Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.39 no.1 Texcoco Jan. 2021 Epub 07-Maio-2021
https://doi.org/10.18781/r.mex.fit.2009-4
Scientific articles
Physicochemical characterization, antioxidant and antifungal activity of three stingless bee pollen aggregate (Apidae: Meliponini) from Soconusco, Chiapas
1Instituto de Biociencias, Universidad Autónoma de Chiapas. Boulevard Príncipe Akishino s/n. Colonia Solidaridad 2000. Tapachula Chiapas CP 30798, México.
2 Escuela de Agronomía, Universidad Dela Salle Bajío. Avenida Universidad #602, Colonia Lomas del Campestre, León, Guanajuato. CP 37150 , México.
Plants-bees interaction can generate bee-hive products with different physicochemical, bioactive and antimicrobial composition. Therefore, in this study we determined the physicochemical composition of pollen aggregates collected from 12 stingless bee-hives established in Chiapas, Mexico, from three different municipalities: Tapachula, Mazatán and Cacahoatán, and with three different species: Melipona beecheii, Scaptotrigona mexicana and Tetragonisca angustula. We also evaluated the effect of pollen aggregate extracts on Colletotrichum gloeosporioides growth. Our results showed differences in the physicochemical composition based on bee species. Pollen from M. beecheii registered the highest phenol content, flavonoids, and free acidity. These results, and the obtained from the antioxidant capacity (Trolox), glucose and pH, were associated with in vitro C. gloesporioides growth. The fungus radial growth rate during the nine-day study was 0.013-0.009 mm h-1 with pollen extracts, which was equivalent to 44% lower value than chlorothalonil. The antifungal activity of pollen extracts depends on bee species; for M. beecheii we registered 65 and 37%, for T. angustula 57 and 16%, and for S. mexicana 60 and 30%, which were higher than the chlorothalonil fungicide.
Key words: Interaction; inhibition; hive products; pollen aggregate; stingless bees
La interacción planta-abeja puede generar productos de la colmena con diferentes características fisicoquímicas, bioactivos y actividad antimicrobiana. Por lo cual, en este trabajo se determinó la composición química de conglomerados o agregados de polen colectados de 12 colmenas establecidas en Chiapas, México, en los municipios Tapachula, Mazatán y Cacahoatán, dentro de tres meliponarios comerciales asociados a las especies: Melipona beecheii, Scaptotrigona mexicana y Tetragonisca angustula. Asimismo, se evaluó el efecto de los agregados de polen en Colletotrichum gloeosporioides. Se encontró una composición química muy diversa independientemente de la especie de abeja. El polen obtenido de colmenas con M. beecheii tuvieron la mayor cantidad de fenoles, flavonoides y acidez libre. Estas propiedades, en adición de la capacidad antioxidante (trolox), glucosa y pH, estuvieron asociados a la inhibición del crecimiento in vitro de C. gloeosporioides. La velocidad de crecimiento radial del hongo durante nueve días fue de 0.013 a 0.009 mm h-1 con extractos de polen, 44 % menor que el efecto del clorotalonil. La actividad antifúngica de los extractos de polen fue de 65 y 37 % para M. beecheii, 57 y 16 % para T. angustula y 60 y 30 % para S. mexicana, respecto al tratamiento testigo y a la dosis más alta de clorotalonil, respectivamente.
Palabras clave: Interacción; inhibición; compuestos bioactivos; agregados de polen; abejas sin aguijón
Bees collect plant products that they use for their growth and development; among the most important products are nectar, pollen, resins, and, in some cases, scent of flowers required in courtship and mating (Borkraâ and Sulaiman, 2010; Dötterl and Vereecken, 2010; Villanueva-Gutiérrez et al., 2015). The plant-bee interaction leads in turn to the development of products with different physicochemical and sensory characteristics, as well as bioactive capacities, which depend on the plant species, nutritional conditions, geographical origin, and taxonomic group of the bee species. The species that have been studied the most are Apis mellifera (Apidae:Apinae) and meliponines (Apidae: Meliponini), which are stingless bees from tropics and subtropics (Ayala et al. 2013; Komosinska et al., 2015; Da Silva et al., 2016).
Since ancient times, beehive products of different species have been used as exchange material, divine offerings, and in the treatment of various diseases (Borkraâ and Sulaiman, 2010). Currently, because of their properties, beehive products have diverse uses, for example, in diabetic foot and wound treatment, as antioxidant and anti-inflammatory, antimicrobial activity, among others (Molan and Bets, 2004; Basim et al., 2006; Vit et al. 2008; Grajales-Conesa et al., 2018). On the other hand, recent studies with stingless bee products have shown a higher rate of epithelialization in wounds and greater anti-inflammatory and antimicrobial effects than those of European or honeybees (Rao et al., 2016). The antimicrobial activity has been evaluated in vitro against different medically important bacteria: Listeria monocytogenes, Escherichia coli, Salmonella enterica, Pseudomonas aeruginosa, Streptococcus pyogenes, Proteus mirabilis, Staphylococcus aureus, and different fungal species of the Candida genus (Cabrera and Montenegro 2013; Zainol et al., 2013; Vit et al., 2016). Methanolic and ethanolic extracts of pollen, as well as honey of A. mellifera and different stingless bee species, have shown an effective antimicrobial activity against fungi of medical and agricultural interest: Alternaria alternata, Botrytis cinerea, Fusarium oxysporum and C. gloeosporioides (Cabrera and Montenegro 2013; Albores-Flores et al., 2018). Of the beehive products, pollen is made up of by-products of the floral male gametangium collected by bees and has a high nutritional content, which is constituted by carbohydrates (13 and 55%), proteins (10 and 40%), lipids (1-10%) and row fiber (0.3 and 20%), as well as minerals, oligoelements, vitamins, carotenoids, phenolic compounds, flavonoids, sterols and terpenes (Bogdanov, 2004). Besides being a source of food for bees, the pollen aggregate is also important in the human diet (Pascoal et al., 2014).
In Soconusco, Chiapas, Mexico, meliponiculture is practiced in areas adjacent to crops, especially coffee (Coffea spp.), mango (Mangifera indica), rambutan (Nephelium lappaceum) and banana (Musa spp.). Several studies have been conducted to characterize the beehive products of the stingless bee species that are most widely bred in the region, such as: Melipona beecheii, M. solani, Scaptotrigona mexicana and Tetragonisca angustula (Grajales-Conesa et al., 2018; Espinoza-Toledo et al., 2018; Albores-Flores et al., 2018).
On the other hand, Colletotrichum gloeosporioides is the fungal species most frequently reported to cause anthracnose in fruit trees and vegetables, in the field and postharvest (Beltrán and García, 2006; Chacini et al., 2013). Despite the specific management practices used to prevent the incidence of this disease, which include application of contact and systemic fungicides, it has not been possible to reduce harvest and postharvest losses of more than 40 and 60%, respectively (Huerta et al., 2009; Trinidad-Ángel, 2017). Because of the tropical and subtropical conditions that prevail in Soconusco, the incidence of this fungus is frequent in crops, especially fruit crops (Huerta et al., 2009).
Based on the above, it is necessary to conduct studies to evaluate the biological effectiveness of new products to control C. gloeosporioides and which also provide an alternative for organic production systems. This study complements previous research on the antifungal effect using honey extracts of the stingless bee species M. beecheii, M. solani and S. mexicana collected in Soconusco (Albores-Flores et al. 2018). This research was focused on characterizing extracts of pollen aggregates at the physicochemical level and identifying potential bioactive compounds with antifungal effects on C. gloeosporioides, using material collected in commercial beehives of M. beecheii, S. mexicana and Tetragonisca angustula, a species whose effects on the fungus had not been previously studied.
MATERIALS AND METHODS
Samples of aggregates of pollen of stingless bees. Samples of pollen aggregates from commercial beehives of three stingless bee species (M. beecheii, S. mexicana and T. angustula) were collected from February 2017 to February 2018. The species were identified based on previous regional studies and using taxonomic information of the subfamily (Espinoza-Toledo et al., 2018; Ayala et al. 2013). The location of the beehives, number of samples per species and dates of collection are shown in Table 1. The pollen aggregate samples were collected using sterile forceps from rational boxes (bee boxes) and then placed in sterile plastic jars that were previously labeled with the corresponding information. The samples were concentrated and stored at -4 ºC at the Biosciences Institute of the Autonomous University of Chiapas, in Tapachula.
Physicochemical analysis. The samples were determined in triplicate using the methods described by the AOAC (2003) and the following parameters: humidity (method 969.38), ashes (945.38), pH (method 962.19) and free acidity (method 962.19). The determination of free acidity was carried out according to Bogdanov (2002) and AOAC (2003) by potentiometric titration with an alkaline buffer up to pH 8.5.
Extraction of total phenols. For this, the protocol of Carpes et al. (2007) with modifications was used. To prepare the pollen aggregates solution, 1 g of aggregate was mixed with 5 mL of methanol:water (1:1), pH 2. For flavonoids, 80% ethanol left to stand for 24 h was used.
Table 1 Stingless bee species, number, and location of commercial meliponine beekeepers, and plant composition that prevails in the area of Chiapas, Mexico, where beehives were sampled.
| Especie (número meliponarios) | Clave de Muestra | Establecimiento de la colmena | Fecha de colecta de polen | Tipo agroecológico (predominante) |
|---|---|---|---|---|
| Tetragonisca angustula | TA1 | Mazatán | Febrero 2017 | Frutales (mango) |
| TA2 | Rancho San Juan, Mazatán | Abril 2017 | ||
| (n=3) | TA3 | Rancho San Juan, Mazatán | Junio 2017 | |
| Scaptotrigona mexicana | SM1 | Cacahoatán | Mayo 2017 | Frutales (rambután) |
| SM2 | Tapachula | Mayo 2017 | ||
| (n=6) | SM3-SM6 | Mazatán | Agosto 2017 | |
| Melipona beecheii | MB1-MB3 | Estancia Agroecológica “Ayol” | Enero-Febrero 2018 | Agrícola (temporal) |
| (n=3) |
Analysis of phenols content. It was determined following the method of Restrepo et al. (2009) with the Folin Ciocalteu reagent. From the extract of pollen aggregates, 1 mL was taken to prepare concentrations at 25, 50, 75 and 100%. Each concentration was mixed with 500 μL of Folin-Ciocalteu reagent diluted with water 1/10, to which 400 μL of Na2CO3 were added. The mixture was kept in total darkness for 15 min to promote the reaction. Later, the absorbance was recorded at 765 nm. A mixture of methanol:water (1:1) was used as blank (control). The calibration curve to estimate the polyphenols content was created with a solution at concentrations of 0, 50, 100, 150, 200, 250 mg L-1 in a methanol:water solution (1:1). The results of the total polyphenols content were expressed in equivalent mg of gallic acid per each 1 g of pollen aggregate.
Analysis of flavonoids content. It was determined using the technique of Restrepo et al. (2009). For this, quercetin dihydrate was used as a standard (1 mg mL-1). The calibration curve was built with a quercetin/methanol solution at different concentrations: 0, 0.002, 0.004, 0.006, 0.008, 0.01 mg mL-1. Afterwards, potassium acetate and aluminum trichloride were added, and the solution was kept in total darkness for 30 min. The absorbance was recorded at 415 nm.
Antioxidant capacity. To quantify the antioxidant activity, a discoloration of the radical 2.2´-azino-bis (3-ethylbenzothiazolin-6-sulfonic acid) was carried out following the ABTS methodology (Overveld et al., 2000). The extracts obtained from pollen aggregates, at concentrations of 25, 50, 75 and 100%, were added with 20 mL of ABTS (2 mM) and 80 μL of potassium persulfate 70 mM. They were left to stand in darkness at 25 ºC for 16 h to produce the ABTS+ radical. The solution absorbance (ʎ = 734 nm) was adjusted to 0.800 ± 0.03 absorbance units in a spectrophotometer (Thermo Scientific Model 4001/4), using a 0.01 M phosphate buffer (Na2PHO4+KH2PO4+NaCl+KCl in 1000 mL of distilled water; pH 7.4 adjusted with NaOH). To obtain the calibration curve, 900 μL of the diluted solution of ABTS+ and 10 μL of Trolox solution (6-hydroxy-2, 5, 7, 8-tetramethylchrome-2-carboxilic acid) were added at different concentrations (0-400 µM in 80% v/v methanol). After 6 min standing, the absorbance was recorded at 734 nm using 80% v/v methanol as blank. The results were expressed as the antioxidant capacity equivalent to Trolox per g of pollen extract.
Antifungal activity. The C. gloeosporioides strain, belonging to the collection of the Biosciences Institute, isolated from Carica papaya fruits, and morphologically identified by Víctor Albores (unpublished data), was reactivated in potato-dextrose-agar (PDA) nutrient medium. The antifungal activity was evaluated by mixing methanolic extracts of the collected pollen aggregates of each bee species with PDA in vitro. The treatments, in triplicate, consisted of mixtures of 100 μL of extracts at 25, 50, 75 and 100% with 20 mL of PDA that was previously prepared. After sowing the strain in 0.5 cm colonial disks in the middle of 52 mm Petri dishes, it was incubated at 32 °C in a stove for 12 days. Mycelial growth was measured every 24 h with a Vernier caliper 0-150 nm capacity for nine days. The C. gloeosporioides colony growth in PDA under the same experimental conditions was used as the absolute control. The inhibition relative controls, in triplicate, were: 1) methanol/water, 2) chlorothalonil at 21 mg mL-1, and 3) chlorothalonil at 56 mg mL-1; the last two according to commercial doses applied to C. papaya crops by the Asociación de Fruticultores del Soconusco, Chiapas.
Rate of absolute growth. It was determined using the following equation: μ = (Db-Da) / (tb - ta). Where: Μ is the rate of the colony growth (mm day-1); Db is the diameter of the colony (mm) in time b; Da corresponds to the diameter of the colony in time a; and tb and ta is the time of absolute growth between two evaluations. For the comparative calculation of C. gloeosporioides growth in the four extracts (1-4) with respect to the relative controls (1-3), the following equation was used: DFC = [(D relative control1-3 - D extract1-4) / (D relative control1-3) ] x 100. Where, DFC is the difference in the diameter of the colony (mm) divided by D relative control1-3 and D extract of the pollen aggregate1-4 expressed as percent.
Analysis of data. The data of all the physicochemical variables were subjected to a multivariate analysis of variance (MANOVA), considering each species-collection as a treatment. The media comparison was carried out using Hotelling’s test (α=0.05). To determine the separation or groups of collections and species, a linear discriminant analysis (LDA) was performed. A principal component analysis (PCA) was carried out to explain the relative weight of the physicochemical variables in the composition of the pollen aggregates within and among species. The relationship of the mycelial growth with phenols, flavonoids, Trolox and the antioxidant activity was estimated using r-Spearman. An ANOVA analysis by bee species and growth evaluation date was conducted in order to compare the effect of the four concentrations of extracts of pollen aggregates with the relative controls. All the analyses were conducted in INFOSTAT.
RESULTS
The result of the physicochemical analysis of pollen aggregates collected by M. beecheii, S. mexicana and T. angustula is shown in Table 2. Overall, the humidity values ranged from 12.2 to 31.4%, ashes from 0.9 to 5.9%, pH from 2.2 to 3.5, free acidity from 117 to 233.4 meq Kg-1, phenols from 1.2 to 2.6 mg EAG g-1, flavonoids from 0.9 to 3.1 µg EQ g-1, Trolox from 4.3 a 8.1 mg, Trolox g-1, and the concentration of glucose from 0.01 a 0.3 mg L-1. The multivariate analysis was significant on bee species in the physicochemical content (p=0.0001). All the species-collection combinations were statistically different (p=0.05); M. beecheii had the highest values, while T. angustula had the lowest. The amount of phenols and flavonoids were positively related to the antifungal activity (p≤0.02), while the antioxidant capacity (Trolox) had a negative r-Spearman (-0.41 a-0.61) (Table 3). In most of the extracts associated with S. mexicana and M. beecheii species, the antioxidant activity had a negative correlation with the antifungal activity.
The discriminant analysis explained 91.1% of variance in the first two components, CAN 1 (80%) and CAN 2 (11.1%). The greatest weight in CAN 1 corresponded to phenols, flavonoids, and free acidity. The formation of groups clearly included the three replications of each species-collection but there was significant variation between intra and inter-species collections. According to MANOVA, the collections from M. beecheii beehives were separated from the other collections and distributed across the positive dimension of CAN 1. The other two species-collections were distributed across CAN 2, which had the greatest weight of pH, ashes, and glucose; S. mexicana had the highest dispersion (Figure 1A).
The analysis of main components explained 58% of the multivariate variance (CP1=43.5; CP2=14.8%) in the two first components. CP1 was mainly explained by flavonoids, pH, and free acidity, while CP2 was determined by ashes and glucose. Phenols had similar weight in both components (Figure 1B). The projection of the collections in this multivariate space unmarked M. beecheii samples from beehives established in an agroecological area where annual agricultural crops prevail (MB3 and MB2). The remaining collections had low dispersion regarding low values of CP1 and CP2.
Table 2 Physicochemical values of pollen collected by Melipona beecheii (Mb), Scaptotrigona mexicana (Sm) and Tetragonisca angustula (Tar) in different regions of Soconusco, Chiapas.
| Variables fisicoquímicas | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clave de muestra | pH | Humedad (%) | Cenizas (%) | Acidez Libre (meq kg-1) | Trolox (mg g-1) | Fenoles (mg EAG g-1) | Flavonoides (mg EQ g-1) | Glucosa (mg L-1) | Prueba hotelling |
| MB1 | 2.2 | 31.4 | 0.9 | 127.1 | 6.8 | 2.2 | 1.8 | 0.04 | C |
| MB2 | 2.6 | 19.6 | 1.1 | 233.3 | 5.8 | 2.6 | 2.9 | 0.1 | B |
| MB3 | 2.5 | 12.2 | 3.4 | 206.2 | 6.4 | 2.5 | 3.1 | 0.01 | A |
| TA1 | 3.3 | 21.1 | 2.5 | 126.6 | 7.9 | 1.9 | 1.3 | 0.2 | J |
| TA2 | 3.4 | 24.7 | 4.1 | 146.4 | 7.4 | 1.8 | 1.7 | 0.2 | K |
| TA3 | 3.3 | 26.2 | 3.9 | 133.2 | 8.0 | 2.2 | 1.6 | 0.3 | L |
| SM1 | 3.3 | 30.6 | 1.8 | 142.8 | 7.4 | 1.2 | 1.5 | 0.1 | F |
| SM2 | 3.3 | 27.0 | 2.1 | 159.9 | 6.8 | 1.5 | 1.2 | 0.01 | E |
| SM3 | 3.5 | 12.2 | 2.4 | 146.4 | 8.1 | 1.4 | 1.3 | 0.02 | D |
| SM4 | 3.3 | 23.7 | 2.8 | 117.9 | 4.3 | 1.3 | 1.0 | 0.3 | I |
| SM5 | 3.5 | 27.5 | 5.9 | 183.1 | 5.3 | 2.1 | 1.1 | 0.06 | H |
| SM6 | 3.5 | 24.3 | 3.4 | 129.9 | 5.5 | 2.0 | 0.9 | 0.03 | G |
xThe reference information of each sample of pollen extract is described in Table 1.
Antifungal activity. The three bee species and four methanolic extracts that were evaluated showed C. gloeosporioides inhibition compared to the controls. From day three of incubation and up to the end, at day nine, statistical differences were observed in the fungus mycelial growth compared to the methanol-water (p<0.0001) (Table 4) control. From day four onwards, the concentrations at 25, 50, 75 and 100% started to differentiate from the same control. The differences ranged from 20 to 64% depending on the growth time rather than the species (Figure 2). From day five or six, the concentrations at 75 and 100% exceeded or equaled the inhibitory effect of chlorothalonil at its highest concentration (56 mg mL-1). The S. mexicana extracts at 50% were the only ones that also exceeded the inhibition caused by the fungicide at that concentration. The T. angustula extracts had the lowest effect compared to chlorothalonil because only at a concentration of 100% exceeded the highest doses (Figure 2). The collection-species had an effect on the fungus inhibitory capacity. For example, M. beecheii MB1 collection had a higher effect than MB1 and MB2 (Figure 3). When the final mycelial growth of the relative control was compared to that of the absolute control (5.1 cm), it was found that methanol/water (3.8 cm) reduced the fungus growth by 25.4% and chlorothalonil by 35.3% (3.3 cm, 21 mg mL-1) and 58.8% (2.1cm, 56 mg mL-1). Overall, the rate of C. gloeosporioides mycelial growth ranged from 0.027-0.041, 0.022-0.037, 0.012-0.025, and 0.009-0.013 mm h-1 at concentrations of 25, 50, 75 and 100%, respectively.
Table 3 Values of the correlation coefficient among the concentration of phenols, flavonoids and Trolox with the antifungal activity of pollen extracts from each bee species.
| Clave de Muestra | Fenoles | Flavonoides | Trolox | |||
|---|---|---|---|---|---|---|
| Spearman | Valor p | Spearman | Valor p | Spearman | Valor p | |
| MB1 | 0.82 | <0.0001 | 0.77 | 0.0018 | -0.51 | 0.0154 |
| MB2 | 0.86 | <0.0001 | 0.81 | 0.0001 | -0.46 | 0.0525 |
| MB3 | 0.86 | <0.0001 | 0.80 | 0.0001 | -0.51 | 0.0154 |
| TA1 | 0.74 | 0.0012 | 0.76 | 0.0018 | -0.44 | 0.0722 |
| TA2 | 0.74 | 0.0012 | 0.72 | 0.0032 | -0.42 | 0.0915 |
| TA3 | 0.78 | 0.0001 | 0.70 | 0.0040 | -0.41 | 0.1458 |
| SM1 | 0.80 | <0.0001 | 0.70 | 0.0040 | -0.44 | 0.0722 |
| SM2 | 0.80 | <0.0001 | 0.70 | 0.0040 | -0.51 | 0.0154 |
| SM3 | 0.81 | <0.0001 | 0.66 | 0.0051 | -0.42 | 0.0915 |
| SM4 | 0.88 | <0.0001 | 0.62 | 0.0073 | -0.61 | 0.0003 |
| SM5 | 0.91 | <0.0001 | 0.62 | 0.0073 | -0.55 | 0.0083 |
| SM6 | 0.88 | <0.0001 | 0.58 | 0.0241 | -0.56 | 0.0083 |
yThe reference information of each pollen extract sample is described in Table 1.
DISCUSSION
The physicochemical composition of the pollen aggregates collected from commercial stingless bee beehives was heterogeneous at intra- and inter-species level because of the bees’ territorial exploration capacity and pollen collection from different plant species (Table 1). However, it was possible to classify the species in groups, observing that M. beecheii was segregated from the other species. The species effect can be associated with vegetal exploitation niches in order to prevent interspecific competence more than some metabolic effect. Previous reports indicate that this is a single-flower species which prefer fabaceae 45% in contrast with M. solani and S. mexicana (Espinoza-Toledo et al., 2018). The physicochemical composition at the pollen aggregates level has an implication in its quality, and nutritional, therapeutic, antioxidant and antimicrobial value (Mărgăoan et al., 2010; Saavedra et al., 2013). Physicochemical heterogeneity has also been reported in studies about honey produced by meliponines in Soconusco (Espinoza-Toledo et al., 2018), and the value of these attributes for ecological purposes has been recognized (Vit, 2008).The correlation from the physicochemical variables with the antioxidant capacity of the extracts of pollen aggregates suggests that phenols, flavonoids and free acidity exert a higher inhibitory action on C. gloesporioides mycelial growth (Table 3) (Mărgăoan et al., 2010). This could be associated with the type of phenolic compounds extracted from pollen aggregates, which do not have the property of donating donate electrons and are acidic and prevent a reaction with the ABTS oxidized radical. This compound acts on polyphenols which donate hydrogen atoms, while the ferric complex in FRAP acts on polyphenols that can donate electrons (Schaich et al., 2015). It was not possible to state that the oxidizing capacity observed in the samples of extracts of stingless bee pollen aggregate was caused by a component in particular. The antioxidant capacity is the result of the interaction between molecules that make up each sample (Paulino-Zunini et al., 2010). Based on this, the low relationship obtained with the antifungal activity would be defined by diverse factors, such as the chemical composition of the sample, geographical region and plant from which the pollen was taken (Bertrams et al., 2013; Duran et al., 2011).
Table 4 Difference values in (DFC) Colletotrichum gloeosporioides colony diameter (expressed as percent values) among the pollen extracts collected by bees and the control treatments (chlorothalonil=Clor). (They are placed only at the concentrations where the pollen extracts of each bee species whose colony size is smaller than most of the control treatments, and the positive values indicate that the colony diameter with the extracts was smaller than that of the control).
| Clave de muestra | Extracto | Metanol/agua (E-a) | Clor (21 mg mL-1)(E-b) | Clor(56 mg mL-1 (E-c) | Clave de muestra | Extracto | Metanol/agua (E-a) | Clor (21 mg mL-1) (E-b) | Clor (56 mg mL-1) (E-c) |
|---|---|---|---|---|---|---|---|---|---|
| MB1 | 75 | 51.9 | 40.4 | 14.4 | SM1 | 75 | 48.1 | 35.7 | 7.6 |
| 100 | 65.5 | 56.5 | 37.5 | 100 | 57.0 | 45.8 | 22.1 | ||
| MB2 | 75 | 60.9 | 51.5 | 30.3 | SM2 | 75 | 46.1 | 33.3 | 4.0 |
| 100 | 65.6 | 56.6 | 37.6 | 100 | 57.7 | 46.6 | 23.3 | ||
| MB3 | 75 | 58.2 | 48.2 | 25.6 | SM3 | 75 | 49.4 | 37.3 | 9.8 |
| 100 | 64.2 | 54.9 | 35.1 | 100 | 60.3 | 49.9 | 28.1 | ||
| TA1 | 75 | 42.8 | 29.1 | -1.9 | SM4 | 75 | 53.3 | 42.1 | 16.8 |
| 100 | 54.7 | 42.9 | 17.9 | 100 | 62.4 | 52.7 | 32.0 | ||
| TA2 | 75 | 43.5 | 29.9 | -0.7 | SM5 | 75 | 54.2 | 43.2 | 18.4 |
| 100 | 53.1 | 40.8 | 14.9 | 100 | 62.8 | 53.1 | 32.6 | ||
| TA3 | 75 | 39.7 | 25.2 | -7.5 | SM6 | 75 | 56.2 | 45.7 | 22.0 |
| 100 | 53.1 | 40.8 | 14.9 | 100 | 61.8 | 51.8 | 30.7 |
zThe reference information of each pollen extract sample is described in Table 1.
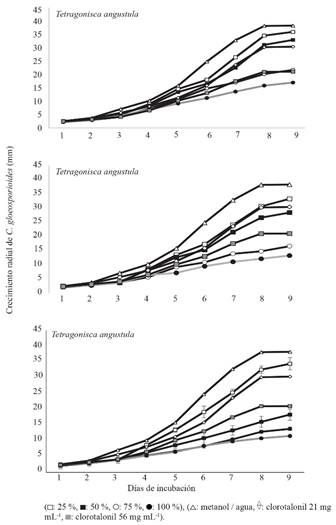
Figure 2 Average values of Colletotrichum gloeosporoides growth in vitro in the presence of methanolic extract at different concentrations of pollen aggregates of Melipona beecheii (Mb), Scaptotrigona mexicana (Sm) and Tetragonisca angustula (Ta).

Figure 3 Colletotrichum gloeosporoides growth in vitro in the presence of methanolic extract of Melipona beecheii (MB100) (100%) pollen aggregate, as well as the controls methanol/water (M-A) and chlorothalonil 56 mg mL -1 (Cl56).
The lowest relationship between the antioxidant and antifungal capacity in the extracts of T. angustula pollen aggregates could be caused by the sample composition (Mărghitaş et al., 2009). On the other hand, it could also be due to a methodological effect, since, according to the solvent, it is possible to determine what molecule was extracted (Muñoz et al., 2015). The inhibitory effect of the extracts of pollen aggregates is consistent with other reports where it is recognized that the type of biomolecule and its bioactivity can vary making it possible to differentiate regions and taxa, but implying the sample effect (Pellati et al., 2011; Li et al., 2011; Ruíz-Montañez et al., 2014).
The extracts of pollen aggregates showed a clear inhibitory effect on C. gloeosporioides compared to chlorothalonil commercial fungicide. The inhibitory action in each extract of pollen aggregate was associated with the extracting action of the solvent and the chemical composition of the pollen samples. The relatively high concentration of phenolic compounds, which may include terpenoids, phenylpropanoids, stilbenes and saponins (Soto and Rosales, 2016), can be involved in the antifungal activity. The hydrophilic extracts of plant cells are strongly related to Alternaria alternata and Fusarium oxysporum cell growth inhibition and conidia germination and can also be toxic in microbial respiration processes (Rodríguez-Maturino et al., 2015). 60% of hydrophilic extracts contain a large amount of flavonoids and phenols (Pietarinen et al., 2006; Okwu and Nnamdi, 2008).
The antifungal property of the pollen aggregates collected by A. mellifera has been reported to inhibit up to 70% of mycelial growth in Aspergillus niger and 99.9% in A. fumigatus (Kacaniova et al., 2012). Similar effects have been reported in Alternaria, Botrytis and Fusarium (Cabrera and Montenegro, 2013). Regarding antifungal properties of pollen aggregates associated with stingless bees, there are reports of T. angustula and 26% inhibition in Candida albicans (Rojas, 2015). In Soconusco, previous studies using meliponines honey showed 40% more effectiveness than chlorothalonil fungicide in C. gloesporioides (Albores-Flores et al., 2018). In contrast, these results, using extracts of pollen aggregates collected by the same species (M. beecheii, S. mexicana), had a higher effect up to 50% than the fungicide in high commercial doses (56 mg mL-1), depending on the extract concentration. This study provides the first report of the antifungal properties of meliponines pollen aggregates. In particular, for Soconusco, Chiapas, stingless bees are an alternative for pollination of different economically important crops, besides honey, pollen aggregates and propolis are medical alternatives for treating diabetic ulcers, and are also used in fruit crops pre- and postharvest processes (Espinoza-Toledo et al., 2018; Albores-Flores et al., 2018, Grajales-Conesa et al., 2018).
CONCLUSIONS
In the pollen aggregates samples, the values of each of the physicochemical properties that were evaluated ranged as follows: pH: 2.2 to 3.5, humidity: 2 to 31 %, ashes: 0.9 a 5.9 %, free acidity: 117 to 133 meq kg-1, phenols: 1.4 to 2.6 mg EAG g-1, flavonoids: 0.9 to 3.1 mg EQ g-1, glucose: 0.01 to 0.3 mg L-1 and Trolox: 4.3 to 8.0 mg g-1. The highest values of pH, ashes, glucose and Trolox corresponded to S. mexicana and T. angustula species. The highest values of free acidity, phenols and flavonoids corresponded to M. beecheii.
The bioactive compounds of the pollen aggregates involved in the antifungal action were free acidity, phenols, and flavonoids. The inhibition values reached by M. beecheii were 38-66% higher in C. gloeosporioides than those of chlorothalonil.
LITERATURA CITADA
Albores-Flores V, Marín SIM, López-García JA, Sánchez GA y Grajales-Conesa J. 2018. Propiedad antifúngica de mieles sobre el desarrollo in vitro de Colletotrichum gloeosporioides. Revista Mexicana de Fitopatología 36(3): 423-431. http://doi.org/10.18781/R.MEX.FIT.1805-3 [ Links ]
Ayala R, González VH and Engel MS. 2013. Mexican stingless bees (Hymenoptera:Apidae): diversity, distribution, and indigenous Knowledge. In: Vit P, Pedro SR and Roubik D (eds.). Honey-Pot: A legacy of stingless bees. New York: Springer. 366p. http://doi.org/10.1007/978-1-4614-4960-7_9 [ Links ]
AOAC (Association of Official Analytical Chemists). 2003. Methods of analysis. Washington, D.C. AOAC. https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55))/reference/ReferencesPapers.aspx?ReferenceID=1994521 [ Links ]
Basim E, Basim H and Özcan M. 2006. Antibacterial activities of Turkish pollen and propolis extracts against plant bacterial pathogens. Journal of Food Engineering 77(4): 992-996. http://dx.doi.org/10.1016/j.jfoodeng.2005.08.027 [ Links ]
Beltrán CMC y García JDJ. 2006. Colletotrichum gloeosporioides fitopatógeno asociado a la nutrición humana. Investigaciones Andinas 8(13): 73-80. https://www.redalyc.org/articulo.oa?id=239017515006 [ Links ]
Bertrams J, Müller M, Kunz N, Kammerer D and Stintzing FC. 2013. Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. Journal of Applied Botany and Food Quality 86(1):143-153. http://dx.doi.org/10.5073/JABFQ.2013.086.020 [ Links ]
Bogdanov S. 2004. Quality and standards of pollen and beeswax. Apiacta 38: 334-341. http://www.researchgate.net/publication/229041189_Quality_and_standards_of_pollen_and_beeswax [ Links ]
Bogdanov S. 2002. Harmonized Methods of the International Honey Commission. International Honey Commission. http://www.researchgate.net/publication/285841406_Harmonised_methods_of_the_International_Honey_Commission [ Links ]
Borkraâ L and Sulaiman S. 2010. Rediscovering the antibiotics of the hive. Recent patents on anti-effective drug discovery 4(3):2006-2013. http://dx.doi.org/10.2174/157489109789318505 [ Links ]
Cabrera C and Montenegro G. 2013. Pathogen control using a natural Chilean bee pollen extract of known botanical origin. Ciencia e Investigación Agraria 40(1): 223-230. http://dx.doi.org/10.4067/S0718-16202013000100020. [ Links ]
Carpes S, Begnini R, de Alencar S and Masson M. 2007. Study of preparations of bee pollen extracts, antioxidant and antibacterial activity. Ciência e Agrotecnologia 31(6): 1818-1825. http://www.scielo.br/pdf/cagro/v31n6/a32v31n6.pdf [ Links ]
Chacini C, Blanco M, Sanchez S y Acevedo I. 2013. Evaluación a nivel de laboratorio del efecto de 7 extractos vegetales para el control de Colletotrichum sp. agente causal de la antracnosis en el cultivo de tomate de árbol. Innovaciencia 1(1): 30-35. https://doi.org/10.15649/2346075X.214 [ Links ]
Da Silva PM, Gauche C, Gonzaga LV, Costa ACO and Fett R. 2016. Honey: Chemical composition, stability and authenticity. Food Chemistry 196: 309-323. http://dx.doi.org/10.1016/j.foodchem.2015.09.051 [ Links ]
Dötterl S and Vereecken N. 2010. The chemical ecology and evolution of bee-flower interactions: a review and perspectives. Canadian Journal of Zoology 88(7): 668-697. http://dx.doi.org/10.1139/Z10-031 [ Links ]
Duran N, Muz M, Culha G, Duran G and Ozer B. 2011. GC-MS analysis and antileishmanial activities of two Turkish propolis types. Parasitology Research 108(1):95-105. http://dx.doi.org/10.1007/s00436-010-2039-z [ Links ]
Espinoza-Toledo C, Vázquez-Ovando A, Torres de los Santos R, López García A, Albores-Flores V and Grajales-Conesa J. 2018. Stingless bee honeys from Soconusco, Chiapas: a complementary approach. Revista de Biología Tropical 66(4): 1536-1546. http:77dx.dor.org/10.15517/RBT.V66I4.32181 [ Links ]
Grajales-Conesa J, Ibarias TC, Ruíz TJ y Sánchez D. 2018. Mieles de abejas sin aguijón en el tratamiento de úlceras de pie diabético. Salud Pública de México 60:102-104. http://doi.org/10.21149/8604 [ Links ]
Huerta G, Holguin F, Benítez C y Toledo J. 2009. Epidemiología de la antracnosis (Colletotrichum gloeosporioides (Penz) Penz and Sacc) en mango (Mangifera indica L.) CV. Ataulfo en el Soconusco, Chiapas, México. Revista Mexicana de Fitopatología 27: 93-105. http://www.scielo.org.mx/pdf/rmfi/v27n2/v27n2a2.pdf [ Links ]
Kacaniova M, Vuković N, Chlebo R, Haščík P, Rovná K, Cubon J and Pasternakiewicz A. 2012. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Archives of Biological Sciences 64(3): 927-934. http://doi.org/10.2298/ABS1203927K [ Links ]
Komosinska VK, Olczyk P, Kaźmierczak J, Mencner L and Olczyk K. 2015. Bee pollen: chemical composition and therapeutic application. Evidence-Based Complementary and Alternative Medicine 2015: 1-6. http://dx.doi.org/10.1155/2015/297425 [ Links ]
Li Y, Skouroumounis GK, Elsey GM and Taylor DK. 2011. Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chemistry 129(2):570-6. http://doi.org/10.1016/j.foodchem.2011.04.068. [ Links ]
Mărgăoan R, Mărghitaş L, Dezmirean D, Mihai CM and Bobiş O. 2010. Bee collected pollen - general aspects and chemical composition. Bull UASVM Animal Science and Biotecnologies 67(1-2): 254-259. file:///C:/Users/RMF/Downloads/5305-19198-1-PB.pdf [ Links ]
Mărghitaş LA, Stanciu OG, Dezmirean DS, Bobiş O, Popescu O, Bogdanov S and Campos MG. 2009. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chemistry 115(3): 878-83. http://doi.org/10.1016/j.foodchem.2009.01.014. [ Links ]
Molan P and Betts N. 2004. Clinical usage of honey as a wound dressing: an update. Journal of Wound Care 13: 353-356. https://doi.org/10.12968/jowc.2004.13.9.26708 [ Links ]
Muñoz CW, Chavez RW, Pabón LC, Rendón FMR, Patricia CM y Otálvaro AAM. 2015. Extracción de compuestos fenólicos con actividad antioxidante a partir de Champa (Campomanesia lineatifolia) Revista CENIC Ciencias Químicas 46: 38-46. http://www.redalyc.org/pdf/1816/181643224027.pdf [ Links ]
Okwu DE and Nnamdi FU. 2008. Evaluation of the chemical composition of Dacryodes edulis and Raphia hookeri Mann and wendl exudates used in herbal madicine in South Eastern Nigeria. African Journal of Traditional, Complementary and Alternative Medicines 5(2): 194-200. http://doi.org/10.4314/ajtcam.v5i2.31273 [ Links ]
Overveld FWPC, Haenen GRMM, Rhemrev J, Vermeiden JPW and Bast A. 2000. Tyrosine as important contributor to the antioxidant capacity of seminal plasma. Chemico-Biological Interactions 127(2): 151-161. http://doi.org/10.1016/S0009-2797(00)00179-4 [ Links ]
Pascoal A, Rodriges S, Teixeira A, Feás X and Estevinho L. 2014. Biological activities of commercial bee pollens: antimicrobial, antimutagenic, antioxidant y antiinflamatorio. Food and Chemical Toxicology 63: 633-639. http://dx.doi.org/10.1016/j.fct.2013.11.010 [ Links ]
Paulino-Zunini M, Rojas C, De Paula S, Elingold I, Alvareda ME, Casanova MB, Iribarne RF, Aguilera MS and Dubin M. 2010. Phenolic contents and antioxidant activity in central-southern Uruguayan propolis extracts. Journal of the Chilean Chemical Society 55(1):141-146. http://dx.doi.org/10.4067/S0717-97072010000100033 [ Links ]
Pellati F, Orlandini G, Pinetti D and Benvenuti S. 2011. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. Journal of Pharmaceutical and Biomedical Analysis 55(5): 934-48. http://doi.org/10.1016/j.jpba.2011.03.024 [ Links ]
Pietarinen SP, Willfor SM, Vikstrom FA and Holmbom BR. 2006. Aspen knots, a rich source of flavonids. Journal of Wood Chemistry and Technology 26(3): 245-258. http://doi.org/10.1080/02773810601023487 [ Links ]
Rao PV, Krishnan KT, Salleh N and Gan SH. 2016. Biological and therapeutic effects of honey produced by honey bees and stingless bees: a comparative review. Revista Brasileira de Farmacognosia 26: 657-664. http://dx.doi.org/10.1016/j.bjp.2016.01.012. [ Links ]
Restrepo SDC, Narváez CCE and Restrepo SPL. 2009. Extracción de compuestos con actividad antioxidante de frutos de guayaba cultivada en Vélez Santander, Colombia. Química Nova 32: 1517-1522. http://www.scielo.br/pdf/qn/v32n6/30.pdf [ Links ]
Rodríguez-Maturino A R, Troncoso-Rojas R, Sánchez-Estrada A, González-Mendoza D, Ruíz-Sánchez E, Zamora-Bustillos R, Cece na-Duran, C, Grimaldo-Juarez O, Avilez-Marin M. 2015. Efecto antifúngico de extractos fenólicos y de carotenoides de chiltepín (Capsicum annum var. glabriusculum) en Alternaria alternata y Fusarium oxysporum. Revista Argentina de microbiología 47(1): 72-77. https://doi.org/10.1016/j.ram.2014.12.005 [ Links ]
Rojas YPM. 2015. Valoración in vitro del potencial antimicrobiano de extractos etanólicos de polen de Apis mellifera y de Tetragonisca angustula, en busca de posibles usos terapéuticos. Tesis magister. Universidad Nacional de Colombia 83 p. http://bdigital.unal.edu.co/51696/1/yurleypaolamonserraterojas.2015.pdf [ Links ]
Ruíz-Montañez G, Ragazo SJ, Calderón SM, Velázquez de la CG, Ramírez de LJ and Navarro OA. 2014. Evaluation of extraction methods for preparative scale obtention of mangiferin and lupeol from mango peles (Mangifera indica L.). Food Chemistry 159: 267-272. http://doi.org/10.1016/j.foodchem.2014.03.009 [ Links ]
Saavedra CKI, Rojas IC y Delgado PGE. 2013. Características polínicas y composición química del polen apícola colectado de Cayaltí (Lambayeque-Perú). Revista Chilena de Nutrición 40: 71-78. http://dx.doi.org/10.4067/S0717-75182013000100011. [ Links ]
Schaich K, Tian X and Xie J. 2015. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. Journal of Functional Foods 14: 111-25. http://doi.org/10.1016/j.jff.2015.01.043 [ Links ]
Soto GM y Rosales CM. 2016. Efecto del solvente y de la relación masa / solvente, sobre la extracción de compuestos fenólicos y la capacidad antioxidante de extractos de corteza de Pinus durangensis y Quercus sideroxyla. Maderas, Ciencia y Tecnología 18(4): 701 - 714. http://dx.doi.org/10.4067/S0718-221X2016005000061 [ Links ]
Trinidad-Ángel E, Ascencio VFJ, Ulloa JA, Ramírez RJC, Ragazzo SJA, Calderon SM y Bautista RPU. 2017. Identificación y caracterización de Colletotrichum spp. Causante de antracnosis en aguacate Nayarit, México. Revista Mexicana de Ciencias Agrícolas 19: 3953-3964. http://www.scielo.org.mx/pdf/remexca/v8nspe19/2007-0934-remexca-8-spe19-3953-en.pdf [ Links ]
Vit P, Gutiérrez MG, Titera D, Bednar M y Rodríguez-Malaver AJ. 2008. Mieles checas categorizadas según su actividad antioxidante. Acta Bioquímica Clínica Latinoamericana 42(2): 237-244. http://www.redalyc.org/pdf/535/53542209.pdf [ Links ]
Vit P. 2008 Review: valorization honey of stingless bees (Meliponini). Brazilian Journal of Pharmaceutical Science 50: 20-28. [ Links ]
Vit P, Santiago B, Silva P, Ruíz J, Maza F, Peña M and Pérez E. 2016. Chemical and bioactive characterization of pot-pollen produced by Melipona and Scaptotrigona stingless bees from Paria Grande, Amazonas State, Venezuela. Emirates Journal of Food and Agriculture 28(2): 78-84. http://doi.org/10.9755/ejfa.2015-05-245 [ Links ]
Villanueva-Gutiérrez R, Roubik D and Porter-Bolland L. 2015. Bee-Plant interactions: Competition and phenology of flowers visited by bees. In: Islebe G, Calmé S, León-Cortés J, Schmook B. (eds). Biodiversity and conservation of the Yucatán Península. Springer, Cham. https://doi.org/10.1007/978-3-319-06529-8_6 [ Links ]
Zainol M, Mohd K and Mohd Y. 2013. Antibacterial activity of selected Malaysian honey. BMC complementary and alternative Medicine 13(129): 1-10. http://doi.org/10.1186/1472-6882-13-129. [ Links ]
Received: September 29, 2020; Accepted: November 19, 2020











 texto em
texto em 



