 1,5- dicafeoilquìnico | Ácido hidroxicinámico | |
| 39.59 | 312.7 | Cirsimaritina | Methoxiflavona | |
| 45.72 | 314.7 | Nepetin | Methoxiflavona | |
| 32.01 | 370.9 | Tangeretina | Methoxiflavona | |
| 31.8 | 370.9 | Tangeretina | Methoxiflavona | |
| 42.59 | 344.7 | Rosmanol | terpenos fenólicos | |
In bold, similar compounds found in each group obtained with different extraction conditions.
Conclusions
In the present study, it was possible to extract rapidly (20 minutes) polyphenolic compounds from Creosote bush, Tarbush and soursop leaves by simultaneously using environmentally friendly methods (ultrasound-microwave) and solvents (water and ethanol). Different polyphenolic compounds were extracted, depending on the plant material, the m/v ratio, the solvent and its concentration. The group of polyphenols CD extracted from Creosote bush showed levels of mycelial growth inhibition of F. solani of 90%. The mycelial growth of Alternaria alternata was inhibited by 50-60% by the groups of polyphenols obtained from tarbush and soursop leaves. In the group profile of CD polyphenols obtained from Creosote bush, various polyphenolic compounds reported to have antioxidant and antimicrobial activity were found, among which the following stand out: epirosmanol, rosmadiol, Apigenin, catechin, and procyanidin C1.











 texto en
texto en 


