Secondary metabolites, such as alkaloids, flavonoids, terpenes and phenols, are molecules synthetized by plants as defense mechanisms against pathogen attacks (Scott et al., 2008). Other molecules, such as chitosan, can be used to elicit secondary metabolite production in plants (López-Moya et al. 2019). Plant-produced secondary metabolites can be used as bioplaguicides (Báez-Valdez et al., 2010), providing environmentally friendly alternatives to the chemical control of pathogens such as Fusarium, a fungus that attacks the vascular system of plants and causes diseases in several vegetable and fruit species (Dweba et al., 2017; McGovern, 2015) leading to serious economic loss. Biological control of Fusarium can be achieved with natural compounds synthetized by some plant species of the genus Piper (Scott et al., 2008), which have been shown to have fungicidal effects on F. solani f. sp. piperis and F. oxysporum f. sp. vanillae (da Luz et al., 2017; Suprapta and Ohsawa, 2007). The fungicidal activity of Piper can be attributed to resistance genes such as those developed by some black pepper (P. nigrum) cultivars that produce secondary metabolites when attacked by F. solani f. sp. piperis (da Luz et al., 2017). Similarly, at least three American Piper taxa were reported being resistant to Fusarium: low mortality of P. divaricatum exposed to Fusarium was attributed to secondary metabolite production, especially phenols (Erisléia-Meireles et al., 2016), the seeds and seedlings of P. aduncum are protected by essential oils (Potzernheim et al., 2012), and P. tuberculatum expresses gene resistance when are treated with F. solani f. sp. piperis, which involves some of the same genes related to the systemic acquired resistance (SAR) in P. nigrum (Nascimento et al., 2009).
SAR is a generalized defense mechanism of plants against a broad range of pathogens generated by different proteins regulating interactions at the interface of the cellular membranes (Ádám et al., 2018). For example, the recognition of fungal chitin triggers a cascade of chemical signals, activating response genes that synthetize proteins such as phenylalanine ammonia-lyase (PAL) responsible for flavonoid and phenol production through a biosynthesis pathway involving the phytohormone salicylic acid as a signal molecule (López-Moya et al., 2019). Salicylic acid and chitosan are used together to induce plant resistance against pathogens. They provide an alternative to fungicides by modulating SAR and by stimulating the production of phenols, terpenes and flavonoids in response to infection by fungi such as Fusarium (Golkar et al., 2019). Therefore, the objectives of this study were to evaluate the production of secondary metabolites of chitosan-treated plant extracts of P. auritum and to determine their in vitro fungicidal activity against F. oxysporum f. sp. vanillae
A wild population of P. auritum was selected in the locality of San Andrés Tlalnelhuayocan, Veracruz, Mexico (-96.9776678 W; 19.29358 N; 1450 m asl), within a cloud forest on an andosol, subject to a wet temperate climate. The population included 72 mature plants with reproductive structures, which were divided into 6 parcels with 4 individuals each. Three parcels were treated with commercial chitosan (trademark VEPINSA, Sinaloa, Mexico) [low molecular weight (22,000 to 33,000 g mol-1), 27 % chitosan in product, deacetylation 80 %, humidity 10 %, cinder 1.8%, viscosity (10g L-1, 25 °C) 30 to 200 cps] dissolved to 1 mg mL-1 concentration and pH 5.5 (Treatment A). The other three parcels were the control, treated with a Tween 80 solution at 0.01 % (v/v) with pH 5.5 (Treatment B). The treatments were applied every week for one month, following the methodology of Benhamou and Thériault (1992). After the treatments, 3 kg of fresh P. auritum (leaves, inflorescences, and stems) were collected and oven dried at 45 ± 5 °C before maceration, separating the treated and control samples by 1.5 m in the drier.
The phytochemical extraction was carried out with a Soxhlet, using 10 g of plant material mixed with ethanol and concentrated with a rotavapor (250 rpm at 45 °C) (Carmona-Hernández et al., 2014). Total alkaloids were measured by spectrophotometry with 544 nm absorbance using the bromocresol green method and the results were expressed in µg of piperine equivalent (PE) per mg of extract (Tiwari et al., 2017). For total flavonoids, the aluminum reduction method was used with 420 nm absorbance and the results were expressed as µg quercetin equivalent (QE) per 10 mg of extract (Blainski et al., 2013). For the total phenols, the Folin-Ciocalteu reagent was used with 760 nm absorbance and the results were expressed as µg tannic acid equivalent (TAE) per 10 mg of extract (Blainski et al., 2013). The total terpenes were obtained following the methodology of Ghorai et al. (2012) and the results were expressed as mg menthol equivalent (ME) per 100 mg of extract. The salicylic acid response hormone was measured using the methodology of Rahman et al. (2016): 1000 µL of ethanolic extract (1 mg mL-1) was added to 50 µL ClFe3 and 950 µL bidistilled water and the absorbance measured at 540 nm. The results were expressed as µg salicylic acid equivalent (SAE) per mg of extract. Five replicas of each analysis were carried out.
The in vitro inhibitory activity of P. auritum extracts was tested using fungal cultures of F. oxysporum f. sp. vanillae in Petri dishes (90 mm diameter) for three replicas with five concentrations of extracts (0.8, 1.6, 2.4, 3.2, 4.0 mg mL-1) and a control (EtOH), added to potato dextrose agar (PDA). The tests were performed by placing 5 mm of mycelium in the center of the Petri dishes. Diameter growth was measured after five days incubation at 27 °C (Rongai et al., 2015). Percentage of inhibition was calculated with the following formula: % growth inhibition = ((control growth - treatment growth) / control growth) *100.
The STATISTICA 10 software was used to perform ANOVAs with post hoc Tukey tests. The median effective concentration (EC50) was estimated with a probit model using the Biostat 6.0 software (Amini and Sidovich, 2010).
Table 1 Mean concentration (with standard deviation and coefficient of variation) of secondary metabolites and salicylic acid in Piper auritum extracts with and without chitosan treatment.
| Metabolite | Treatment | Mean concentration | Standard deviation | Coefficient of variation |
|---|---|---|---|---|
| Alkaloids | A | 84.5a | 1.1 | 0.8 |
| B | 148.2b | 0.8 | 0.9 | |
| Flavonoids | A | 12.8b | 0.1 | 1.0 |
| B | 12.4a | 0.1 | 0.5 | |
| Phenols | A | 12.6b | 0.0 | 1.7 |
| B | 2.3a | 0.2 | 1.9 | |
| Terpenes | A | 16.3b | 0.3 | 2.7 |
| B | 11.6a | 0.4 | 2.7 | |
| Salicylic acid | A | 2.2b | 0.1 | 7.5 |
| B | 1.3a | 0.0 | 1.2 |
A: with chitosan, B: without chitosan.
Alkaloids: μg EP mg-1, Flavonoids: μg EQ mg-1, Phenols: μg Eat 10 mg-1, Terpenes mg EM/100 mg, Salicylic acid: μg ESA mg-1.
Different superscript letters indicate significant differences (p<0.05) in mean metabolite concentration.
There were significant differences in the secondary metabolite production between the P. auritum plant extracts with and without chitosan treatment (Table 1). In line with previous studies with other plant species, the concentrations of flavonoids, terpenes and phenols were significantly higher (P<0.05) in the chitosan-treated plant extracts. For example, the application of chitosan (200 mg) to Hypericum perforatum induced flavonoid production (Brasili et al., 2014), terpene production increased with chitosan addition (1 g) in Mentha piperita (Chang et al., 1998), and phenol concentrations were higher with chitosan treatment (200, 500 and 1000 ppm) in extracts of Origanum vulgare spp. hirtum (Yin et al., 2012). According to López-Moya et al. (2019), the chitosan treatment increased the production of salicylic acid in plants; which can trigger the production of phenylalanine ammonia-lyase (PAL), in turn exacerbating the production of flavonoids, terpenes, and phenols, which might contribute to generate the SAR (Wiesel et al., 2014). Contrary to the positive effect on flavonoids, terpenes and phenols, the chitosan treatment significantly decreased the concentration of alkaloids (P < 0.05). Such an effect has previously been reported in Stemona curtisii (Pitta-Alvarez et al., 1999) at concentrations of chitosan higher than 0.1 mg mL-1, in less than 4 weeks.
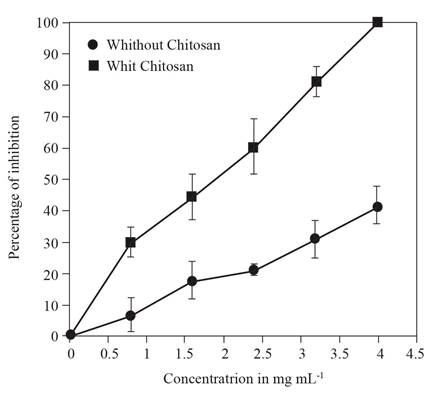
The median effective concentration (EC50) of P. auritum extracts against F. oxysporum f. sp. vanillae was significantly lower with chitosan treatment (EC50 = 1.473 mg mL-1; standard error = 0.288) than in the control (EC50 = 5.123 mg mL-1; standard error = 0.646) (P<0.001). The inhibition curve shows that the highest concentration P. auritum ethanolic extract (4 mg/mL) caused 100 % inhibition of Fusarium growth with chitosan treatment, compared to 41% without chitosan (Figure 1). Fungicidal activity of P. auritum against F. oxysporum and other phytopathogenic fungi has previously been reported by Pineda et al., 2012), which could be attributed to high concentrations of phenols, flavonoids and terpenes. The American Piper species are known to possess resistance genes associated with the production of phenylpropanoids (Erisléia-Meireles et al., 2016; Nascimento et al., 2009; Potzernheim et al., 2012). The results presented here show that chitosan has an eliciting effect on the production of phenols, flavonoids, terpenes and salicylic acid in the plant extracts of P. auritum, but an antagonistic effect on alkaloid production. The fungicidal activity of P. auritum ethanolic extracts against F. oxysporum f. sp. vanillae was markedly improved by chitosan addition.











 text in
text in