In Mexico, the production of pepper seedlings is severely affected by the disease known as ‘damping off’, with losses ranging between 10 and 100%. The disease is caused by a complex of species of Fusarium, Rhizoctonia, Phytophthora and Pythium (Hernández, 2018), although the Pythium species are considered the main causal agent (Elshahawy et al., 2018; Hernández, 2018). Pythium is an oomycete which is differentiated from true fungi in that it contains cellulose and β-glucans as the constituents of its cell wall. This genus is distributed globally, and it is favored by climates with high humidity levels and highly varied temperatures, depending on the species (Schroeder et al., 2013). The initial infection can be triggered by the fragment of a mycelium in growth or the germination of zoospores, oospores and even a sporangium. In the stage of penetration, the Pythium species require high humidity levels and they introduce themselves through lesions or exerting mechanical pressure and via the enzymatic degradation of the plant tissues. In young roots it penetrates through the tips of the rootlets (Agrios, 2005). This microorganism can survive as a saprophyte in the remains of plants and as a parasite in over 60 genera of crops belonging to the Solanaceae, cucurbit families, and others (Agrios, 2005; Díaz et al., 2011; Valdés et al., 2011). In the establishment of trays or seedbeds, the damage by Pythium spp. presents itself in the stage of pre- and post-emergence (Gómez et al., 2011; Grijalba and Ridao, 2017), in which it causes the rotting of seeds, strangling of the base of the stem, root necrosis, wilt, lodging and the death of seedlings (Gómez and Melero, 2011; D’Angelo, 2016; Rivera and Fernández, 2017). Adult plants are generally affected at the level of roots with small lesions in the epidermis, although it may damage the fruits exposed to high levels humidity or in postharvest (Fry et al., 2010; Díaz et al., 2011; Hernández et al., 2018). This genus has a high growth rate (Schroeder et al., 2013) of up to 7-34 mm a day, depending on the species, the temperature and the substrate in which it develops (Van der Plaats-Niterink, 1981).
Approximately 160 species have been documented from the genus Pythium (Grijalba and Ridao, 2017), out of which the most important are P. aphanidermatum, P. ultimum, P. debaryanum, P. irregulare, P. myriotylum and others (Díaz et al., 2011; Grijalba et al., 2015; Ríos and de Rocha, 2018; Grijalba et al., 2020). The morphological identification of Pythium spp. is based on the shape and dimensions of the sexual and asexual reproductive structures, such as sporangia, oogonia, type of oospore (aplerotic or plerotic) and the number and position of the antheridium when attaching with the oogonium. Due to the high variation of these structures among species, their morphological identification is difficult, therefore we resort to molecular identification to obtain a more reliable characterization (Díaz et al., 2011; Schroeder et al., 2013). During a visit in July, 2019, to greenhouses with pepper plants in the municipal area of Parras de la Fuente, Coahuila, Mexico, dead seedlings were found. In this region there are no records of pathogens in pepper causing damping off, therefore we decided to identify the causal agent; preliminary studies indicated that the oomycete related to the disease displayed characteristics of the genus Pythium. Due to this, the present study was carried out with the following aims: A) to identify the causal agent of the deaths of pepper plants in the greenhouses in Parras, Coahuila, Mexico and B) to evaluate its pathogenicity in seeds and seedlings, as well as its behavior and growth rate in different culture media in the laboratory.
Location of the experiment. This work was carried out in the Microbiology Laboratory of the Department of Agricultural Parasitology of the Universidad Autónoma Agraría Antonio Narro (Antonio Narro Autonomous Agrarian University - UAAAN) located in Calzada Antonio Narro 1923, Buenavista, Saltillo, Coahuila, Mexico.
Isolation of the phytopathogen. Serrano pepper seedlings with the typical symptoms of ‘damping off’ were collected from three greenhouses in the area of Parras de la Fuente, Coahuila. The seedlings were taken to the laboratory, where they were washed with tap water and the roots were separated from the stems. Later, using a sterilized scalpel, they were cut into small pieces which were then disinfected with a 1% sodium hypochlorite solution for 3 min, washed in three steps of sterile distilled water and left to dry on brown paper under aseptic conditions in a laminar flow cabinet. The pieces of roots and stems were placed in Petri dishes with a V8-Agar (V8A) culture medium and incubated at 26 ± 2 °C for 4 days, where cenocytic hyaline mycelium, oogonia, antheridia and typical sporangia of the genus Pythium (Van der Plaats-Niterink, 1981) appeared. Pieces of mycelium were transferred to water agar culture medium (AA) and 24 h later, the isolation was purified by transferring hypha tips into Petri dishes with V8A medium and incubated at 26± 2 °C with a light period of 12:12 (light: darkness) for seven days for its identification, measurement of its growth rate and pathogenicity tests.
Morphological identification. This was carried out with direct observations of the mycelial growth, formation of sporangia, oogonia and antheridia developed over a sterilized seed, inoculated with a 3 mm piece of Pythium spp. cultivation placed on a Petri dish with AA culture medium and incubated for seven days in which the mentioned structures of this oomycete would form. Observations were carried out using a Keyence VHX-7000 digital microscope and a Motic BA210E compound microscope, in which microscopic preparations were observed. The identification was carried out following the descriptive keys by Van der Plaats-Niterink (1981).
Molecular characterization. The confirmation of the identity of the species of Pythium sp. was carried out based on the internal transcribed spacer (ITS’s) sequences 1 and 4, including gene 5.8S rDNA, obtained in the National Agricultural, Medical and Environmental Biotechnology Laboratory (Laboratorio Nacional de Biotecnología Agrícola, Médica y Ambiental - LANBAMA) in San Luis Potosí, Mexico. The samples were sequenced with the marked dideoxynucleotide method in the 3130 Genetic Analyzer (Applied Biosystems) sequencer in an automatic Genic Analyzer series 3500. To amplify the genes, we used a Veriti thermocycler for Endpoint PCR (Applied Biosystems) with oligos ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) (Al-Sheikh, 2010; Jahén Rivera et al., 2020).
Pathogenicity tests. These were carried out in vitro in Petri dishes with AA culture medium, using two methods: in pre-emergence and post-emergence. The former was carried out with the technique used by Apodaca et al. (2002), which consisted in placing 10 Platino hybrid serrano pepper seeds in Petri dishes containing AA and inoculating them with a mixture of mycelium, oogonia, oospores and Pythium zoospores. The inoculated seeds were incubated at 26 ± 2 °C with a light period of 12:12 (light: darkness) and the severity was evaluated every 24 h for 12 days. The second method was carried out using the technique proposed by Sánchez et al., (1975) with slight modifications. Fifty seeds of the same hybrid were germinated in AA medium. After five days and once the hypocotyl (hp) developed, they were transferred axenically pure in groups of three seeds into new Petri dishes containing AA, two days later. When the development of their roots and cotyledons were observed, a piece of Pythium mycelium was transferred to the center of the Petri dishes along with culture medium, 3 mm in diameter, with 7 day’s growth, from the Petri dishes with V8A. The Petri dishes were incubated at 26 ± 2 °C with a light period of 12:12 (light: darkness) and the severity was evaluated five days after inoculation. In both methodologies, the visual severity scale described by Apodaca et al. (2002) was used, with modifications, where 0= no symptoms, healthy plant, 1= few small necrotic spots in the hp or on cotyledons, 2= necrotizing on the base of the hp, 3= hp necrotized by up to 50%, 4= seed, hp or plant completely necrotized. Both treatments (one with inoculation, the other without inoculation) were distributed in a completely random arrangement with three repetitions and two treatments. The data underwent an analysis of variation (ANOVA) and a Tukey means comparison test (p≤0.05) in the R statistical program, version 3.3.1.
Development of Pythium in different culture media. The culture media used were V8- Agar (V8A), Maize Agar (MA), Maize Potato Agar (MPA), Potato Dextrose Agar (PDA), Czapek (Czp) and Oat Agar (AvA), in Petri dishes, all 9 cm in diameter. In each one of the media, a piece of Pythium mycelium, 3 mm in diameter, was placed after two days growing in V8A. The mycelial growth was measured 24 h after planting. A completely randomized design was used, with four repetitions (four Petri dishes). The data underwent an analysis of variance (ANOVA) and a Tukey means comparison test (p≤0.05) in the R statistical program, version 3.3.1.
Morphological identification of Pythium sp. The isolation obtained from pepper plants with symptoms of damping off presented hyaline coenocytic toruloid hyphae with diameters between 5 and 7 µm (Figure 1-Aa and Da), spherical terminal oogonia, flat ones with a diameter of 25-28 µm (Figure 1- Ba, Ca and Ea), with an aplerotic oospore with a diameter between 24 and 26 µm (Figure 1Cc and Eb), dicline or monocline antheridia, one per oogonium (Figure 1-Bbc and Cb), filamentous, lobulated and irregular sporangia, (Figura 1-Fa) and zoospores (Figure 1Ab). These characteristics coincided, when using the taxonomic keys by Van der Plaats-Niterink (1981) and Tsuneo (2010), with those of Pythium aphanidermatum. However, although the morphological characteristics observed in Pythium coincide with those described by these authors, there are differences with the sizes of some structures. For example, Van der Plaats-Niterink (1981) describes the hypha as having a 10 µm diameter, oogonia that measure 20-25 µm with an aplerotic 18-22 µm oospore and one or two antheridia per oogonium, whereas this study found hyphae with diameters of 5-7 µm, oogonia with diameters of 25-28 µm with aplerotic oospores of 24-26 µm and with only one antheridium per oogonium. The characteristics observed in this study were similar to those observed by Díaz et al. (2011), who found oogonia with a diameter of 27.5 µm and only one antheridium per oogonium, as well as Al-Sheikh (2010), who reported irregular sporangia, spherical terminal oogonia with an average diameter of 24 µm, with an aplerotic oospore measuring 24-26 µm and a dicline or monocline antheridium.
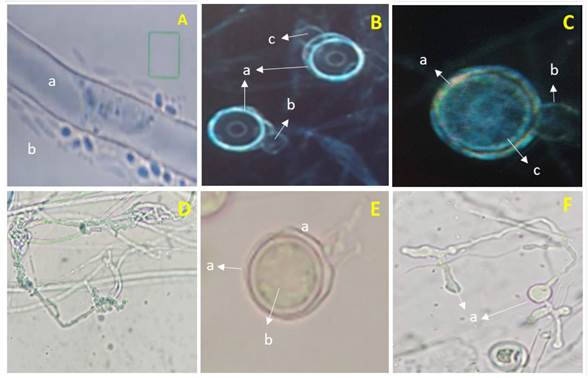
Figure 1 Pythium aphanidermatum. A) a- cenocytic hypha and b- Zoospores. B) a- Smooth spherical terminal oogonia, b and c- Dicline antheridia. C) a- Oogonium, b- Monocline antheridium and c- Aplerotic oospore. D) a- Toruloid hypha. E) a- Terminal oogonium and b-Aplerotic oospore. F) a- Lobulated sporangia.
Molecular identification of Pythium sp. The sequencing of the region of the rDNA ITS-1- of gene 5.8S- ITS-4 of the oomycete displayed a similarity of 99.15% with accession key sequence JN695786.1., of the GenBank, corresponding to P. aphanidermatum. This provided certainty regarding the identity of the causal agent of the damping off found in pepper plants in greenhouses in Parras de la Fuente. Other investigators, in order to characterize this species, have used different oligos such as ITS4 and ITS5, ITS1 and ITS2. Such was the case for Al-Sheikh, (2010) and Díaz et al. (2011), whereas Grijalba et al. (2015) used oligos ITS4 and ITS5, confirming the identity of the species P. aphanidermatum as the cause of damping off in wheat, tomato and ornamental plants, unlike this study, which reports the isolation of plantlets in pepper plants in greenhouses.
Pathogenicity tests. In pre-emergence, the seeds inoculated with P. aphanidermatum displayed the growth of whitish mycelia 24 h after inoculation, which did not occur in the control seeds. Five days after inoculation, the formation of the hypocotyl (hp) was observed in inoculated and non-inoculated treatments, but only the control treatment developed a root (Figure 2A and C). Twelve days after inoculation, the treatments inoculated with P. aphanidermatum did not display the growth of cotyledons or roots, and its hypocotyl was necrotized and contained mycelia (Figure 2D). Out of the 30 seeds inoculated, only three displayed no development of the hypocotyl, whereas in the control treatment, the seeds developed roots, stems and cotyledons without signs or symptoms of the microorganism (Figure 2B).

Figure 2 P. aphanidermatum pathogenicity test´s in serrano pepper seeds and seedlings. A) Control treatment, seed five days after planting (dap), B) Treatment control 12 dap, C) Seeds inoculated with P. aphanidermatum five days after inoculation (dai), D) Seed inoculated with P. aphanidermatum, 12 dai, E) Necrosis of cotyledons and abundant mycelium in the root 3 dai, F) Total necrosis of seedlings 5 dai, G) Control treatment with symptom-free seedlings.
In the case of post-emergence, in all treatments inoculated with a piece of mycelium with 3 mm in diameter from the Pythium culture, mycelial growth was observed 24 h after inoculation, except in the control. Three days after inoculation, plants displayed symptoms of necrosis in cotyledons and roots, as well as abundant mycelia (Figure 2E) and after five days, they displayed total necrosis (Figure 2F), whereas the controls presented no symptoms (Figure 2G). The isolation characterized as P. aphanidermatum displayed a high pathogenicity in pre- and post-emergence, since it caused a mortality rate of 100% in serrano pepper seeds and seedlings, which is similar to the data reported by Grijalba et al. (2015) and Grijalba et al. (2020) in tomato and soybean seedlings, respectively. At a temperature above 25 °C, as in reports by Al-Sheikh (2010), P. aphanidermatum caused 100% of ‘damping off’ in both pre- and post-emergence in wheat. Meanwhile, Valdez et al. (2011) reported a reduction of 65% in the germination of Jatropha seeds inoculated in vitro with the same oomycete.
According to the severity scale by Apodaca et al. (2002), the damage observed in the inoculated treatments was placed in the highest level (4) of the scale, since necrosis was found, along with seed rot and death of the seedling, whereas the controls were located in level 0 of the scale, due to the absence of signs or symptoms of the microorganism. Given this, the isolation identified as P. aphanidermatum was considered a severe causal agent of damping off in pre- and post-emergence in pepper seedlings and seeds.
Development of Pythium aphanidermatum in different culture media.Table 1 shows that 24 h after the microorganism was planted, it presented a significant difference in mycelial growth in the different treatments, V8A being the medium in which P. aphanidermatum achieved the highest growth rate, with 67.7 mm/day, making it statistically higher than the rest of the treatments with an 18% increase in regard to the MA treatment, which had the lowest growth rate, with 57.3 mm/day. These growth rates were higher than the one reported by Van der Plaats-Niterink (1981), who mentions a growth rate of 30 mm/day for this species, which may be a factor involved in the high pathogenic ability observed on pepper seeds and seedling.
Table 1 P. aphanidermatum mycelial growth rate after 24 h in different culture media.
| Medio de Cultivo | Media del Crecimiento (mm ± DS) |
|---|---|
| V8A | 67.7± 0.7 az |
| MPA | 60.2± 0.7 b |
| PDA | 58.3± 0.3 bc |
| Czp | 58.2± 0.5 bc |
| AvA | 58.1± 0.3 c |
| MA | 57.3± 1.8 c |
z Means with different letters are significantly different (Tukey; p≤0,05).
The behavior of P. aphanidermatum after 32 h in the different culture media appears in Figure 3, in which white and cotton-like mycelia are visible, with the exception of the Czp medium (Figure 3A), in which mycelium was scarce and superficial, at the level of the culture medium. In the MA medium (Figure 3B), P. aphanidermatum presented dense mycelium, yet lower growth than in MPA (Figure 3C), V8A (Figure 3F) and AvA (Figure 3D). In the latter medium, the oomycete developed the most abundant and dense mycelia. However, it was in V8A where cellular differentiation took place, and sexual and asexual reproduction structures were observed.

Figure 3 Growth of Pythium aphanidermatum after 32 h of incubation; A) Czp medium, B) MA medium, C) MPA medium, D) AvA medium, E) PDA medium, F) V8A medium.
It is worth highlighting that the formation of reproductive structures in Pythium aphanidermatum such as sporangia, oogonia and zoospores only took place in the V8A medium 96 h after planting, and no other treatments studied (culture media) developed sexual or asexual reproductive structures before 168 h of incubation. Therefore, the amount of nutrients in V8A promoted the growth and formation of sexual and asexual structures in P. aphanidermatum, as reported by Ko (1998) and González et al. (2014), who claim that culture media with high sugar levels (glycerides) and vitamins promote the production of Pythium and Phytophthora oospores and antheridia. Also observed was the growth of cotton-like mycelia, typical of P. aphanidermatum, in the PDA culture medium (Grijalba et al., 2015; Al-Sheikh, 2010). This phytopathogen, due to its growth in Czapek medium, displayed poor development, as it did in PDA, being AvA and V8A where the growth of cotton-like mycelia was promoted.
A strain of the oomycete Pythium aphanidermatum, which caused the death of pepper seedlings in a greenhouse in Parras de la Fuente in Coahuila, Mexico, was isolated and morphologically and molecularly identified. The isolated strain presented a high virulence in vitro in pre-emergence over the seeds and in post-emergence in serrano pepper seedlings. The isolated strain has a quick development in different culture media, being V8A the medium in which it displayed a better development. In addition, this culture medium, which is composed of tomato juice, plant extracts and CaCO3, allowed it to form sexual and asexual reproductive structures.











 texto en
texto en 


