Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.38 no.3 Texcoco Set. 2020 Epub 27-Nov-2020
https://doi.org/10.18781/r.mex.fit.2005-7
Phytopathological Notes
Characterization of strawberry associated fungi and in vitro antagonistic effect of Trichoderma harzianum
1 Centro de Agroecología, Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla, San Pedro Zacachimalpa, 72960, Puebla, México;
2 Producción de Semillas, Colegio de Postgraduados, Campus Montecillo, Texcoco, Estado de México, México;
3 Jardín Botánico Universitario, Benemérita Universidad Autónoma de Puebla Colonia San Manuel, 72590 Puebla, Puebla. México.
Mexico is the third largest strawberry producer in the world, where the production of this crop is of economic importance and generates foreign exchange in the country. The objective of the present investigation was to identify and morphologically characterize the fungi associated with a commercial strawberry field, as well as to determine the in vitro antagonistic capacity of the T-H4 strain of Trichoderma harzianum in isolated fungi. Samples of plants from the strawberry crop with fungal symptoms were collected, sown in PDA medium and monosporic cultures were generated for their morphological characterization. The identified fungi and the strain T-H4 were compared in a dual way. Were identified and characterized three fungi associated with the fruit (A. niger, Colletotrichum sp. and R. stolonifer), three on leaves and stem (Pestalotiopsis sp., Curvularia sp. and Alternaria sp.) and two associated fungi on the root (Rhizoctonia sp. and Fusarium sp.). The T-H4 strain presented an antagonistic level suitable for Colletotrichum sp., Pestalotiopsis sp., Alternaria sp., Rhizoctonia sp. and Curvularia sp., in vitro. It is suggested to carry out biological control evaluations with these isolates in the greenhouse and in the open field, as well as to determine their pathogenicity.
Key words: Antagonist; taxonomic identification; mycelial growth; inhibition.
México es el tercer productor de fresa a nivel mundial, donde la producción de este cultivo es de importancia económica y generación de divisas en el pais. El objetivo de la presente investigación fue identificar y caracterizar morfológicamente los hongos asociados a enfermedades en un cultivo de la fresa, así como determinar la capacidad antagónica in vitro de la cepa T-H4 de Trichoderma harzianum con los hongos identificados. Se colectaron muestras de plantas del cultivo de fresa con síntomas de enfermedades fúngicas, se sembraron en medio PDA y se generaron cultivos monospóricos para su caracterización morfológica. Los hongos identificados y la cepa T-H4 se confrontaron mediante cultivos duales. Se identificaron tres hongos asociados al fruto (A. niger, Colletotrichum sp. y R. stolonifer), tres en hojas y tallo (Pestalotiopsis sp., Curvularia sp. y Alternaria sp.) y dos hongos asociados a la raíz (Rhizoctonia sp. y Fusarium sp.). La cepa T-H4 presentó un nivel antagónico adecuado para Colletotrichum sp., Pestalotiopsis sp., Alternaria sp., Rhizoctonia sp. y Curvularia sp., in vitro. Se sugiere realizar evaluaciones de control biológico con estos aislamientos en invernadero y a campo abierto, así como determinar su patogenicidad.
Palabras clave: Antagonismo; identificación taxonómica; crecimiento micelial; inhibición
The planting of strawberry (Fragaria × ananassa) is widely distributed in the world due to its genotypic diversity, its highly heterozygotic nature, and the wide range of environmental adaptations (Vikas-Kumar and Anil, 2019). In Mexico, the states of Jalisco, Tlaxcala, Michoacán, Baja California, Baja California Sur and Guanajuato are the main producers, providing 99% of the country’s production. The state of Puebla is ninth in strawberry production (SIAP, 2019). Despite its importance, the crop presents phytosanitary problems, mostly caused by fungi, some of the most important of which include Fusarium solani (Baiswar and Ngachan, 2018), Pestalotiopsis sp. (Morales-Mora et al., 2019), Curvularia inaequalis, C. spicifera (Ayoubi et al., 2017), Colletotrichum fragariae, C. acutatum (Chung et al., 2019), Rhizopus stolonifer (Oliveira et al., 2019) and Aspergillus niger (Chiotta et al., 2009), to mention a few. These phytopathogens, whether alone or in combination, cause 70% of the production to go to waste, leading to economic losses (Lafuente-Rincón et al., 2016). Chemical synthesis products have been used for decades to control these diseases (Gan and Wickings, 2017). However, their use is related to the creation of resistance and damages to both the environment and to human health (Andrade-Hoyos et al., 2019). Under this premise, biological control is considered an efficient and environmentally feasible practice for the development of a sustainable agriculture (Pérez-Torres et al., 2018).
The genus Trichoderma contains the most relevant antagonistic species, capable of controlling a large number of fungi that affect plants of agricultural interest (Romero-Arenas et al., 2017). Its success and use in agriculture are due to its action mechanisms, such as competition for space, mycoparasitism, antibiosis and the production of volatile compounds (Nawrocka et al., 2018; Hernández-Melchor et al., 2019). Consequently, the goals of the present work were 1) to identify and morphologically characterize fungal isolations related to a strawberry plantation of the Camino Real variety in Atlixco, Puebla, Mexico, at a genus level, and 2) to determine the antagonistic ability and the percentage of inhibition of radial growth in vitro of the strain T-H4 of Trichoderma harzianum against isolated fungi of the strawberry crop.
Isolation zone. Plant tissue samples were taken from the “Camino Real” strawberry crop variety in a 2,000 m2 plot in the town of Xalpatlaco, Atlixco, Puebla (18°56’9.26” N y 98°26’16.56” W; 2,350 masl), with backgrounds of high incidence of fungal diseases during the 2018-2019 spring-summer production cycle. The sampling was directed, colleting samples with suspicious fungus symptoms; all samples were stored in plastic bags inside an ice cooler until they were transported to the laboratory.
Isolation of fungi of plant tissues. The samples were cut into 0.5 cm2 sections of living and dead tissue. They were disinfested with 1.5% sodium hypochlorite and washed three times with distilled water, dried with sterile paper and finally planted in Petri dishes with a Potato Dextrose Agar (PDA) medium at a temperature of 25 ± 2 °C with ambient light for three days. The cultures developed were isolated and purified using monosporic cultures or the transfer of hyphae tips, and kept in a 20% glycerol solution at -84 °C.
Morphological characterization and mycelial growth. The identification of the fungi at a genus level was carried out by comparing the morphological characteristics of the culture (texture, type of mycelia, color, type of hyphae) and measurements of shape and size of anamorphic structures, which were compared using taxonomic identification keys by Barnett and Hunter (1998) in a microculture system using an optical microscope (Carl Zeiss, Jena, Germany) at a magnification of 1000x (Samson et al., 2014). For the evaluation of the mycelial development rate, 0.25 cm2 pieces of agar were placed, along with 10-day old mycelia from each characterized fungus were placed in Petri dishes with PDA; they were incubated in the dark at 27 °C for 10 days, and the mycelial diameter was measured every 12 h to estimate the speed of growth (cm), which was calculated with the linear growth function y=mx + b (where ‘y’ is distance, ‘x’ is time and ‘b’ is the constant factor) and expressed in centimeters per day (cm d-1) (Zeravakis et al., 2001). The diameter was measured using a digital caliper (CD-6 Mitutoyo), kept always in the same direction in triplicate, which was established at random for each repetition; only the average was used to calculate the development rate and the speed of mycelial growth per day.
Antagonism of T. harzianum towards fungi isolated from strawberry crop. The antagonism was evaluated with the strain T-H4 of T. harzianum, isolated from the root of Persea americana, the sequence of which was included in the National Center for Biological Information (NCBI) data base, with Access number MK779064.1, which is deposited in the Eco-Campus Valsequillo laboratory of the Science Institute, Benemérita Universidad Autónoma de Puebla (BUAP). The dual culture technique was used following Andrade-Hoyos et al. (2019) in triplicate to determine the percentage of inhibition of radial growth applying the formula PICR= [(R1-R2/R1) x 100] for each assay evaluated in a lapse of 7 to 10 days. To complete the evidence of the antagonism, each assay was compared and classified using the scale established by Bell et al. (1982); I) Growth of Trichoderma sp. covered the entire surface of the medium and reduced the pathogen culture, II) Growth of Trichoderma sp. covered at least 2/3 of the medium, III) Trichoderma sp. and phytopathogen both grew to cover half of the Surface of the medium, did not overgrow each other, IV) Pathogenic fungus grew to cover at least 2/3 of the medium and resisted the invasion by Trichoderma sp. and V) Growth of the pathogen covered the entire surface of the medium.
Statistical analyses. The data were analyzed with an ANOVA (two-way) in the IBM SPSS Statistics statistical package, version 25. Growth speed, rate of development and PICR were the response variables with three repetitions is a totally random statistical design. The experiment was carried out twice for validation. A comparison of averages was carried out using the Tukey-Kramer method with a level of likelihood of p≤0.05.
Thirty plant tissue samples were taken: 10 root samples, 10 leaf samples and 10 stem samples with the presence of mycelia, as well as 20 mature strawberry fruits with disease symptoms, in which two root-related fungi, three leaf and stem-related fungi and three strawberry fruit-related fungi were identified.
Fusarium sp. This was isolated from the crown of the plant and related to symptoms of wilting. The fungal cultures developed abundant white aerial mycelia with a cotton-like texture. In the reverse, the culture was pink and it stained the agar with a purple-to-violet color (Rentería-Martínez et al., 2018). Regarding microscopic characteristics, slightly curved macroconidia were observed (Figure 1A) with one to five septa (Gordon, 2017), along with oval-shaped microconidia, are born from lateral monophyllids (Figure 1B); they can form masses (simulating heads) but never chains. The cultures grew at a speed of 0.83 ± 0.03 cm d-1 (Table 1), a range which agrees with the study by Groenewald et al. (2006).
Rhizoctonia sp. This fungus was related to symptoms of wilting and root rot. The culture in the PDA culture medium initially presented a white color, and later, maroon and brown on the edges, with a velvetlike and waxy texture, with scarce aerial mycelia and a development rate of 1.05 cm per day (Table 1). Hyalin and septated hyphae were observed, some of which presented darker tones, with the formation of a right angle in some areas in which hyphae crossed (Figures 1 C and D), criteria to locate the fungus in the Rhizoctonia genus (González et al., 2006).
Pestalotiopsis sp. The fungus was isolated from areas with necrosis, mainly on leaves and stems; the leaves presented acervuli, mainly on the necrotic areas, and the Pestalotiopsis genus was characterized (Morales-Mora et al., 2019). The cultures displayed rosetted growth and scarce aerial mycelia, a white to creamy color on the inside, and on the Surface of the culture medium, bright black acervuli developed (Figure 1E). Conidia, typical to the Pestalotiopsis genus were observed, similar to those reported by Maharachchikumbura et al. (2014), dark, slightly curved, with six cells with the basal and the hyalin terminal, the latter of which was pointy with three to five apical hyaline appendages, as well as ellipsoidal (Figure 1F). In addition, hyalin conidiophores were observed, irregularly ramified, septated, smooth and short. The cultures presented an average mycelial development of 0.28 ± 0.027 mm h-1 (Table 1).
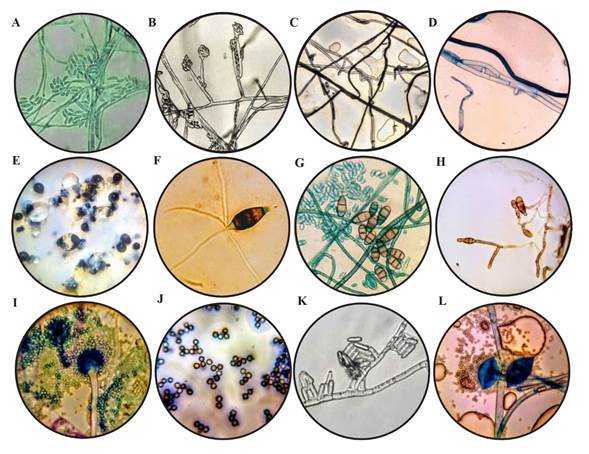
Figure 1. Características morfológicas, (A) Macroconidios septados (40 X) de Fusarium sp. teñidos con verde de malaquita; (B) Microconidios aglomerados en cabezas falsas sobre monofiálides con presencia de septos (100 X) característicos de Fusarium sp.; C) Micelio con ramificación y constricción en ángulo recto (40 X) de Rhizoctonia sp.; (D) Hifas septadas, con hifa naciente en un ángulo recto (100 X), de Rhizoctonia sp. teñidas con azul de metileno; (E) Conidiomas de color negro de Pestalotiopsis sp. en la superficie de la colonia; (F) Conidio (100 X) de Pestalotiopsis sp.; (G) Microconidios teñidos con verde de malaquita y presencia de conidios maduros (100 X) de Curvularia sp.; (H) Conidios ovoides a oblongos con celdillas (100 X) y sostenidos por hifas septadas de Alternaria sp.; (I) Conidióforo teñido con azul de metileno y métula hialina de Aspergillus niger (40 X); (J) Conidios globosos con crestas en su contorno (100 X) característicos de Aspergillus niger; (K) Conidios (100 X) de Colletotrichum sp.; (L) Esporangio maduro teñidos con azul de metileno de Rhizopus stolonifer (100 X).
Table 1. Type of antagonism according to Bell et al. (1982), percentage of inhibition of radial growth and rate of development of different fungi related to strawberry crops in Atlixco, Puebla.
| Nombre | Tasa de desarrollo (mm/h) * | Velocidad de crecimiento ( cm d-1 ) * | PICR* | Clase Antagonismo |
|---|---|---|---|---|
| Colletotrichum sp. | 0.28 ± 0.007 c | 0.75 ± 0.01 e | 87.56 ± 1.60 a | II |
| Pestalotiopsis sp. | 0.28 ± 0.027 c | 0.94 ± 0.08 d | 71.11 ± 1.18 d | II |
| Alternaria sp. | 0.18 ± 0.006 d | 0.47 ± 0.01 f | 81.33 ± 0.77 c | II |
| Rhizoctonia sp. | 0.46 ± 0.052 b | 0.47 ± 0.02 f | 70.22 ± 5.46 de | III |
| A. niger | 0.51 ± 0.067 b | 1.33 ± 0.07 c | 43.56 ± 6.73 f | III |
| R. stolonifer | 0.56 ± 0.017 b | 2.08 ± 0.28 a | 28 ± 12.72 f | IV |
| Curvularia sp. | 0.31 ± 0.027 c | 1.52 ± 0.10 b | 84 ± 0.77 b | II |
| Fusarium sp. | 0.16 ± 0.013 d | 0.83 ± 0.03 d | 63.65 ± 1.50 e | III |
| T. harzianum | 1.67 ± 0.01 a | 1.86± 0.22 a | ||
*Different letters mean significant difference between treatments according to Tukey-Kramer for p≤0.05.
Curvularia sp. The fungus was isolated from leaves with oval stains with light to dark chestnut tones, with yellowish edges. Some species of Curvularia are known to cause smut on the leaf and rotting of the strawberry (Ayoubi et al., 2017). The fungal culture displayed abundant aerial mycelia, greenish black in color, and radial and expansive vegetative mycelia, dark brown in color and greyish white towards the edges of the reverse part of the culture. Septated hyphae, hyaline, light brown and ramified, 1.5-4 µm in width. Sympodial, spindle-shaped conidia with oval ends, with three or four divisions, gold-brown to pale brown in color, with divisions in the slightly hyaline ends, measuring between 10-25 μm in length and 7-10 μm in width. Hyalin conidiogenous cells, 7-12 μm in length by 5-10 μm in width, proliferating in a sympodial shape with the detachment of conidia (Figure 1G). This characterization matches some Curvularia species described by Madrid et al. (2014). Average growth speed was 1.52 ± 0.10 cm d-1. Similar results were reported by Almaguer et al. (2013).
Alternaria sp. The fungus was isolated from leaves with irregularly-shaped, dark brown foliar spots with a pale yellow edge, symptoms which are associated with Alternaria infections. The culture presented a dense, abundant cotton-like texture with tones between white and gray, and later dark gray, as reported by Mehmood et al. (2018) for strawberry crops. On both sides of the Petri dish, the agar acquired a color between Green and black. The strain presented septated hyphae, hyaline and oval to oblong-shaped conidia, septated transversally and longitudinally (Figure 1H), with three to five divisions in the conidia. This fungus presented a mycelial growth rate of 0.18 ± 0.006 mm h-1 (Table 1).
Aspergillus niger. The fungus was isolated from fruits with signs of “strawberry rot” and characterized as A. niger (Chiotta et al., 2009). The culture presented mycelia with black scattered growth, with a dense, grainy texture. Microscopic characteristics observed included biseriated and radial conidial heads from aerial hyphae, de 5-7 µm in diameter, with thick, smooth, hyaline walls, pale maroon in color, an almost spherical vesicle, 10 µm in diameter, in which metulas develop, covering the entire surface. Its maroon globular conidia (Figure 1 I-J) are normally rugged with uneven crests and bulges (Krijgsheld et al., 2013). Growth speed was of 1.33 ± 0.07 cm d-1 (Table 1). Jørgensen et al. (2011) reported a vegetative growth of A. niger N402 in a maltose medium of 0.22 to 0.24 mm h-1.
Colletotrichum sp. The fungus was isolated from fruits with sunken necrotic lesions in the shape of concentric rings. The main fungi related to these disease symptoms belong to the genus Colletotrichum (C. acutatum, C. gloeosporioides y C. fragariae) (Howard et al., 1992). The cultures presented pale orange to salmon orange mycelia, and developed an aerial mycelium with white to pink tones. In addition, bright orange agglomerations formed on the surface of the culture, and conidia developed inside. These structures are described as acervular conidiomas (Dai et al., 2006). Specifically, the isolated culture developed epidermal acervula, cylindrical conidia with rounded ends, which were generated directly from the monophyllids from the septated hyphae (Figure 1K) (Freeman and Katan, 1997). The cultures grew at a speed of 0.75 ± 0.01 cm d-1 (Table 1), which matches reports by Gutiérrez-Alonso et al. (2001), who evaluated different isolations of C. gloeosporioides.
Rhizopus stolonifer. The fungus was isolated from rotting fruits. The culture presented white and later gray cultures, cottonlike in texture, with rapid growth and with aerial mycelia. Growth speed was 2.08 ± 0.28 cm d-1 (Table 1); Hernández-Lauzardo et al. (2005) reported a higher growth rate (2.3 mm h-1) of R. stolonifer during the four-day incubation period; higher results to those reported in the present investigation. Dark brown sporangiophora developed from a knot of rhizoids, spherical zygospores with thick, naked walls. This fungus is easily recognizable thanks to its hyaline or brownish side shoots, its numerous brown rhizoids and its black and lustrous sporangia (Figure 1L) (Farrera et al., 2007).
Antagonism of T. harzianum towards fungi isolated from strawberry crop. Areas of interaction were observed between T. harzianum and the evaluated fungi, in which parasitism was displayed in all cases. The reduction in growth rate of the fungi in dual cultures is an indicator of the antagonistic ability of Trichoderma (Guigón-López et al., 2010). The percentage of inhibition of radial growth (PIRG) varied in a range of 28 to 87.6%, with significant differences (p<0.05). The greatest percentages of inhibition were recorded in the confrontations with the strains of Colletotrichum sp., Alternaria sp. and Curvularia sp. (Table 1). No growth was observed in the confrontation with Alternaria sp. at the moment of contact with the antagonist. This effect was reported against A. porri when confronting T. harzianum in in vitro tests, which also displayed a strong mycoparasitic activity and a high ability of competition for space and nutrients (Mazrou et al., 2020).
When studying T. harzianum, Gaviria-Hernández et al. (2013) obtained antagonism values of 65% for C. gloeosporioides and 79% for C. acutatum, results below those reported in the present investigation. On the other hand, Benhamou and Chet (1993) indicated strong aggression from the antagonist T. harzianum and susceptibility of R. solani from the second day after inoculation. These results coincide with those by Hernández-Lauzardo et al. (2005), who, under similar conditions, obtained a PIRG of 58% when they evaluated T. harzianum A-34 against R. solani, results lower than those reported in the present investigation. However, Andrade-Hoyos et al. (2019), found a PIRG of 87.9% for R. solani using T. harzianum.
In another study, Guédez et al. (2009) compared the myceliar growth of T. harzianum with R. stolonifer (3.22 cm), R. solani (3.1 cm) and A. niger (1.72 cm) and obtained significant differences (p<0.01), similar results to those in the present investigation. Michel-Aceves et al. (2005) found that evaluating the antagonistic effect of native Trichoderma spp. isolations on the growth of F. oxysporum and F. subglutinans were 47.6% and 73%, respectively, similar results to those in the present investigation. On the other hand, a recent investigation by Andrade-Hoyos et al. (2019) mentions that T. harzianum inhibits the myceliar growth of F. oxysporum by up to 35%.
The antagonistic activity of T. harzianum was lower with A. niger and R. stolonifer, where the percentages of inhibition were 43.56 ± 6.73% and 28 ± 12.72%, respectively, values classified in classes III and IV (Figure 2), according to the scale obtained by Bell et al. (1982). Corrêa et al. (2007), observed a null antagonistic effect for T. harzianum and T. aureoviride strains when faced with Sclerotium rolfsii. This could indicate that the S. rolfsii isolations are able to release substances into the culture medium when they come in contact with the antagonist, which hinder its progress and/or detox the metabolites secreted by Trichoderma, as mentioned by Duarte-Leal et al. (2017), therefore these aspects must be investigated further for other fungi, as in the case of A. niger and R. stolonifer.
Reyes et al. (2008) noted that one of the significant characteristics of Trichoderma is its high growth speed. García-Espejo et al. (2016) mentioned that the way in which T. harzianum probably inhibits the growth of the pathogen is due to the production of inhibiting compounds that spread into the culture medium; antibiosis by the production of volatile and non-volatile metabolites, which include pyrones, isocyanates, peptics and trichocines; in addition, the production of extracellular diffusible enzymes such as pectinases, cutinases, glucanases and chitinases. In this sense, it is possible to notice that Alternaria sp., Colletotrichum sp. and Curvularia sp. are more susceptible to the antagonistic fungus. Antagonism tests reflect the ability and genetic variability of the antagonist and the phytopathogen to resist antagonism, allowing the preliminary selection for their evaluation under field conditions, as well as to complement and determine their biocontrolling activity (Fraire-Cordero et al., 2003). In this area of study, potential perspectives open up, since the antagonistic microorganisms have been used as biocontrol agents in diseases in fresh fruit and crops with good results (Elad et al., 1983).

Figure 2 Antagonismo de la cepa T-H4 de T. harzianum en escala de Bell et al. (1982), (A) Aspergillus niger, (B) Colletotrichum sp., (C) Rhizopus stolonifer, (D) Pestalotiopsis sp., (E) Curvularia sp., (F) Alternaria sp., (G) Fusarium sp. y (H) Rhizoctonia sp.
The present investigation identified Colletotrichum sp., A. niger and R. stolonifer in relation to the strawberry fruit, Pestalotiopsis sp., Curvularia sp. and Alternaria sp., present in leaves and stems, and Rhizoctonia sp. and Fusarium sp. in relation to the root of the strawberry plant, of the Camino Real variety. The strain of Trichoderma harzianum (T-H4) displayed antagonistic activity in vitro against Colletotrichum sp., Pestalotiopsis sp., Alternaria sp., Rhizoctonia sp. and Curvularia sp., but it did not inhibit the development of R. stolonifer.
Literatura Citada
Almaguer, M., Rojas, TI., Dobal, V., Batista, A., Rives, N. and Aira, MJ. 2013. Effect of temperature on growth and germination of conidia in Curvularia and Bipolaris species isolated from the air. Aerobiología 29: 3-20. https://doi.org/10.1007/s10453-012-9257-z [ Links ]
Andrade-Hoyos, P., Luna-Cruz, A., Hernández, EO., Gayosso, EM., Valenzuela, NL. and Cureño, HJB. 2019. Antagonismo de Trichoderma spp. vs hongos asociados a la marchitez de chile. Revista Mexicana de Ciencias Agrícolas 10(6): 1259-1272. https://doi.org/10.29312/remexca.v10i6.1326 [ Links ]
Ayoubi, N., Soleimani, MJ., Zare, R. and Zafari, D. 2017. First report of Curvularia inaequalis and C. spicifera causing leaf blight and fruit rot of strawberry in Iran. Nova Hedwigia 105: 75-85. https://doi.org/10.1127/NOVA_HEDWIGIA/2017/0402 [ Links ]
Baiswar, P. and Ngachan, SV. 2018. First report of root and collar rot of strawberry (Fragaria× ananassa) caused by Ceratobasidium sp. AG-B (o) (Binucleate Rhizoctonia) in India. Plant Disease 102(5):1035. https://doi.org/10.1094/PDIS-08-17-1285-PDN [ Links ]
Barnett, HL. and Hunter, BB. 1998. Illustrated genera of imperfect fungi. St. Paul, Minnesota, USA: The American Phytopathological Society. 218 p. [ Links ]
Bell, DK., Wells, HD. and Markham, CR. 1982. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology 72: 379-382. https://doi.org/10.1094/Phyto-72-379. [ Links ]
Benhamou, N. and Chet, I. 1993. Hyphal interactions between Trichoderma harzianum and Rhizoctonia solani: ultrastructure and gold cytochemistry of the mycoparasitic process. Phytopathology 83: 1062-1062. https://doi.org/10.1094/phyto-83-1062 [ Links ]
Corrêa, S., Mello, M., Ávila, ZR., Minaré, B., Raquel, R. and Gomes, D. 2007. Cepas de Trichoderma spp., para el control biológico de Sclerotium rolfsii Sacc. Fitosanidad 11(1): 3-9. https://www.redalyc.org/pdf/2091/209116144001.pdf [ Links ]
Chiotta, ML., Ponsone, ML., Combina, M., Torres, AM. and Chulze, SN. 2009. Aspergillus section nigri species isolated from different wine-grape growing regions in Argentina. International Journal of Food Microbiology 136(1): 137-141. https://doi.org/10.1016/j.ijfoodmicro.2009.08.013 [ Links ]
Chung, PC., Wu, HY., Ariyawansa, HA. and Chung, CL. 2019. First report of anthracnose crown rot of strawberry caused by Colletotrichum siamense in Taiwan. Plant Disease 103(7): 1775. https://doi.org/10.1094/PDIS-12-18-2167-PDN [ Links ]
Dai, FM., Ren, XJ. and Lu, JP. 2006. First report of anthracnose fruit rot of strawberry caused byColletotrichum acutatumin China. Plant disease 90(11): 1460-1460. https://doi.org/10.1094/PD-90-1460A [ Links ]
Duarte-Leal, Y., Lamz-Piedra, A. and Martínez-Coca, B. 2017. Antagonismo in vitro de aislamientos de Trichoderma asperellum Samuels, Lieckfeldt y Nirenberg frente a Sclerotium rolfsii Sacc. Revista de Protección Vegetal 32(3): 1-11. http://scielo.sld.cu/pdf/rpv/v32n3/rpv03317.pdf [ Links ]
Elad, Y., Chet, I., Boyle, P. and Henis, Y. 1983. Parasitism of Trichoderma spp. On Rhizoctonia solaniand Sclerotium rolfsii, scanning electron microscopy and fluorescence microscopy. Phytopathology 73(1): 85-88. https://doi.org/10.1094/Phyto-73-85 [ Links ]
Farrera, PRE., Zambrano, VAE. and Ortiz, MFA. 2007. Identificación de hongos asociados a enfermedades del fruto de la fresa en el municipio Jáuregui del estado Táchira. Revista de la Facultad de Agronomía 24(2): 269-281. http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-78182007000200005 [ Links ]
FAOSTAT. 2019. Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/ [ Links ]
Fraire-Cordero, M., Yánez-Morales, M., Nieto-Ángel, D. and Vázquez-Gálvez, G. 2003. Hongos patógenos en fruto de fresa (Fragaria x ananassa Duch) en postcosecha. Revista Mexicana de Fitopatología 21: 285-91. https://www.redalyc.org/pdf/612/61221307.pdf [ Links ]
Freeman, S. and Katan, T. 1997. Identification of Colletotrichum species responsible for anthracnose and root necrosis of strawberry in Israel. Phytopathology 87: 516-521. https://doi.org/10.1094/PHYTO.1997.87.5.516 [ Links ]
Gan, H. and Wickings, K. 2017. Soil ecological responses to pest management in golf turf vary with management intensity, pesticide identity, and application program. Agriculture, Ecosystems & Environment 246: 66-77. https://doi.org/10.1016/j.agee.2017.05.014 [ Links ]
García-Espejo, CN., Mamani-Mamani, MM., Chávez-Lizárraga, GA. and Álvarez-Aliaga, MT. 2016. Evaluación de la actividad enzimática del Trichoderma inhamatum (BOL-12 QD) como posible biocontrolador. Journal of the Selva Andina Research Society 7(1): 20-32. http://www.scielo.org.bo/pdf/jsars/v7n1/v7n1_a04.pdf [ Links ]
Gaviria-Hernández, V., Patiño-Hoyos, LF. and Saldarriaga-Cardona, A. 2013. Evaluación in vitro de fungicidas comerciales para el control de Colletotrichum spp., en mora de castilla. Ciencia & Tecnología Agropecuaria 14(1): 67-75. https://doi.org/10.21930/rcta.vol14_num1_art:344 [ Links ]
González, GV., Portal, OSM. and Rubio, V. 2006. Review. Biology and systematics of the form genus Rhizoctonia. Spanish Journal of Agricultural Research 4(1): 55-79. https://doi.org/10.5424/sjar/2006041-178 [ Links ]
Gordon, TR. 2017. Fusarium oxysporum and the Fusarium wilt syndrome. The Annual Review of Phytopathology 55: 23-39. https://doi.org/10.1146/annurev-phyto-080615-095919 [ Links ]
Groenewald, S., Van den Berg, N., Marasas, WF. and Viljoen, A. 2006. Biological, physiological and pathogenic variation in a genetically homogenous population of Fusarium oxysporum f. sp. cubense. Australasian Plant Pathology 35(4): 1-40. https://doi.org/10.1071/AP06041 [ Links ]
Guédez, C., Cañizález, L., Castillo, C. and Oliva, R. 2009. Efecto antagónico de Trichoderma harzianum sobre algunos hongos patógenos postcosecha de la fresa (Fragaria spp.). Revista de la Sociedad Venezolana de Microbiología 29(1): 34-38. http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S131525562009000100007&lng=es&tlng=es. [ Links ]
Guigón-López, C., Guerrero-Prieto, V., Vargas-Albores, F., Carvajal-Millán, E., Ávila-Quezada, GD., Bravo-Luna, L., Roucco, M., Lanzuise, S., Woo, S., Lorito, M. 2010. Identificación molecular de cepas nativas de Trichoderma spp. su tasa de crecimiento in vitro y antagonismo contra hongos fitopatógenos. Revista Mexicana de Fitopatología 28(2): 87-96. https://www.redalyc.org/articulo.oa?id=612/61218468002 [ Links ]
Gutiérrez-Alonso, JG., Nieto-Ángel, D., Téliz-Ortiz, D., Zavaleta-Mejía, E., Vaquera-Huerta, H., Martínez-Damián, T. and Delgadillo-Sánchez, F. 2001. Características de crecimiento, germinación, esporulación y patogenicidad de aislamientos de Colletotrichum gloeosporioides Penz. obtenidos de frutos de mango (Mangifera indica L.). Revista Mexicana de Fitopatología 19(1): 90-93. https://www.redalyc.org/articulo.oa?id=612/61219113 [ Links ]
Hernández-Lauzardo, AN., Bautista-Baños, S., Velázquez del Valle, MG., Rodríguez- Ambriz, SL., Corona-Rangel, ML., Solano-Navarro, A. and Bósquez-Molina, E. 2005. Potencial del quitosano en el control de las enfermedades postcosecha. Revista Mexicana de Fitopatología 23: 198-205. [ Links ]
Hernández-Melchor, DJ., Ferrera-Cerrato, R. and Alarcón, A. 2019. Trichoderma: importancia agrícola, biotecnológica, y sistemas de fermentación para producir biomasa y enzimas de interés industrial. Chilean Journal of Agricultural & Animal Sciences 35(1): 98-112. https://dx.doi.org/10.4067/S0719-38902019005000205 [ Links ]
Howard, CM., Maas, JL., Chandler, CK. and Albregts, EE. 1992. Anthracnose of Strawberry caused by the Colletotrichum complex in Florida. Plant Disease 76: 976-981. https://doi.org/10.1094/PD-76-0976 [ Links ]
Jørgensen, TR., Nielsen, KF., Arentshorst, M., Park, J., Van den Hondel, CA., Frisvad, JC., Ram, AF. 2011. Submerged conidiation and product formation by Aspergillus niger at low specific growth rates are affected in aerial developmental mutants. Applied and Environmental Microbiology 77(15): 5270-5277. https://doi.org/10.1128/AEM.00118-11 [ Links ]
Krijgsheld, P., Bleichrodt, RJ., Veluw, GJ., Wang, F. and Müller, WG. 2013. Development of Aspergillus. Studies in Mycology 74: 1-29. https://doi.org/10.3114/sim0006 [ Links ]
Lafuente-Rincón, DF., Barboza-Corona, JE., Salcedo-Hernández, R., Abraham-Juárez, R., Valadez-Lira, JA., Quistián-Martínez, D., De la Fuente-Salcido, NM. 2016. Identificación molecular de hongos fitopatógenos de fresa por PCR (its y ef-1α) y susceptibilidad a bacteriocinas de Bacillus thuringiensis. Investigación y Desarrollo en Ciencia y Tecnología de Alimentos 1(1): 417-422. http://www.fcb.uanl.mx/IDCyTA/files/volume1/1/3/72.pdf [ Links ]
Madrid, H., Cunha, KC., Gené, J., Dijksterhuis, J., Cano, J., Sutton, DA., Guarro, J. and Crous, PW. 2014. Novel Curvularia species from clinical specimens. Persoonia 33: 48-60. https://doi.org/10.3767/003158514X683538 [ Links ]
Maharachchikumbura, SS., Hyde, KD., Groenewald, JZ., Xu, J. and Crous, PW. 2014.Pestalotiopsis revisited. Studies in Mycology 79: 121-186. https://doi.org/10.1016/j.simyco.2014.09.005 [ Links ]
Mazrou, YS., Makhlouf, AH., Elseehy, MM., Awad, MF. and Hassan, MM. 2020. Antagonistic activity and molecular characterization of biological control agent Trichoderma harzianum from Saudi Arabia. Egyptian Journal of Biological Pest Control 30(1): 4e. https://doi.org/10.1186/s41938-020-0207-8 [ Links ]
Mehmood, N., Riaz, A., Naz, F., Hassan, I., Jaabeen, N., Anwaar, S. and Gleason, ML. 2018. First report of strawberry leaf spot caused by Alternaria alternata in Pakistan. Plant Disease 102(4): 820 https://doi.org/10.1094/PDIS-09-17-1464-PDN [ Links ]
Michel-Aceves, AC., Otero-Sánchez, MA., Darío, R., Rebolledo-Domínguez, O., Lezama-Gutiérrez, R. and Ariza-Flores, R. 2005. In vitro mycoparasitic activity of Trichoderma spp. Against Fusarium subglutinans (Wollenweb and Reinking) PE Nelson, TA Toussoun, and Marasas, and F. oxysporum Schlechtend. Revista Mexicana de Fitopatología 23(3): 253-261. https://www.cabdirect.org/cabdirect/abstract/20073103350 [ Links ]
Morales-Mora, LA., Martinez, SSJ., Andrade-Hoyos, P., Valencia de Ita, MA., Silva-Rojas, HV. and Romero-Arenas, O. 2019. First report of leaf spot and anthracnosis caused by Pestalotiopsis sp., on strawberry in Puebla, Mexico. Plant Disease 103(10): 2668 https://doi.org/10.1094/PDIS-05-19-1010-PDN [ Links ]
Nawrocka, J., Szczech, M. and Malolepsza, U. 2018. Trichoderma atroviride enhances phenolic synthesis and cucumber protecction against Rhizoctonia solani. Plant Protection Science 54(1): 17-23. https://doi.org/10.17221/126/2016-PPS [ Links ]
Oliveira, J., Parisi, MC., Baggio, JS., Silva, PPM., Paviani, B., Spoto, MHF. and Gloria, EM. 2019. Control of Rhizopus stolonifer in strawberries by the combination of essential oil with carboxymethyl cellulose. International Journal of Food Microbiology 292: 150-158. https://doi.org/10.1016/j.ijfoodmicro.2018.12.014 [ Links ]
Pérez-Torres, E., Bernal-Cabrera, A., Milanés-Virelles, P., Sierra-Reyes, Y., Leiva-Mora, M., Marín-Guerra, S. and Monteagudo-Hernández, O. 2018. Eficiencia de Trichoderma harzianum (cepa a-34) y sus filtrados en el control de tres enfermedades fúngicas foliares en arroz. Bioagro 30(1): 17-26. [ Links ]
Rentería-Martínez, ME., Guerra-Camacho, MA., Ochoa-Meza, A., Moreno-Salazar, SF., Varela-Romero, A., Gutiérrez-Millán, LE. and Meza-Moller, AC. 2018. Multilocus phylogenetic analysis of fungal complex associated with root rot watermelon in Sonora, Mexico. Mexican Journal of Phytopatholgy 36(2): 233-255. https://doi.org/10.18781/r.mex.fit.1710-1 [ Links ]
Reyes, Y., Martínez, B. and Infante, D. 2008. Evaluación de la actividad antagónica de trece aislamientos deTrichoderma spp. sobreRhizoctonia sp. Revista Protección Vegetal 23(2): 112-117. [ Links ]
Romero-Arenas O, Amaro JL, Damián MA, Valencia de Ita MA, Rivera A and Huerta M. 2017. Bio-preparados de Trichoderma spp. para el control biológico de Phytophthora capsici en el cultivo de tomate de Puebla, México. ITEA 113(4): 313-324. https://doi.org/10.12706/itea.2017.019 [ Links ]
Samson RA, Visagie CM and Houbraken J. 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology 78:141-173. https://doi.org/10.1016/j.simyco.2014.07.004 [ Links ]
Servicio de Información Agroalimentaria y Pesquera (SIAP) 2019. Atlas Agroalimentario 1080-2019. [ Links ]
Vikas-Kumar S and Anil KG. 2019. Growth responses of strawberry (Fragaria × ananassa duch.) plants grown at different planting density using pvc pipe under protected cultivation. Bangladesh Journal of Botany 48(1): 1-7. [ Links ]
Zeravakis, G., Philippoussis, A., Ioannidou, S. and Diamantopoulou, P. 2001. Mycelium growth kinetics and optimal temperature conditions for the cultivation of edible mushroom species on lignocellulosic substrates. Folia microbiologica 46(3): 231e. https://doi.org/10.1007/BF02818539 [ Links ]
Received: May 26, 2020; Accepted: August 14, 2020











 texto em
texto em 


