According to some estimates, the world population will reach 9.7 billion by 2050. Agricultural production will have to increase to meet the increased demand for food (United Nations, 2019). A worldwide problem working against increased agricultural production is the damaging effect of diseases caused by pathogens that reduce harvest yields and in extreme cases cause the death of crops (Jain et al., 2019). These pathogens include nematodes, phytoplasmas, viruses, bacteria, and fungi (Ortiz-Martínez et al., 2022). Phytopathogenic fungi are one of the main infectious agents of crops during pre-harvest, harvest and post-harvest stages (transport, storage and marketing), causing very significant economic losses (Juárez et al., 2010). Among the species of phytopathogenic fungi that are considered of agricultural importance because they infect a wide variety of crops and affect the quality, nutritional value and sensory characteristics of agricultural products are Alternaria spp., Botrytis spp., Epicoccum sorghinum, Fusarium spp., Lasiodiplodia spp., Monilinia spp., Mucor spp., Penicillium spp., Rhizopus spp., Colletotrichum spp., Aspergillus spp., Phomopsis spp., and Phytophthora spp. (De Oliveira et al., 2021). Many agricultural products are susceptible to these pathogens, which cause necrotic lesions on the leaves (leaf spots), gummosis, vascular wilt, rot, decay, and extensive necrosis (Nachilima et al., 2020; Palma-Guerrero et al., 2021). Chemical fungicides are the most widely used method for controlling these phytopathogenic fungi due to their quickness and effectiveness. However, frequent application of these compounds has led to the development of resistance in various fungal strains and has negative effects on human health and the environment (Zubrod et al., 2019). In Mexico, there are an estimated 10,000-13,000 endemic plants that have various biological properties (antioxidant, antibacterial, anticancer, and antifungal activity, among others). The extracts or compounds (alone or in combination) obtained from them are important for the pharmaceutical, food and agricultural industries. The latter is constantly looking for new fungicides of natural origin that can be used in the field and that can bring added value to endemic plants. Scientific interest in the use of plant products such as essential oils, organic extracts and purified compounds has increased in recent years. These products are recommended as safe alternatives to synthetic fungicides and as an important element of sustainable agricultural practices (Tapia- Quirós et al., 2020; Mui Yun Wong et al., 2020). The antifungal potential of various plant extracts (Larrea tridentata, Tagetes lucida, Flourensia cernua, Acaciella angustissima, Jatropha cuneata, Yucca filifera) was evaluated against Fusarium oxysporum, Botrytis cinerea, Colletotrichum spp., Aspergillus flavus, Rhizoctonia solani, Alternaria alternata, Sclerotium spp., among others, and it was reported that they induced 30-100% inhibition of mycelial growth (Asael et al., 2018).
Agaves are plants with high social, ecological, and economic importance. They are used in more than 100 different ways, such as food, drink, medicine, decoration, fiber, fuel, fertilizer, and construction material, among others. Their ecological importance lies in their ability to limit rainwater runoff, reduce soil erosion, capture carbon, and offer shelter and food to a wide range of animal species, from insects to mammals. Their most important use is as raw material for the production of distilled beverages such as mezcal, tequila, sotol, charanda, and bacanora (García-Mendoza, 2007; Barrientos-Rivera et al., 2019). These plants are characterized by synthesizing secondary metabolites such as fatty acids, terpenes, coumarins, triterpenes, steroids, tannins, flavonoids, glycosides, fructans, saponins, and alkaloids, which are responsible for their various biological activities (El-Hawary et al., 2020; Soto- Castro et al., 2021). The antifungal activity of the genus Agave has been evaluated against fungi of agricultural importance such as Postia placenta, Aspergillus niger, B. cinerea, Mucor spp., Fusarium spp., and Penicillium spp. (El-Hawary et al., 2020; Soto-Castro et al., 2021).
In Guerrero, Mexico, the “piña” of A. angustifolia and A. cupreata is traditionally used for the production of mezcal. However, the leaves, which represent 46% of the total weight of the plant, are not used in any other process and become a waste product (Iñiguez-Covarrubias et al., 2001). Recent studies have shown that the leaves of A. cupreata contain anthrones, anthraquinones, coumarins, alkaloids, essential oils, pungent compounds, and saponins, which have antibacterial and anti-inflammatory activity (Salazar-Pineda et al., 2017). Triterpenes (β-sitosterol), steroidal saponins (aglycones or glycosylated), and fructans have been isolated from the leaves of A. angustifolia and their antibacterial, anti-inflammatory, antiulcerogenic, and molluscicidal activity has been evaluated (López-Salazar et al., 2019; Camacho-Campos et al., 2020). There are no previous reports on the antifungal activity of A. cupreata and A. angustifolia. Thus, the present study aimed: 1) to determine the phytochemical profile of A. angustifolia and A. cupreata, and 2) to evaluate the antifungal effect of the extracts of these two botanical species on the mycelial growth and spore production of five phytopathogenic fungi of agricultural importance.
Materials and methods
Plant material. Leaves of A. angustifolia and A. cupreata were collected in the state of Guerrero, Mex., in the towns of Coacán, municipality of Atenango del Río (Latitude: 18.139444, Longitude: -49.187778) and in Totomochapa municipality of Tlapa de Comonfort, Guerrero (Latitude: 17.541111, Longitude: -98.459167) respectively. The identification of A. cupreata and A. angustifolia was carried out by Dr. Abisaí J. García Mendoza of the National Herbarium of Mexico (MEXU). The assigned accession numbers were: MEXU-2050 for Agave cupreata and ARC for Agave angustifolia.
Obtaining extracts from Agave spp. The collected leaves were cleaned with water. With the help of a knife, they were then cut into fractions of an approximate size of 3 cm. The aqueous extract of A. angustifolia (EAAa) was obtained by mixing the plant material (300 g) with water at 60 °C and leaving it to stand for 3 h. The mixture was then filtered and the obtained aqueous extract was frozen at ˗40 °C until lyophilization, which was carried out in a Free Zone 4.5 L system (Labconco, Kansas, USA) at a vacuum of 0.045 mBar and a temperature of -52 °C. The obtained powder was stored at ˗20 °C for later use in the antifungal activity test. The acetone extract of A. cupreata (EAAc) was obtained by direct maceration of the leaves (3500 g) with acetone (Golden Bell, Mexico) for 48 h. It was then filtered and the organic phase was evaporated in a rotary evaporator (digital rotary 410, Puebla, Mexico) at 80 rpm and 60 °C. Once the extract was obtained, it was stored at room temperature in amber bottles protected from light. The yield percentage of the extracts was calculated using the following formula:
Qualitative phytochemical Profile. The phytochemical profile was determined using thin-layer chromatography, as proposed by Salazar-Pineda et al. (2017). The extracts (5 mg) were diluted in 1 mL of extraction solvent and placed, using a capillary tube, on chromatoplates with silica gel TLC-F254 (5 cm high x 5 cm wide; Merck) that were eluted with hexane-acetone (7:3). The chromatoplates were observed under UV light (254 nm) and sprayed with the following chemical developers: Dragendorff (alkaloids), Polyethylene glycol (flavonoids), Liebermann-Burchard (triterpenes and saponins), and vanillin-phosphoric acid (terpenes, lignans, and curcubitacins) for the qualitative identification of secondary metabolites.
Phytopathogenic fungi and preparation of colonies. The fungi used were obtained from the collection of the Laboratory of Fruit Diseases of the Phytopathology Program of the Colegio de Postgraduados, based in Motecillo, Texcoco, Mexico. The species used in the assays were: E. sorghinum, Fusarium subglutinans, Lasiodiplodia viticola, L. iraniensis, and Colletotrichum sp., all of which were pathogenic for their primary hosts. Colonies of each fungal species were used for in vitro tests. The colonies were obtained from monosporic isolates and incubated for 72 h in Petri dishes with PDA medium (Potato Dextrose Agar 5%, Bioxon, Mexico).
Effect of Agave spp. extracts on mycelial growth. The agar dilution technique was used (González-Alvarez et al., 2015), with some modifications. The EAAc and EAAa extracts were dissolved in sterile water to concentrations of 2-16 mg mL-1 and placed in sterile Petri dishes (60 x 15 mm) containing 3 mL of unsolidified PDA agar. Once the agar gelled, a PDA disk with mycelial growth (5 mm in diameter) was placed in the center of the Petri dishes for each experimental fungal species. The dishes were incubated at 25 °C and the diameter of mycelial growth was monitored at 24, 48 and 72 h until the negative control colony (PDA medium plus fungi without extracts) covered the surface of the Petri dish completely. The antifungal Mancozeb (Manzate®) was used as a positive control at a concentration of 16 mg mL-1. All tests were performed in triplicate. The percentage of mycelial growth inhibition was determined at 72 h and was estimated using the following formula:
Where D1 is the average value of the diameter of the negative control colony and D2 is the average value of the diameter of the colony inhibited by the extracts.
Effect of Agave spp. extracts on spore production. The effect of the EAAc and EAAa extracts on sporulation at 72 h of growth was determined in the same Petri dishes that were used to determine antifungal activity at a concentration of 16 mg mL-1. The mycelium in the dishes was sprinkled with sterile distilled water (5 mL) and scraped with a bacteriological loop. The obtained suspension was filtered through a Pasteur pipette containing cotton as a filter (sterile) to remove fragments of culture medium and mycelium. Subsequently, 20 µL of the filtered suspension were placed in a Neubauer chamber. The spores were counted with the help of an optical microscope (10x; Olympus). The spore concentration was expressed in spores mL-1. All experiments were performed in triplicate.
Statistical analysis. The results were expressed as the mean of three repetitions ± standard deviation. Mycelial growth inhibition was assessed using an analysis of variance (ANOVA) followed by a Tukey’s test. The inhibition of spore production was assessed using Dunnett’s test with the negative control used for comparison. A value of p ˂0.05 was considered statistically significant. The statistical package Sigma Plot version 11.0 was used for all analyses.
Results and discussion
Phytochemical profile. The amount of extract obtained for EAAc was 20 g (0.57%) and 2.8 g (0.93%) for EAAa. Different phytochemical compounds were identified in the EAAc and EAAa extracts (Table 1). The variability is mainly due to the polarity of the solvent used in the extraction since the extractability of the compounds depends on their nature and solubility (Bui et al., 2021). The flavonoids present in the EAAa are generally extracted using highly polar solvents such as water, while the alkaloids detected in the EAAc are insoluble or slightly soluble in water, so they are extracted with other organic solvents such as ethanol, methanol, acetone, and chloroform. The presence or absence of certain compounds is also due to the biotic or abiotic factors to which the plants have been subjected (Pavarini et al., 2012). The saponins detected in A. cupreata have already been reported by Salazar-Pineda et al. (2017) and Urbina et al. (2018). Steroidal saponins have also been identified in methanolic extracts of the leaves of A. angustifolia (Pereira et al., 2017). Some authors have pointed out that these compounds are very common in the genus and yucagenin, hecogenin, tigogenin, diosgenin, ruizgenin and chlorogenin have been identified in species such as: A. lechuguilla, A. americana, A. inaequidens, A. hookeri, A. fourcroydes, A. brittoniana, and A. macroacantha (López-Salazar et al., 2019; González-Madariaga et al., 2020). With respect to triterpenes, Ahumada-Santos et al. (2013) reported the presence of p-cymene, limonene, β-trans ocimene, linalool, α-terpineol, nerol, geraniol and trans-nerolidol in extracts of A. angustifolia. Salazar-Pineda et al. (2017) reported the qualitative presence of triterpenes in extracts of the leaves of A. cupreata. The presence of α-linalool, α-terpinene, limonene, geraniol, nerol, α-cubebene, cadinene and trans-nerolidol, among others, has also been detected in extracts of Agave salmiana, Agave tequilana, and Agave potatorum (Soto-Castro et al., 2021). Regarding flavonoids, Ahumada-Santos et al. (2013) mentioned that the concentration of flavonoids in A. angustifolia was low. Kaempferol, myricetin rhamnoside, and quercetin arabinoside have been identified in the methanolic extracts of the leaves of Agave attenuata and qualitatively in A. potatorum (Soto-Castro et al., 2021).
Table 1 Qualitative phytochemical profile of the aqueous and acetone extracts of Agave angustifolia and Agave cupreata
| Revelador | Metabolito identificado | Disolvente | |
|---|---|---|---|
| EAAay | EAAcz | ||
| Dragendorff | Alcaloides | - | + |
| Polietilenglicol | Flavonoides | + | - |
| Liebermann-Burchard | Triterpenos, Saponinas | + | + |
| Vainillina ácido fosfórico | Terpenos, Lignanos, Curcubitacinas | - | - |
yEAAa: extracto acuoso de Agave angustifolia, zEAAc: extracto de acetona de Agave cupreata; +: Presencia, - Ausencia.
Effect of Agave spp. extracts on mycelial growth. The obtained extracts showed different effects on the mycelial growth of the fungi under study (Figure 1). In general, the application of these extracts was associated with a decrease in colony growth (Figure 1 A2-E2), presence of aerial mycelium (Figure 1 B2 and 3; D2 and 3), cottony (Figure 1 A5, C5, D5, E5) and deformed (Figure 1 A2, A5, C5, E5), as well as pigment production (Figure 1 A2, A3, A4, C5 and D3). The colonies in the negative control grew with normal shape, size and texture (Figure 1 A1-E1).
Even though no previous studies exist on the negative effect of A. angustifolia and A. cupreata on the morphology of the fungi under study, Siddhapura et al. (2011) mentioned that the methanolic extracts of Agave ferox, A. marginata, A. americana, A. montana and A. scabra affected the development of Postia placenta. The observed effects on the morphology, texture, vertical expansion of the mycelium, dehydration and pigment production have already been reported (De la Cruz-Ricardez et al., 2020). However, it is important to indicate that the mechanism by which they are produced is related to the action of metabolites associated with the cell wall. For example, the essential oils of Lavandula angustifolia and Eucalyptus globulus induced the formation of rough folds, torsion, peeling, fractures, and dehydration of the hyphae of Monilinia fructicola and Rhizopus stolonifer (da Silva et al., 2020; De la Cruz- Ricardez et al., 2020).
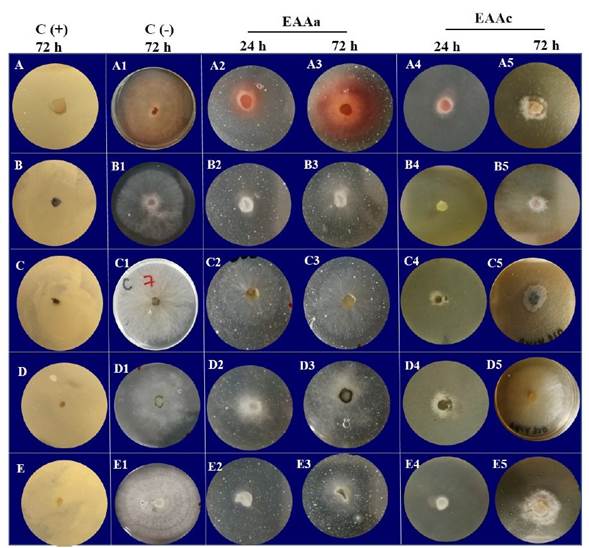
Figure 1 Effect of the aqueous extract from the leaves of A. angustifolia (EAAa) and the acetone extract of A. cupreata (EAAc) on the growth of five phytopathogenic fungi incubated in 5% PDA for 24 and 72 hours of exposure. A) E. sorghinum; B) F. subglutinans; C) L. viticola; D) L. iraniensis; E) Colletotrichum sp. C (+): antifungal Mancozeb (Manzate®) at 16 mg mL-1; C(-): 5% PDA agar.
Table 2 shows the antifungal effect of different concentrations of EAAa and EAAc against phytopathogenic fungi. The inhibition of mycelial growth of EAAc (39-76%) was significantly different (p≤0.05) compared to that of EAAa (20-40%). The table shows that the species L. viticola, E. sorghinum and Colletotrichum sp. were sensitive to EAAc, with mycelial growth inhibition percentages between 38 and 76% at concentrations of 16 and 8 mg mL-1 (p≤0.001).
In the case of EAAa, the percentages of mycelial growth inhibition in E. sorghinum and Colletotrichum sp. did not show statistically significant differences (p≥0.064) at concentrations of 4-16 mg mL-1. However, this extract was the only one that showed inhibitory activity against F. subglutinans, at 16 mg mL-1. Exposure to EAAa had no effect on L. viticola and L. iraniensis.
The results obtained demonstrate for the first time the antifungal activity of the polar extracts EAAa and EAAc on phytopathogenic fungi of economic interest. However, other authors have indicated the antifungal potential of polar extracts obtained from different species of Agave (A. montana, A. americana, A. marginetta, A. lechuguilla, A. scabra and A. ferox) against Fusarium oxysporum, F. solani, F. udum, and Colletotrichum gloeosporioides, reporting mycelial growth inhibition percentages ranging from 15 to 100% (De Rodríguez et al., 2011; Maharshi and Thaker, 2014; González-Alvarez et al., 2015).
Table 2 Inhibition percentage of mycelial growth of five phytopathogenic fungi exposed to aqueous and acetonic extracts from the leaves of A. angustifolia and A. cupreata
| Extracto | Concentración(mg mL-1) | % de inhibición del crecimiento micelial | ||||
|---|---|---|---|---|---|---|
| Epicoccumsorghinum | Fusarium subglutinans | Lasiodiplodia viticola | Lasiodiplodia iraniensis | Colletotrichum sp. | ||
| 2 | --- | --- | --- | --- | --- | |
| EAAay | 4 | 39.4b | --- | --- | --- | --- |
| 8 | 39.4b | --- | --- | --- | --- | |
| 16 | 40.0b | 20.0a | --- | --- | 41.6b | |
| 2 | --- | --- | 17.3b | --- | --- | |
| EAAcz | 4 | 13.3c | --- | 16.6b | --- | 32.0c |
| 8 | 39.4b | --- | 74.0a | 12.0b | 38.0b | |
| 16 | 59.6a | --- | 76.6a | 17.8a | 60.8a | |
| Control (+) Manzate | 16 | 100 | 100 | 100 | 100 | 100 |
yEAAa: extracto acuoso de Agave angustifolia acuoso, zEAAc: extracto de acetona de Agave cupreata; ---: No Presentó inhibición del crecimiento micelial. Medias con letras distintas en una misma columna son significativamente diferentes (Tukey; p≤0.05).
The results of the present study show variability in the phytochemical profile (Table 1) and mycelial growth inhibition activity (Table 2) of the Agave extracts under study when used on phytopathogenic fungi. The biological effect of each compound on different microorganisms has been reported before. Lagrouh et al. (2017) mentioned the antifungal effect of alkaloids and attributed to them the ability to intercalate with DNA, stop protein synthesis, induce apoptosis, and inhibit carbohydrate metabolism enzymes. Zhao et al. (2021) indicated that the alkaloid lycorine damages the integrity of cell membranes, reduces cell viability and affects the expression of GTPase BcMPS1, Bccdc, BcRac and BcRas1 in B. cinerea. Furthermore, flavonoids have been reported to have antifungal activity against Aspergillus fumigatus and Candida albicans through their effect on protein precipitation and the formation of complexes with the nucleophilic amino acids of proteins, which inactivates them and causes developmental arrest. In contrast, the effect of flavonoids on B. cinerea is attributed to the inhibition of fungal endo-1,3-β-glucanase (Jin, 2019; Tapia-Quirós et al., 2020). In R. solani, terpene compounds affect the permeability of the pathogen’s membrane, causing metabolic disorders and inhibiting mycelial growth. They also disrupt the potential of the mitochondrial membrane (causing loss of structure and function), inhibit the cycle of tricarboxylic acids, reduce ATP content and inhibit ATPase (Yan et al., 2020). Regarding saponins, it has been determined that these compounds form complexes with sterols in the cell membranes, causing them to disintegrate through the formation of pores and the triggering of cell lysis (Zaynab et al., 2021). Ito et al. (2007) mentioned that α tomatine induces apoptosis mediated by the accumulation of reactive oxygen species in F. oxysporum.
Effect of Agave spp. extracts on spore production.Figure 2 shows the effect of the extracts under study on the production of spores of each of the fungal strains tested. The most surprising results were obtained with the EAAa extract, which showed 40% inhibition of mycelial growth against F. subglutinans and Colletotrichum sp. However, spore production decreased by 92 and 86.6% compared to the negative control, respectively (Figure 2 B and E). This result is relevant for the control of these phytopathogens because it would prevent their reproduction and dissemination. On the contrary, EAAa stimulated the production of the spores of E. sorghinum and L. viticola (Figure 2 A and C), causing an increase of 407.5 and 73.2%, respectively, compared to the negative control (p≤0.001). No effect was observed on the production of spores L. iraniensis (Figure 2D). Regarding the EAAc extract, which showed a greater inhibiting effect on the mycelial growth of L. viticola, E. sorghinum and Colletotrichum sp. (Table 2), caused a decrease of only 35.6% in the production of spores of L. viticola (Figure 2C), compared to the negative control (p≤ 0.001), while no effects were observed on the spore production of E. sorghinum, F. subglutinans, L. iraniensis and Colletotrichum sp. (Figure 2 A, B, D and E), compared to the negative control (p≥0.05).
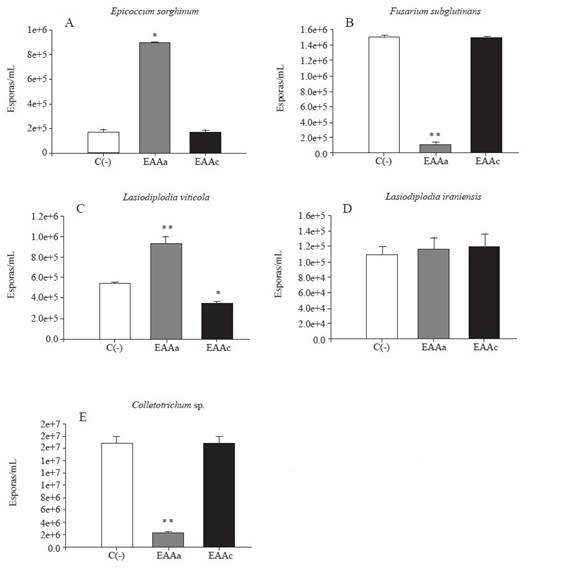
Figure 2 Effect of the aqueous extract from the leaves of A. angustifolia (EAAa) and the acetone extract of A. cupreata (EAAc) on the spore production of five phytopathogenic fungi incubated in 5% PDA for 72 hours of exposure. C(-): negative control, 5% PDA agar. The results are expressed as the average of three repetitions ± standard deviation. Dunnet’s statistical test with respect to C(-). * = significant (p˂0.05); highly significant (** p˂0.001).
Although the inhibitory effect of extracts from different agaves on the mycelial growth of various species of fungi has been evaluated before, studies on the inhibition of sporulation are still scarce. Lozano-Muñiz et al. (2011) found that the aqueous and methanolic extracts from the leaves and flowers of A. americana, A. asperrima, A. lechuguilla, A. tequilana negatively affected the conidiogenesis of Aspergillus parasiticus and Aspergillus flavus. They reported that the methanolic extracts of A. asperrima and A. americana caused notable changes in conidiophore morphology, which may affect conidiogenesis. Other authors pointed out that the inhibition or stimulation of spore production are possible responses of fungi when exposed to different extracts. For example, Achimon et al. (2020) reported that the essential oils of Pimenta dioica and Curcuma longa (1000 mg L-1) inhibited the mycelial growth of Fusarium verticillioides M3125 by 87.2 and 55.5%, respectively, but the percentages of conidia production were 3046.4 and 341% respectively. The authors also reported that oils from Rosmarinus officinalis showed little growth inhibitory activity (24%), but inhibited conidia production by 27% compared to the control, which is similar to what was observed in the present study.
Conclusions
The phytochemical profile of the extracts of A. angustifolia and A. cupreata confirmed the presence of alkaloids, flavonoids, saponins and triterpenes.
The aqueous extract of A. cupreata showed an antifungal effect against L. viticola, Colletotrichum sp. and E. sorghinum inhibiting 76, 60 and 59 % of mycelial growth, respectively, at concentrations of 8 and 16 mg mL-1 (p≤0.05), while spore production decreased by 86% in Colletotrichum sp. Therefore, this extract has the potential to be used as an alternative source of new natural antifungal compounds.
The acetone extract of A. angustifolia inhibited 40% of the mycelial growth of E. sorghinum at 16 mg mL-1 (p≤0.05) without reducing its sporulation (p≥0.05).











 texto en
texto en 


