The mango fruit is consumed all over the world for its exceptional flavor, but it also has antioxidant, immunomodulatory, antiallergic, anti-inflammatory, antitumor, antidiabetic, and antiparasitic properties, among others (Swaroop et al., 2018; Kim et al., 2021). This tropical fruit is of economic importance (Altendorf, 2019) and Mexico is the main exporter worldwide. Its main markets are the United States, Canada and Japan (SADER, 2020). Fungal diseases are the main phytosanitary problem for mango crops, affecting the yield and quality of the fruit (Elqassas and Abu-Naser, 2018). One of the most important diseases of mango trees is dieback, which is caused by various species of fungi (Rodríguez-Gálvez et al., 2017). The incidence of this disease has been increasing; in some countries, such as Oman, the incidence is as high as 89% (Al-Adawi et al., 2006), in Pakistan 83.3% (Khaskheli et al., 2011), in Peru 29% (RodríguezGalvez et al., 2017). In Mexico, there have been no studies on the incidence of mango dieback, a lack that should be addressed. Sandoval-Sanchez et al. (2013) reported cases of dieback in mango trees in Mexico and their association with some phytopathogenic microorganisms. The present work assumed this premise and aimed to increase our knowledge of this disease in Manila mango trees in Mexico.
The characteristic symptoms of dieback in trees are gummosis, rotting of branches and trunk, decay, and chlorosis of the leaves (Marques et al., 2013; Rodríguez-Gálvez et al., 2017). The main fungal species associated with dieback belong to the family Botryosphaeriaceae and, recently, to the family Sporocadaceae (de-Oliveira et al., 2010; Li et al., 2021; Santos et al., 2021). The dominant genus of the family Botryosphaeriaceae is Lasiodiplodia, the most important species of which include L. theobromae, L. pseudotheobromae, L. brasiliensis, L. iranensis, L. mahajangana, and L. hormozganensis (Rodríguez-Gálvez et al., 2017). In the family Sporocadaceae, the most significant genera are Pestalotiopsis and Neopestalotiopsis (Rodríguez-Gálvez et al., 2020; Santos et al., 2021). Chemical control of this disease has turned out to be a complex issue because the fungi are found in the xylem or parenchyma of branches and trunk (Al-Saadoon et al., 2012; Fan et al., 2019).
Coinfections are caused by multiple plant pathogens (Abdullah et al., 2017) and dieback can be caused by various fungi (Chen et al., 2012; Kwon et al., 2017; Marques et al., 2013). The effect of coinfections in plants has been little studied due to the complexity of these pathosystems. The assessments that have been conducted so far have focused on virus-virus, bacterium-fungus and fungus-fungus coinfections (Tollenaere et al., 2016). Some studies have shown that fungal coinfections increase the prevalence and severity of diseases and spore production (Fang et al., 2021; Susi et al., 2015). However, plant pathogens may inhibit each other (Orton and Brown 2016; Lerch-Olson and Robertson, 2020). In mango trees, dieback has been caused by species of Lasiodiplodia and Pestalotiopsis; however, the effect of coinfection with both phytopathogens on the severity of this disease in branches is still unknown.
Various species of Lasiodiplodia and Pestalotiopsis are of interest for different industries due to the antimicrobial, anti-inflammatory, mycotoxic and cytotoxic properties, among others, of the compounds that can be extracted from them (Qian et al., 2021; Salvatore et al., 2020). However, the effect of these compounds on other phytopathogenic fungi is unknown. Therefore, the present study aimed to isolate and identify the phytopathogenic fungi associated with dieback of Manila mango trees to determine their in vitro interaction and their effect on the coinfection of Mangifera indica branches.
Materials and methods
Study site
A commercial orchard of mango var. Manila with a plantation age of 40 years, located at 19° 30.161″ N and 096° 35.460″ W in Actopan, Veracruz, Mexico. Using directed sampling, ten trees with dieback symptoms (gummosis, rotting of branches and trunk) were selected. Three segments of 15 cm were cut and collected from 5 cm thick secondary branches from each tree for a total of thirty samples. The samples were stored in sterile plastic bags for later analysis.
Isolation of fungi and morphological characterization
The thirty collected samples were cut into 2 cm long chips, which were disinfected in a solution of sodium hypochlorite and water at 1% v/v for 2 min. They were then rinsed three times with sterile distilled water and dried on sterile paper. Two chips of each sample were seeded in plates with potato-dextrose agar (PDA) plus ampicillin (40 mg mL-1), at 3 cm from the plate center and incubated at 28 °C for 9 days. The monosporic fungal cultures obtained were reseeded on PDA until 40 pure cultures were obtained. The fungi were morphologically classified by the shape and color of the colony into 15 groups. To observe the microscopic structures and identify the genus of each fungus, the plates were cultured by alternating white and black light every 12 h for 15 days. The shape and color of the mycelium were observed in the plates at days 1, 3, 5, 7 and 9. The structures of the fungi were stained with cotton blue (Jackson and Johnson-Cicalese, 1988) and examined with a phase contrast microscope Axio Lab.A1 HAL 35, FL-LED, 5x H. The identification was done using the taxonomic keys of Clendenin (1896) and Maharachchikumbura et al. (2014) according to color of pycnidia (Lasiodiplodia), color and shape of conidiomata (Pestalotiopsis), shape and color of hyphae, and shape, color, and size of conidia.
Molecular identification
The molecular identification of the fungi that showed greater aggressiveness was conducted by extracting genomic DNA according to the protocol of Lee (1990). The genomic DNA was used to amplify the following: 1) The internal transcribed spacer (ITS) region of the rDNA (ITS1-5.8S-ITS2 group) with the primers ITS1F (5’ TCCGTAGGTCAACCTGCGG 3’) and ITS4 (5’ TCCTCCGCTTATTGATATGC 3’), according to the method and PCR conditions described by Manter and Vivanco (2007); 2) A partial β-tubulin gene (benA) with primers Bt-2a (5’ GGTAACCAAATCGGTGCTGCTTTC3’) and Bt-2b (5’ ACCCTCAGTGTAGTGACCCTTGGC 3’) according to the method and PCR conditions described by Úrbez-Torres et al. (2008). The PCR products obtained were sent to the Instituto Potosino A.C. (IPICYT) for sequencing. The sequences obtained were analyzed with the Cromas Pro1.7.6 software. Identity was verified with the NCBI (National Center for Biotechnology Information) BLASTn (Basic Local Alignment Search Tool) system. The amplified ITS and β-tubulin rDNA sequences were deposited in the Gen Bank (http://www.ncbi.nlm.nih.gov).
Pathogenicity test
The pathogenicity test was performed on branches of mango var. Manila obtained from a newly planted commercial orchard free of dieback disease located in the town of Los Idolos in Actopan, Veracruz, Mexico. The health of the branches was verified visually and through cultures in PDA medium (See the section on isolation and characterization of the isolates). In addition, control branches inoculated with PDA medium were used to rule out disease development. The branches were cut into 15 cm long segments and disinfected with 70% alcohol for 30 s; they were then rinsed three times with sterile water. The isolates were grouped into fifteen groups according to the morphological characteristics of the mycelium. Of the fifteen groups, only 5 of the genus Lasiodiplodia and 2 of the genus Pestalotiopsis showed pathogenicity. A wound was made in the middle of each mango branch using a dissection needle. An 8-mm-diameter mycelial plug of each fungus, previously cultivated on PDA plates for 7 days at 28 °C, was placed in each wound. A plug of PDA medium without phytopathogen was inoculated into each wound as control. The branches were incubated in glass plates for 7 days at 28 °C with a relative humidity of 70-80%. The presence of necrosis and gum was observed in the branches. The lesion area (mm2) was measured at days 1, 3, 5 and 7. The percentage of incidence (%I) was determined through the formula %I = x/N × 100, where x represents the number of diseased branches and N is the total number of branches evaluated. The experiment was conducted with a completely randomized experimental design with five replications. The fungi were re-isolated on PDA plates to confirm Koch’s postulates.
Evaluation of in vitro growth by dual culture technique
The in vitro tests were performed on the strain S10 of L. pseudotheobromae and strain MN2 of P. mangiferae, which were the most virulent in the pathogenicity test. The in vitro evaluation was performed according to the modified protocol of Lawrence et al., (2018). The fungi were seeded on PDA plates for seven days at 28 °C. Previous observations had found differences in the growth rate of both phytopathogens; therefore, two experiments were proposed. The first consisted of placing two 8-mm plugs of P. mangiferae MN2 on the lateral part of each plate with PDA and incubating them for two days at 28 °C. Subsequently, two 8-mm plugs of L. pseudotheobromae S10 were inoculated 2 cm from the P. mangiferae MN2 colony and incubated for nine days at 28 °C. The second experiment consisted of performing a simultaneous inoculation of two 8-mm plugs (one of each phytopathogen) in the same plate with PDA. As a control, the fungi were also seeded individually in other PDA plates and a group of plates were inoculated with a plug of PDA medium without phytopathogen. The plates were incubated for 9 days at 28 °C. The coloration of the mycelium was observed in both fungi on days 1, 3, 5, 7 and 9. The presence of conidiomata was assessed in P. mangiferae MN2 on the same days. The stereoscopic microscope was used to check for any crossing of hyphae or the presence of an inhibition halo between them. Four replications of each treatment were performed with a completely randomized experimental design.
Assessment of fungal filtrates from L. pseudotheobromae and P. mangiferae
The effect of fungal filtrates on the growth of P. mangiferae MN2 and L. pseudotheobromae S10 was evaluated following the procedure described by Naglot et al. (2015) and Hajieghrari et al. (2008). Fungi were cultivated separately in potato dextrose broth in a shaker at 150 rpm for 14 days at 28 °C. The culture medium was centrifuged at 6,000 rpm for 30 min and subsequently filtered through a 0.20 μm sterile membrane (Whatman). Plates were prepared with PDA medium plus the filtrates of each phytopathogen at a concentration of 50% v/v. The fungi had been previously sown on PDA plates for seven days at 28 °C. The plates containing the filtrate of L. pseudotheobromae S10 were inoculated in the center with an 8 mm plug with P. mangiferae MN2. The plates with the filtrate of P. mangiferae MN2 were inoculated in the center with an 8 mm plug with L. pseudotheobromae S10. As a control, the fungi were inoculated individually in PDA medium. As an absolute control, a PDA plug was inoculated in the PDA plates containing the filtrate of each fungus. The plates were incubated for 9 days at 28 °C. Four replicates per treatment were performed using a completely randomized experimental design. The coloration of the mycelium and, in the case of P. mangiferae MN2, the presence of conidiomata were recorded. The inhibition percentage (I) was measured according to the formula of Singh (2006): I = (C-t)/C × 100, where C represents the diameter of the control colony and t the diameter of the colony with filtrate.
Fungal coinfection on mango branches
The effect of coinfection on mango branches was assessed using a modified protocol of Lawrence et al. (2018). P. mangiferae MN2 and L. pseudotheobromae S10 were previously seeded on PDA plates for 7 days at 28 °C. The branches were cut into 15 cm long segments, the surface was disinfected with 70% alcohol for 30 s and rinsed three times with sterile water. Two wounds, separated by 2 cm, were made in the central part of each branch with a sterile needle. An 8-mm-diameter mycelial plug of P. mangiferae MN2 was placed in the first wound, and an 8-mm-diameter mycelial plug of L. pseudotheobromae S10 was placed in the second one. For control purposes, branches were inoculated only with each fungus and another one was inoculated with plugs containing only PDA medium, without phytopathogen. The branches were incubated for seven days at 28 °C and a relative humidity of 70-80%. Five replicates were performed for each treatment in a completely randomized experimental design. The presence of necrosis and gum in the branches was recorded and the lesion area (mm2) was measured using the ImageJ program. The fungi were re-isolated on PDA plates to confirm Koch’s postulates.
Statistical analysis
The statistical analysis of the obtained data was based on analysis of variance (ANOVA) using the SPSS statistical software. The analyzed data corresponded to lesion area and inhibition percentage. Significant differences (p ≤ 0.05) between means were determined using Tukey’s post hoc test.
Results
Isolation and morphological identification of fungi
Forty isolates were obtained, and 15 groups of fungi were formed from the sampled trees with dieback symptoms (Figure 1A-C). A representative of each group was characterized, resulting in 13 fungi of the genus Lasiodiplodia and two of the genus Pestalotiopsis. Seven isolates were selected for morphological identification according to their level of aggressiveness: five of them belonged to the species L. pseudotheobromae and two to the species P. mangiferae. The following morphological characteristics were observed for each fungus:
In the first three days, the five isolates of L. pseudotheobromae showed a white cottony mycelium (Figure 2D). In the fourth day, the mycelium showed a grayish color, and from day seven the color changed to grayish-black (Figure 2E). When the plates were exposed to black and white light, dark brown pycnidia were formed. The hyphae were filamentous, septate, with constricted septum, and dark brown in color. The conidia were ellipsoidal, with a rounded apex and base, two dark brown septa and an average size (N=100) of 23.4 μm in length × 14.25 μm in width (Figure 2F). The five isolates were identified as L. pseudotheobromae according to Clendenin’s taxonomic keys (1896).
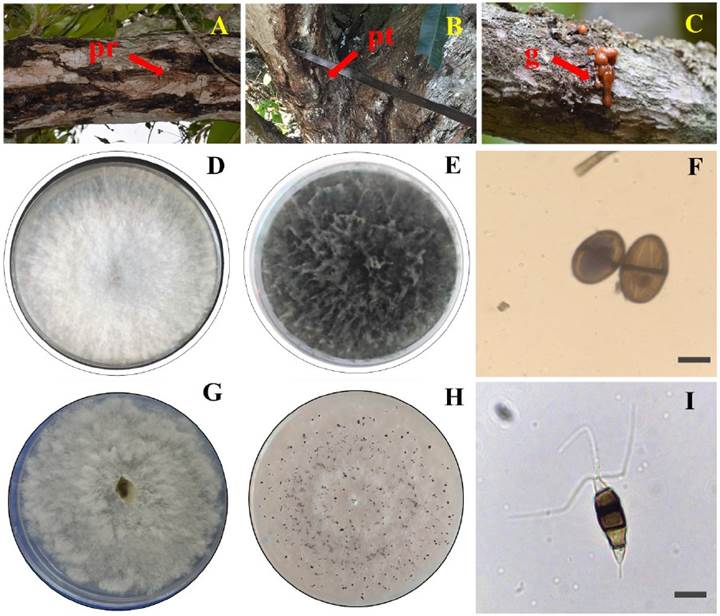
Figure 1 Symptoms of dieback in mango trees var. Manila and morphological characterization of Lasiodiplodia pseudotheobromae and Pestalotiopsis mangiferae. A) Rotting on branches (pr), B) Rotting on trunk (pt), C) Gummosis (g), D) Mycelial growth of L. pseudotheobromae cottony white, E) Micellar growth of L. pseudotheobromae greyish-black, F) Conidia of L. pseudotheobromae dark brown (100x), G) Cottony white mycelial growth of P. mangiferae, H) Presence of P. mangiferae conidiomata e I) Conidia of P. mangiferae dark brown-black (100x).
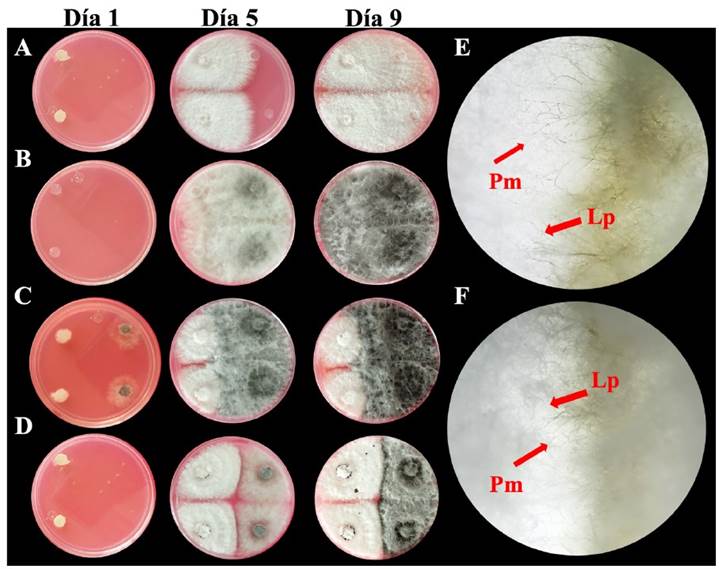
Figure 2 Mycelial growth of Lasiodiplodia pseudotheobromae and Pestalotiopsis mangiferae and hyphal interaction during the dual inoculation technique. A) Pestalotiopsis mangiferae MN2, B) Lasiodiplodia pseudotheobromae S10, C) P. mangiferae MN2 and L. pseudotheobromae S10, D) Crossing of hyphae of P. mangiferae MN2 and L. pseudotheobromae S10 inoculated two days later, E) Crossing of hyphae of P. mangiferae MN2 and L. pseudotheobromae S10 and F) Interaction between P. mangiferae MN2 and L. pseudotheobromae S10 inoculated two days later.
When subjected to alternating white and black light, the mycelial growth of P. mangiferae from days one to seven was irregular, cottony and diffuse, with a white coloration (Figure 2G). A cream coloration developed in the mycelium before the appearance of conidiomata. On day nine, black pycnidial and globose conidiomata (Figure 2H) and hyaline and septate hyphae were observed. The indistinct brown conidia were slightly curved ellipsoid in shape with 4 septa and between 2 and 3 flagella. The average conidia size was 26.5 μm long × 7.2 μm wide (Figure 2I). The two isolates were identified as P. mangiferae according to the taxonomic keys of Maharachchikumbura et al. (2014).
The sequences of the ITS and β-tubulin regions of three Lasiodiplodia isolates, as well as an ITS sequence from Pestalotiopsis, were deposited in GenBank. L. pseudotheobromae S10 (MH181156, OL988629), L. pseudotheobromae SN (MH179101, OL988630), Lasiodiplodia II3 (OK648476, OL988631), and Pestalotiopsis mangiferae MN2 (MH179308). The resulting amplifications were 550 bp for the ITS and 430 bp for β-tubulin. Analysis of the sequences in the GenBank database identified the species Lasiodiplodia pseudotheobromae and Pestalotiopsis mangiferae in the ITS regions by 100% and in the β -tubulin regions by 99%. Lasiodiplodia pseudotheobromae and Pestalotiopsis mangiferae were identified based on their morphological and molecular characteristics.
Pathogenicity tests
Necrosis and gummosis were observed after seven days in the mango branches inoculated with L. pseudotheobromae and P. mangiferae. Significant differences (P<0.05) were observed in the lesion area between the branches inoculated with each phytopathogen (Table 1). On day seven, isolate S10 of L. pseudotheobromae showed the highest aggressiveness compared to the rest of the isolates, generating a lesion area of 28.22 mm2. The MN2 isolate of P. mangiferae showed a larger lesion area of 8.44 mm2 compared to the SN1 isolate. Isolates SN, LB2, S10 and II3 of the genus Lasiodiplodia caused a larger lesion area compared to the two isolates of Pestalotiopsis. The incidence rate of the disease was 100% in all the branches inoculated with both phytopathogens. Lasiodiplodia pseudotheobromae and P. mangiferae were re-isolated from the branches, confirming Koch’s postulates.
Table 1 Lesion area in branches of mango var. Manila inoculated with isolates of L. pseudotheobromae and P. mangiferae.
| Aislamiento | Incidencia (%) | Día 1 | Día 3 | Día 5 | Día 7 |
|---|---|---|---|---|---|
| Área de lesión (mm2)y | |||||
| L. pseudotheobromae SN | 100 | 3.77±0.5 dz | 7±0.88cdz | 12.77±0.83cz | 21.55±0.5dz |
| L. pseudotheobromae LB2 | 100 | 2.44±0.38c | 6.33±0.88c | 10.11±0.50b | 17.33±0.57c |
| L. pseudotheobromae S10 | 100 | 5.66±0.66 e | 8.27±0.58d | 15.44±0.83d | 28.22±1.7e |
| L. pseudotheobromae AA | 100 | 0.88±0.38a | 1.56±0.32a | 2.27±0.9a | 3.33±0.4a |
| L. pseudotheobromae II3 | 100 | 2.44±0.38c | 3.46±0.17b | 8.22±0.5b | 19.22±0.5cd |
| P. mangiferae MN2 | 100 | 2.33±0.33 c | 2.78±0.1ab | 4.22±1.5a | 8.44±1b |
| P. mangiferae SN1 | 100 | 1.5 ±0.29 b | 2.1±0.2 ab | 2.56±0.6a | 3.86±0.4a |
y Each value represents the mean ± standard deviation. z Means with different letters in each column are statistically different (Tukey, p≤0.05).
Evaluation of in vitro growth by dual culture technique
The results of the dual inoculation treatment did not show differences in the coloration of the mycelium between P. mangiferae MN2 and L. pseudotheobromae S10. The presence of conidiomata in P. mangiferae MN2, which was inoculated two days before L. pseudotheobromae, was observed on the seventh day. Individual inoculations did not present conidiomata. In the plates inoculated two days later, the growth of both fungi seemed to auto-equalize. A crossing between the hyphae of both species was observed in the microphotographs (Figure 2E-F). No inhibition halos were observed in the contact zone between P. mangiferae MN2 and L. pseudotheobromae S10 (Figure 2A-D).
In vitro evaluation of fungal filtrates
The percentage of inhibition of P. mangiferae MN2 and L. pseudotheobromae S10 showed significant differences (P<0.05) between the treatments. No changes were observed in the coloration of the mycelium during the growth of both fungi. P. mangiferae MN2 did not show conidiomata. The filtrate of L. pseudotheobromae S10 inhibited the growth of P. mangiferae MN2 by 41.38%. The percentage of inhibition of the filtrate of P. mangiferae MN2 on the growth of L. pseudotheobromae S10 was 2.68%.
Coinfection of Lasiodiplodia pseudotheobromae and Pestalotiopsis mangiferae in mango branches
Gummosis and necrosis were observed in mango branches inoculated with L. pseudotheobromae S10 and P. mangiferae MN2 (Figure 3). There were significant differences in lesion area between the coinfection and individual infection treatments. In coinfection treatments, the lesion area was larger compared to individual inoculations (Table 2). The lesion area was clearly seen to increase significantly in coinfection treatments. The control branches did not show a lesion area. The presence of gummosis, a characteristic symptom of dieback, was not observed. Necrotic tissue was obtained from each branch and the species L. pseudotheobromae S10 and P. mangiferae MN2 were re-isolated, confirming Koch’s postulates.
Discussion
The fungus Lasiodiplodia pseudotheobromae has been reported as the causative agent of mango dieback individually and as fungal complexes in various parts of the world such as Brazil, Egypt, Peru, Korea, and Australia, among others (Sakalidis et al., 2011; Ismail et al., 2012; Kwon et al., 2017; Rodríguez-Gálvez et al., 2017). In Mexico, the presence of L. pseudotheobromae has been associated with the death of mango trees of the Tommy Atkins and Ataulfo varieties in the states of Colima, Guerrero, Jalisco and Michoacán (Sandoval-Sánchez et al., 2013). The present study reports for the first time the presence of L. pseudotheobromae in mango trees var. Manila with dieback symptoms in Veracruz. In recent years, the importance of the genus Pestalotiopsis has increased due to the damage it causes to plants of commercial importance such as peach and blueberry plants (Chen et al., 2012; Borrero et al., 2018; Rodríguez-Gálvez et al., 2020). In mango, P. mangiferae has been associated with leaf spot (Rakesh et al., 2020). However, so far it has not been reported as part of the complex that causes dieback, unlike other species of the genus such as P. theae (de-Oliveira et al., 2010; Sandoval-Sánchez et al., 2013). The present study provides the first report of P. mangiferae as part of the complex causing dieback in mango var. Manila.
Table 2 Coinfection of Pestalotiopsis mangiferae MN2 and Lasiodiplodia pseudotheobromae S10 in mango branches after 5 days.
| Tratamiento | Área de lesión (mm2)y |
|---|---|
| Control (PDA) | 0.0±0.1a |
| L. pseudotheobromae S10 individual | 24.7±3.7b |
| P. mangiferae MN2 individual | 2.7±1.2a |
| L. pseudotheobromae S10+ | |
| P. mangiferae MN2 coinfección | 39.3±2.5c |
y Each value represents the mean ± standard deviation
z Means with different letters are statistically different (Tukey, p≤0.05)
In different ecosystems, plants interact with multiple groups of microorganisms, including phytopathogenic fungi, which interact with each other forming different ecological relationships (Liu et al., 2019). Although there are various reports on phytopathogenic fungi that associate to form complexes and cause diseases in various crops, little is known about their interaction and the mutual effect they have on their growth (Abdullah et al., 2017; 2018). The results of the present study using dual culture suggest a neutral interaction between L. pseud*otheobromae and P. mangiferae when hyphal crossing is observed instead of an area of antagonism. The results coincide with those that were previously reported on the interaction between Seimatosporium vitifusiforme and Diplodia seriata in grapevines. (Lawrence et al., 2018). The interaction between fungi in a particular space and time could give rise to the formation of complexes that cause diseases in various plants, including dieback in mango (Junaid et al., 2020).
Various phytopathogenic fungi produce antifungal compounds, such as phenols, terpenes, and flavonoids, which can inhibit the growth of various microorganisms, including fungi. This has been used as a biotechnological control strategy (Nalin et al., 2019; Reveglia et al., 2020). In the present study, the filtrate of L. pseudotheobromae S1O significantly inhibited P. mangiferae MN2. There are various reports of species of the genus Lasiodiplodia that produce mellein, an antifungal phenolic compound that causes cell lysis, inhibiting conidia germination and mycelial growth in various fungi (Qian et al., 2014; Cimmino et al., 2017; Morales-Sánchez et al. al., 2021) such as Botrytis cinerea, Fulvia fulva and Lasiodiplodia theobromae (Wang et al., 2014; Abro et al., 2019). Studies of fungal filtrates focused on the control of phytopathogens could provide useful alternatives if their active compounds, metabolic production, fungicidal and/or fungistatic capacity, among others, are characterized (Pradeep et al., 2013). This deserves special attention since the dual culture assay showed no evidence of antagonism. This could be attributed to an increase in the production of antifungal metabolites in liquid culture medium compared to the solid culture medium, as has been observed in other species (Pradeep et al., 2013). Another probable explanation could be the decrease or suppression from metabolites of L. pseudotheobromae when it recognizes P. mangiferae. The development in solid medium and in the branches was the same, corroborating the interaction between both species. The present study opens an opportunity to address the importance of secondary metabolites produced during interaction with various dieback-causing species.
In recent years, various studies have focused on investigating plant diseases caused by co-infections of phytopathogenic fungi and their interactions with the host (Susi et al., 2015; Lerch-Olson and Robertson, 2020; Hoffmann et al., 2021). Mango branches co-inoculated with L. pseudotheobromae and P. mangiferae exhibited greater severity of dieback overall compared to individual infections. Fungal interactions are complex and involve processes of mutualism, antagonism, coexistence, among others (Fang et al., 2021). In addition, the severity of diseases caused by co-infections of phytopathogens depends on various factors, mainly growth rate, virulence, metabolite production, interactions, host, among others (Müller et al., 2012; Boddy, 2016; Luo et al., 2017). Future studies will aim to analyze the fungal-host pathosystem and develop integrated management strategies for dieback.
Conclusions
The present study provides the first report of the causal agents associated with dieback in Manila mango trees in the state of Veracruz. Lasiodiplodia pseudotheobromae and Pestalotiopsis mangiferae were morphologically and molecularly identified based on the results obtained in the field and in the laboratory. In co-infection, the disease lesion area was larger in branches co-inoculated with L. pseudotheobromae and P. mangiferae, compared to individual infections. P. mangiferae filtrates had no effect on the growth of L. pseudotheobromae, while L. pseudotheobromae filtrates had an inhibitory effect on P. mangiferae growth.











 text in
text in 



