Enzymes belong to a great diversity of molecules from natural origin, and within their attributes, some of them possess antimicrobial activity (Oh and Park, 2018). Enzymes present particular characteristics that make them excellent biologic catalysts and can accelerate the rate at which specific biological reactions are carried out to form or degrade a new product (Sheng et al., 2016). In microorganisms, as in other types of living organisms, enzymes play an essential role to perform their biological functions such as cell division and cell development, defense, and adaptation to unfavorable conditions in a given environment (Gow et al., 2017). On the other hand, the ability of the microorganisms to colonize specific habitats has led to the emergence of various economic and health problems worldwide (Gálvez-Iriqui et al., 2019). However, plants and organisms are not defenseless against the microbial offensive, since enzymes participate extensively in defense processes.
Currently, the antimicrobial enzymes that have been studied, are among others, the proteolytic, the oxidative, and those capable of hydrolyzing polysaccharides, including the amylases, lyases, Dispersin B, and lysozymes (Thallinger et al., 2013). In particular, the activity of lysozymes against several microorganisms has been extensively reported due to their ability to hydrolyze the polymers present in the bacteria cell wall, specifically those that belong to the Gram (+) group (Phillips, 1967). In recent studies, the ability of this enzyme to hydrolyze the cell wall of fungi and yeast has also been reported (Manikandan et al., 2015; Zhou et al., 2017; Hernández-Téllez et al., 2018). Its enhancement as an antibacterial and antifungal agent by combining them with other active compounds has been reported (Hernández-Téllez et al., 2018; Wu et al., 2018a). Biopolymeric molecules such as chitosan have been used to prepare functional materials or composites formulated with lysozyme. Chitosan is a polycationic biopolymer capable of retarding or inhibiting bacterial and fungal development by interacting with cellular components present in the membrane and cell wall (Bautista-Baños et al., 2016).
The lysozyme and chitosan combination has been used for developing materials in the form of solutions, films, fibers, hydrogels, and micro and nanoparticles, which have been proposed for controlling microorganisms within the fields of human health, agriculture, and food processing and preservation industry (Wu et al., 2018a). Therefore, it is possible to assume that lysozyme alone or combines with chitosan is capable of acting on different biopolymer and peptides present in fungi and yeast cells inducing cell death or abnormalities during growth. Hence, the following review aims to present the most important aspects and characteristics of lysozymes and their technological applications as antimicrobial enzymes.
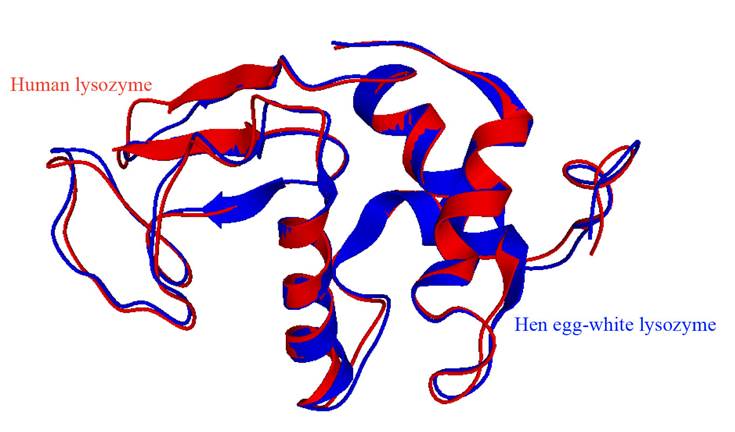
Lysozyme overview. Lysozyme or muramidase is part of the group of glycosidic hydrolases and catalyzes the hydrolysis of the β (1-4) bond between N-acetylglucosamine and N-acetylmuramic acid of bacterial cell walls (Jana et al., 2017). Its primary structure consists of one single polypeptide chain, which varies on the number of amino acid residues, 130 for the human lysozyme (hLyz), and 129 for the chicken lysozyme (cLyz) (Cao et al., 2015). However, they are very similar in structure. The cLyz is an enzyme with 14.6 kDa and a high isoelectric point of 11.16 (Yu et al., 2018). Meanwhile, the hLyz has 14.7 kDa and an isoelectric point of 9.28, however, aspartate-52 and glutamate-35 are the critical amino acids to the enzymatic activity (Cao et al., 2015). Besides, the tertiary enzyme structure of both lysozymes has similarities, however, they differ in one beta region. Figure 1 shows the four alpha-helices, four random coils, and a double antiparallel beta-pleated sheet for human lysozyme, and a triple-stranded and antiparallel beta-pleated sheet for the chicken lysozyme (Strader, 2018). Generally, it folds into a compact and globular tertiary structure with a long slit on its surface (Sheng et al., 2016; Jana et al., 2017; Strader, 2018). The enzyme structure has six tryptophan (Trp-28, Trp-62, Trp-63, Trp-108, Trp-111, and Trp-123) and three tyrosines (Tyr-20, Tyr-23, and Tyr-53), where three Trp residues are situated at the substrate-binding site, two are on the hydrophobic part, and one is at the edge of the lysozyme. Within these amino acids, Trp-62 and Trp-108 are found in the substrate-binding site, performing an essential function of substrate binding and enzyme structure stabilization (Figure 2) (Sheng et al., 2016; Wu et al., 2019).
Lysozyme substrate. Peptidoglycan is an essential component of the prokaryotic cell wall outside the cytoplasmic membrane (Jana et al., 2017; Monteiro et al., 2018). This polymeric structure is crucial for the bacterial cells; it carries out the maintenance of its morphology, serves as a scaffold to anchor other components on the cell envelope such as proteins, deals with the mechanical stress, and allows the diffusion of nutrients towards the plasma membrane containing the transporters (i.e., proteins, porins, permeases, among others) required for their translocation into the cytosol to achieve the survival of bacteria (Jana et al., 2017).
Peptidoglycan or murein is a biopolymer consisting of linear glucan chains, cross-linked by short peptides with different compositions depending on the type of bacteria. They are assembled by N-acetylglucosamine (GlcNAc) and N-acetyl muramic acid (MurNAc) residues linked by β (1-4) bonds (Jana et al., 2017; Monteiro et al., 2018). Pathogenic bacteria have developed resistance to the bacteriolytic activity of host secreted lysozyme during infection; the primary mechanism is the modification of its peptidoglycan glycan backbone (Sukhithasri et al., 2013) (Figure 3).
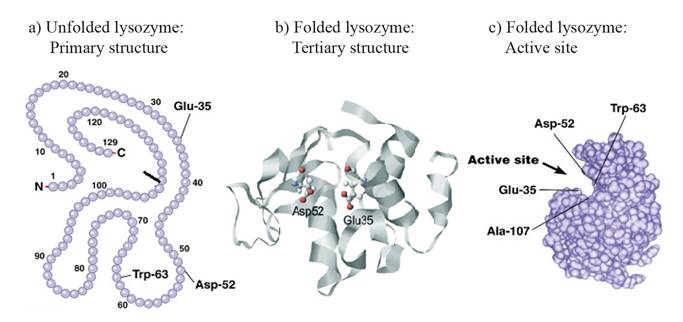
Figure 2. Chicken lysozyme primary (a) and tertiary structure (b). Active site with the amino acids responsible for the lysozyme activity (c). (Held and Van Smaalen, 2014; Hardin et al., 2016).
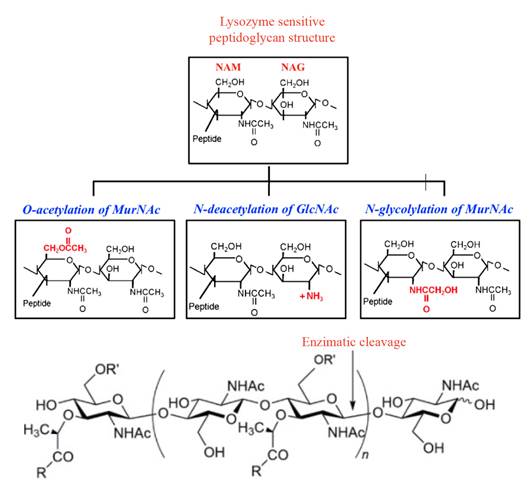
Figure 3. Structure of lysozyme sensitive and lysozyme resistant peptidoglycan (a) and enzymatic cleavage of lysozyme, (b) Pathogenic bacteria modify this structure by O-acetylation of NAM at C-6 position, N-deacetylation of NAG at C-2 position and N-glycosylation of muramic acid at C-2 position (Sukhithasri et al., 2013;Taylor et al., 2019).
Recently, the synthesis of monosaccharides and disaccharides from acetylmuramic acid (NAM) and the possibility that these compounds can bind to the lysozyme’s active site, resulting in their hydrolysis, has been reported (Jana et al., 2017). The arrangement in the space of the functional groups of these compounds can impede or promote the binding to the active site of the enzyme, in this case, methoxy (-OCH3), acetyl (-COCH3), and methyl (-CH3) groups. This study suggests that the substrates related to peptidoglycan, which can adopt the necessary conformation for interacting with the active site, will be hydrolyzed on the o-glycosidic bonds by the lysozyme.
This brings the query of how lysozyme and other polysaccharides different from the peptidoglycan can interact, especially those present in the cell wall of lysozyme-sensitive fungi or yeast, such as chitin and chitosan, which are structurally related, or even other polysaccharides such as β-glucans. However, these microorganisms do not have peptidoglycan. Interestingly, how it interacts at the molecular level with these polysaccharides has not been reported yet (Sowa-Jasiłek et al., 2016; Zhou et al., 2017; Hernández-Téllez et al., 2018;). Chitin (a long-chain polymer of N-acetylglucosamine) or chitosan (composed of randomly distributed β-(1→4)-linked D-glucosamine and N-acetyl-D-glucosamine residues) may play an important role in the interaction of lysozyme with the fungal cell wall (Han et al., 2012).
Mechanism of catalysis. The catalysis mechanism of lysozyme was the first to be studied in the glycosidases group. It catalyzes the cleavage of β-1,4-glycosidic bonds between N-acetyl muramic acid and N-acetyl glucosamine in peptidoglycan (Yu et al., 2018). Lysozyme has two carboxylic acid residues, aspartate-52, and glutamate-35, strategically positioned on the glycosidic bond and catalyze the cleavage in two steps (Figure 3, 4 and 5).

Figure 4. Enzyme-substrate complex for chicken lysozyme, where can be appreciated the catalytic residues Glu-35 and Asp-52 in the active site. Figure edited from Hardin et al., 2016.
In general, the catalytic process initiates with a fragment of six residues of the polysaccharide (subsites A-F); this fragment binds to the active site of the enzyme, where all monomers of the fragment are readily embedded, except one (D). The D monomer undergoes a half-chair conformation, which changes to enter to a specific point of the active site of the enzyme. This site contains two ionic amino acids, glutamic (Glu-35) and aspartate acids (Asp-52). These two residues carry out quite different functions, where Glu-35 acts as an acid-base residue and the Asp-52 as an enzymatic nucleophile (Jana et al., 2017).
Phillips (1967) established in his work the distance between the glycosidic oxygen-linking residues D and E. The nearest oxygen of Glu 35 is almost 3 Å, the nearest oxygen of Asp 52 is about 3 Å from the C(1) atom of sugar residue D, and about the same distance from the ring oxygen atom of that residue.
In the first step (glycosylation), D is strained into a conformation similar to a transition state. The acid-base residue Glu-35 acts by donating a proton to the oxygen, which participates in the glycosidic bond of residues D and E; therefore, the reduction of the oxygen, induces the cleaver of the C-O bond. Glu-35 now has a negatively charged oxygen, and D is an unstable, positively charged ion. At the same time, the deprotonated carboxylate of Asp-52 performs a nucleophilic attack on the anomeric carbon, and through resonance, stabilizes the positively charged carbon. In the second step (deglycosylation), the deprotonated Glu-35 acts as a general base where it accepts a proton (deprotonate). This proton comes from a directly positioned water molecule, performing a nucleophilic substitution in the glycosyl enzyme. The resulting hydroxide can bond with residue D, neutralizing the charge and causing its release from the cleft. Then the nucleophile is regenerated in the active site (Figure 5) (Stick et al., 2009; Jana et al., 2017; Strader, 2018; Wu et al., 2019).
Catalytic differences among types of lysozymes. Lysozyme and enzyme-like lysozyme (low muramidase activity) have been isolated and purified from natural sources and organisms. These enzymes can induce changes in cell structure, leading microorganisms to death (Chen et al., 2016). In animals, there are four main types of lysozymes, referring to the source: type-c (chicken or conventional), type-g (goose), type-i (invertebrate), and type-ch (chalaropsis) (Zhou et al., 2017). Types -c and -g are found in all vertebrates, while invertebrates typically produce type-i. Different types of lysozyme have been reported in some invertebrates, for example, type-c lysozymes in Arthropoda, type-g in Mollusca, and type-ch in Nematodes (Zhou et al., 2017; Yu et al., 2019).

Figure 5 Catalytic mechanism of lysozyme mediated by Glu-35 and Asp-52 amino acids (Stick et al., 2009).
Table 1 summarizes diverse types of lysozymes and enzyme-like lysozymes from animals, plants, and those obtained from genetic recombination. In both vertebrates and invertebrates, lysozymes and enzyme-like lysozymes are multifunctional enzymes, which serve as non-specific innate immunity molecules responsible for antibacterial defense (pathogens and non-pathogens) and/or digestion (Zhou et al., 2017; Wu et al., 2019). Notwithstanding, few studies report a previous stimulation with a bacterial or fungal infection in the host organisms to induce the expression of lysozymes and enzyme-like lysozymes (Zhou et al., 2017; Yu et al., 2019); Once were isolated some of them had low or null lysozyme or muramidase activity, however, exhibit high peptidase activity or were overexpressed on the digestive tract. This behavior suggests that in certain living organisms lysozymes or lysozyme-like enzymes have a primary function related to digestive functions and nutrition (Zhou et al., 2017; Yu et al., 2019).
The catalytic differences among the diverse types of lysozyme suggest that the lack of muramidase activity of the lysozymes type-i concerning type-c may be due to differences in the amino acid sequence. Zhou et al. (2017) obtained and purified a type-i recombinant lysozyme from mud crab (Scylla paramamosain), which presented 54% similarity with type-i isolated from shrimp (Litopenaeus vannamei); this was highly expressed in both, the digestive tract and the hemocytes. The amino acid sequence analysis revealed that this enzyme lacked the two amino acids responsible for muramidase activity, aspartic and glutamic acids. Despite this, type-i lysozyme homologs in invertebrates, Splys-i enzyme, showed antimicrobial activity against both Gram (+) and Gram (-) bacteria, as well as on fungal cells, when crabs were previously exposed to them. The authors suggest that the mechanism of action against these microorganisms is not related to muramidase activity, but to the isopeptidase activity and the protein agglutination phenomena on the microbial surface, making it possible to bind to cellular components such as peptidoglycan and lipopolysaccharide.
Table 1 Properties of some purified lysozymes isolated from animals, plants and genetic recombination.
| Source of lysozyme | Characteristic | pH | Molecular Weight | Biological activity | Reference |
|---|---|---|---|---|---|
| Pupae(Cameraria ohridella) | Crude extract with lysozyme activity | Experimental conditions: 6.4 | 15 & 28 kDa | Antibacterial activity against Micrococcus luteus, Saccharomyces cerevisiae, Bacillus megaterium | Fiołka et al., 2005 |
| Mung bean (Phaseolus mungo) | SLA = 355 U mg-1 at 30 °C pI: 9.7 Optimum temperature of 55 °C | Optimum at 5.5 | 14.4 kDa | Antifungal activity against Fusarium oxysporum, F. solani, Sclerotium rolfsii, Pythium aphanidermatum, B. cinerea | Wang et al., 2005 |
| Clam (Ruditapes philippinarum) | SLA = 3.76×105 U mg-1 | 5.5 | 13.4 kDa | N/D | Kim et al., 2012 |
| Cauliflower (Brassica oleracea) | EA = 133.0 μg Optimum temperature of 40 °C | 3.0-9.0 Optimum at 8 | 22.0 kDa | Antimicrobial activity against Pseudomonas syringae, Xanthomonas campestris, E. carotovora, F. solani, Leptosphaeria maculans, B. cinerea, Cheilomenes lunata, Rhizoctonia solani, A. alternata | Manikandan et al., 2015 |
| Lyz-i2 recombinant from white shrimp (Litopenaeus vannamei) | Physicochemical parameters = N/D | Experimental condition: 6.2 | 16.9 kDa | Antibacterial activity against E. coli, Micrococcus lysodeikticus, Vibrio vulnificus, V. harveyi, V. alginolyticus, V. parahaemolyticus | Chen et al., 2016 |
| Lyz type-c from silkworm (Galleria mellonella) | Physicochemical parameters = N/D | Experimental condition: 6.4-7.4 | N/D | Activity against Candida albicans | Sowa-Jasiłek et al., 2016 |
| Lyz type-i recombinant from mud crab (Scylla paramamosain) | Lys with a 54% identity to Lys of L. vannamei Physicochemical parameters = N/D | Optimum at 8 | 16 kDa | Low muramidase activity, moderate antimicrobial activity against Gram (+) and Gram (-) bacteria and C. albicans | Zhou et al., 2017 |
| Lyz recombinant from whitefly (Bemisia tabaci) | Type-c: Btlysc, Type-I: Btlysi1-Btlysi2 | Optimum at 6 | 15 kDa | Antibacterial activity against Bacillus subtilis and Staphylococcus aureus | Wang et al., 2018 |
| Lyz type-i from earthworm (Eisenia andrei) | Isopeptidase activity. Chain of 226 amino acid residues | N/D | 22.2 kDa | Important for the digestive system | Yu et al., 2019 |
aEA = enzymatic activity; bSLA = specific lysozyme activity, cpI = isoelectric point, dN/D = not determined.
The lysozyme type-i obtained from the earthworm Eisenia andrei showed a polypeptide sequence of 226 amino acid residues, with 14 cysteine residues (Cys). The authors theorize that these Cys residues are possibly involved in the high numbers of sulfur bridges that distinguish the type-i from other lysozymes (Yu et al., 2019). An important aspect of this enzyme sequence (Ea-iLys) is that it contains three residues of aspartic and glutamic acids and serine, promoting the activity of muramidase, alanine, and histidine-mediated isopeptidase. The 14 Cys residues form seven disulfide bridges, which block the few cleavage sites for trypsin (< 10), possibly making this enzyme resistant to digestive proteinases secreted by the intestinal glands. These differences suggest that this enzyme activity mainly focuses on digestive functions, instead of being part of the effectors of the immune system (Yu et al., 2019). Finally, Wang et al. (2018) obtained from the whitefly (Bemisia tabaci) three genes for three recombinant lysozymes, one type-c (Btlysc), and two type-i (Btlysi1 and Btlysi2). These enzymes showed variations in the number of amino acids in their sequence, 146 for type-c and 156 and 160 for both type-i, respectively. Type-c contained eight conserved cysteine residues, while Btlysi1 and Btlysi2 had twelve conserved cysteine residues capable of forming six disulfide bridges.
These findings agree with Yu et al. (2019) and Zhou et al. (2017). They suggested that type-i lysozymes contain large numbers of disulfide bridges, due to their relation with the digestive function of E. andrei and S. paramamosain. Further, type-c lysozyme was expressed before and after the whitefly was infected with Beauveria bassiana entomopathogenic fungus, without changing their behavior pattern; this suggested that the type-c lysozyme plays a role as an effector of the immunological system. The two types-i enzymes changed their expression pattern when the feeding of the flies varied and did not suffer any infection with pathogenic microorganisms, which indicates that these enzymes are closely related to digestive processes.
Effect of pH and temperature on the enzymatic activity. The amino acids responsible for the polysaccharide cleavage are susceptible to variations in the acidity of the medium. The pH and temperature affect the enzyme’s catalytic activity and, therefore, the reaction rate, which performs the mechanism of action. Depending on the source of isolation and purification, the lysozyme may exhibit slight variations in its optimum pH and temperature (Table 1). Most studies show that the optimal pH ranges from slightly acidic (5.5-6.7) for type-c (Fiołka et al., 2005; Kim et al., 2012; Chen et al., 2016; Wang et al., 2018) to alkaline (7.4-8) for type-i (Manikandan et al., 2015; Zhou et al., 2017; Yu et al., 2019;). The optimal temperature of lysozymes varies depending on the source, but most studies indicate that it is around 40 °C for insects and animals.
Antibacterial and antifungal activity of lysozyme. The biological activity of lysozyme leads to a disturbance of the bacterial growth, especially Gram (+). Furthermore, the discovery of the lysozyme structure promoted more research studies about its possible application. In general, the lysozyme activity is determined using Micrococcus sp. strains, which serve as a substrate for visualizing the activity by turbidimetry. Currently, the Micrococcus sp. assay is considered a gold standard for measuring lysozyme activity (Fiołka et al., 2005; Kim et al., 2012).
Fiołka et al. (2005) isolated and purified a lysozyme from the pupae of Cameraria ohridella, allowing the lysis of Bacillus megaterium cells, caused a decrease in the optical density of the bacterial growth compared with the lysozyme control. According to the authors, lysozymes may play two roles, a digestive way breaking down the ingested bacteria in the gut and a defense response against pathogens that enter the hemocoel. Wang et al. (2005) obtained similar results with a purified lysozyme from mung beans (Phaseolus mungo), which showed antibacterial and antifungal activities. The mung bean lysozyme seems to have more effectiveness in inhibiting Fusarium solani and Botrytis cinerea. Furthermore, it was able to interrupt the radial growth of F. solani and induced abnormalities in its cellular structure, such as detachment of cell membrane and cell wall of hypha, and leaking out of its cytoplasm, which suggested the vital role of lysozyme in the constitutive host defense mechanisms against microbial pathogens (Wang et al., 2005). A lysozyme, isolated and purified from a cauliflower plant tissue, affected the growth of phytopathogenic fungi and bacteria such as F. solani and Erwinia carotovora that usually infects this crop (Manikandan et al., 2015).
Regarding the activity against phytopathogenic fungi, Acremonium obclavatum showed higher enzymatic sensitivity, followed by Leptosphaeria maculans and Alternaria alternata (Manikandan et al., 2015). All these studies suggest that lysozymes from plant and insect tissues inhibited bacteria and fungi; however, in some cases, the antibacterial activity of hen egg lysozyme remains more efficient than other types of lysozymes previously reported. Although the mechanism of action in which the lysozyme exerts the antibacterial activity has been already enlightened, to date, there is little information about the exact mechanism of action that the lysozymes follow to cause damage to the fungal cell surface (Hernández-Téllez et al., 2018; Wu et al., 2017).
Lysozyme isolated from Pithecellobium dulce seeds exerted antifungal activity against Macrophomina phaseolina, with a rather high thermal stability at up to 80 °C for 15 min, at pH=8.0 (Sawasdipuksa et al., 2011). The activity of hen egg-white lysozyme on the growth of dimorphic fungi such as Paracoccidioides brasiliensis (Lopera et al., 2008) and the anti-biofilm effect on Candida albicans (Sebaa et al., 2017) have also been reported. When P. brasiliensis yeast cells were exposed to lysozyme, the multiple-budding ability was impaired. Also, ultra-structural changes like a fusion of lipid vacuoles, lamellar structures, subcellular degradation, and interruption of fibrillar layers were observed in conidia after exposure to the enzyme. Thus, lysozyme seems to exert a dual role as part of the antifungal defense mechanisms (Lopera et al., 2008). Human lysozyme displayed >80% of antifungal activity against Aspergillus fumigatus, Penicillium sp., Acremonium sp., Candida parasilopsis, and C. albicans, commonly identified in patients with chronic rhinosinusitis (Woods et al., 2011).
It has been suggested that lysozyme can hydrolyze the (β-1,4)-glycosidic bonds between the N-acetylglucosamine monomers which integrate the chitin, affecting the cell wall function (Sowa-Jasiłek et al., 2016; Hernández-Téllez et al., 2018). Sowa-Jasiłek et al. (2016) indicated that the lysozyme affected the growth of Candida albicans since they enter in an apoptotic state, caused by a loss of the membrane potential in the mitochondria; however, the mechanism was not discussed. Various effects undergoing in the cell nucleus level, such as chromatin condensation and DNA fragmentation, were also observed. Hernández-Téllez et al. (2017, 2018) reported that chitosan-lysozyme nanoparticles and their separate components could disrupt the first stage of Aspergillus parasiticus growth. The authors found low percentages of cell viability at particle concentrations of 200 to 300 μg mL-1. The germination and diameter of spores, reducing sugar production, and membrane integrity, were also affected.
Until now, data about the mechanism of action of how lysozyme affects fungal development is still insufficient. Structural affectations could be associated with the β-1,3-glucanase production, which agree with the low reducing sugar production detected in Aspergillus parasiticus after exposure to chitosan-lysozyme nanoparticles (Hernández-Téllez et al., 2017). It should be noted that these studies have been carried out using pure enzymes, without combination with other antimicrobial agents; therefore, it is necessary first to establish how the enzyme works on fungi and yeast, in order to achieve their technological applications in the pharmaceutical, food and agricultural industries.
Lysozyme immobilization in chitosan-based antimicrobial nanomaterials. The enzymes’ industrial application is highly desirable due to their selectivity and specificity under moderate conditions (Figure 6).
Furthermore, the enzymes can improve a large number of industrial processes in a sustainable and environmentally-friendly way, since their performance could reduce the use of chemical compounds and their inherent toxicity generated during the reactions (Bilal and Iqbal, 2019; Wang and Jiang, 2019). However, there are still some disadvantages to their application at the industrial level, such as high costs (depending on the process), low operational stability, deactivation by solvents, and the lack of recovery or recyclability, among others.
Regarding their biological origin, enzymes are soluble and could be inhibited by substrates and reaction products, metabolites, and other compounds in the media (Bilal and Iqbal, 2019). One proposed strategy is the immobilization of the enzymes in polymeric matrices designed to increase their antibacterial activity and prolong their activity and effectiveness. To date, this procedure has been carried out with different solid supports and has allowed overcoming the previously mentioned problems effectively but at a lower scale (Huang et al., 2012; Wang and Jiang, 2019). Immobilization can improve the control of the reaction, prevents the contamination of the product by the enzyme, which is notably relevant in the food industry, allowing the use of different reactors. The enzyme immobilization technology can promote and improve enzyme stability.
Moreover, it supposes that enzymes immobilized can be reused. Even the immobilization technique can be used for enzyme isolation and purification purposes. Besides, this process is usually necessary when achieving optimal performance in non-aqueous media is crucial (Bilal and Iqbal, 2019). To date, the pre-existing solids to immobilize the enzymes is the most widespread strategy. Therefore, many polymers and their derivatives have been recommended, including alginate, cellulose, dextran, agarose, agar-agar, carrageenan, gelatin, guar gum, pectin, chitosan, and others (Bautista-Baños et al., 2016; Bilal and Iqbal, 2019).
The most-reported synthesis methods to perform the enzyme immobilization can be classified as physical and chemical. In the physical methods, the enzymes bind to the support with hydrogen bonds or by entrapment in the polymer matrix, while, in the chemical methods, the enzymes are closely united in the support by covalent bonds (Bilal and Iqbal, 2019). When using these methods, the enzyme immobilization is carried out by adsorption on insoluble materials, entrapment in polymeric gel networks, encapsulation, crosslinking using bifunctional agents, covalent binding to an insoluble vehicle, and the formation of enzyme aggregates by crosslinking. The enzyme immobilization generally provides better accessibility and additional stabilization denaturation processes. Immobilized enzymes have shown higher thermal and operational stability to pH, extreme temperature conditions, and organic solvents (Bilal and Iqbal, 2019). However, there still some practical problems to solve during enzyme immobilization, such as low load capacity on solid supports, low recovery rate, and the loss of enzymatic activity by the inhibitors (Wang and Jiang, 2019).
It is crucial to know the right immobilization technique, followed by selecting the support matrix to achieve the optimal enzyme immobilization. It is necessary to check out that the enzyme support matrix is robust and biocompatible with recoverable characteristics (Bilal and Iqbal, 2019). For this purpose, the natural polymeric matrices have been mostly studied due to their desired characteristics such as non-toxicity, biocompatibility, biodegradability, flexibility, sustainability, and the available number of reactive sites (Bilal and Iqbal, 2019; Wang and Jiang, 2019).
Chitosan is a widely used polymer, with all these characteristics, which makes it attractive for the food industry, pharmaceuticals, electro-chemicals, biosensors, textiles, and coatings (Hernández-Téllez et al., 2018; Wu et al., 2018b). Several macro and nano-scale materials have been prepared from lysozyme and chitosan, whose use has been proposed to inhibit or retard the microbial growth. Among these materials, solution mixtures, hydrogels, fibers and nanofibers, and nano and microparticles, have been studied (Hernández-Téllez et al., 2018; Wu et al., 2018b; Yu et al., 2018). The most-reported matrix to achieve lysozyme immobilization is chitosan, carried out by electrostatic interaction between the positive charges of chitosan and the negative charge of tripolyphosphate (TPP) (Yu et al., 2018). However, the incorporation of other counter ions such as sodium alginate, carrageenan, and glutaraldehyde has allowed the synthesis of these materials, without losing its biological activity (Hernández-Téllez et al., 2018; Wu et al., 2018c).
Another highlighting aspect is that, due to the complexity of the enzymes’ isolation and purification from natural sources, these materials are commonly synthesized with commercial lysozymes from chicken egg-white, reducing their application to the biological activity (Table 2). Some reports suggest that lysozymes isolated from plants, even if they are type-c or type-i, can affect a broad spectrum of phytopathogenic fungi, especially crop pathogenic species, where the enzyme was isolated (Wang et al., 2005; Manikandan et al., 2015). Although the research on chitosan and lysozyme has expanded in recent years, the functional materials developed from them have currently opened a new line of research worldwide.
Table 2 Technological applications and biological activity of lysozyme.
| Source of lysozyme | Immobilization | Characteristics | Technological application and biological activity | References |
|---|---|---|---|---|
| Commercial from chicken egg white | CS-TPP nanoparticles by ionic complexation | 20000 U mg-1, D: 50-280 nm, EE (%): 47.3, PIEA (%): 85 | Possible antimicrobial material, evaluation not reported | Deng et al., 2006 |
| Commercial from chicken egg white | Nanofibers by electrospinning: deposition of the components by layers | 25,000 U mg-1 Z: +26.4 mV | Evaluated as antimicrobial packaging. S. aureus and E. coli were inhibited 60-80% | Huang et al., 2012 |
| Commercial from chicken egg white | CS-TPP nanoparticles by ionic complexation | ~100,000 U mg-1, D: 159 ± 24 nm, Z: +41.6 ± 1.1 mV, EE (%):10 ± 0.2 | Antimicrobial agent or possible drug. Evaluated against S. epidermidis | Piras et al., 2014 |
| Purified from egg white | CS beads crosslinked with glutaraldehyde | N/D | Control Oenococcus oeni during the winemaking process | Liburdi et al., 2016 |
| Commercial from chicken egg white | CS-TPP nanoparticles by ionic complexation | D:297.9 nm, Z: + 39 mV, EE (%): 29.1 | Activity against Bacillus subtilis, M. luteus, Serratia marcescens and E. coli | Matouskova et al., 2016 |
| Commercial from chicken egg white | CS-TPP nanoparticles by ionic complexation | D: 488.8 ± 10.22 nm, Z: +21.10±0.84 mVPDI: 0.71 ± 0.17, EE (%): 80% | Antibacterial material against S. aureus and E. coli | Wu et al., 2017 |
| Commercial from chicken egg white | CS and sodium alginate hydrogels | 5000 U mg-1, spherical shape, Z: -55 a -30 mV, SI: 45.66 ± 7.62, RRC(%): 87.72 ± 3.96 | Controlled release material to control foodborne microorganisms. Complete inhibition of E. coli and S. aureus | Wu et al., 2018b |
| Commercial from chicken egg white | CS-carrageenan-lysozyme nanoparticles by ionic complexation | 40000 U mg-1, D: 250 ± 8.1 nm, Z: +21.6 ± 0.8 mV, EE (%):70 ± 1.7 | Antifungal activity against Aspergillus parasiticus | Hernández-Téllez et al., 2018 |
| Commercial from chicken egg white | CS nanoparticles by ionic complexation with sodium alginate and calcium ions | 4000 U mg-1, D: 901.8 ± 75.50 nm Z: +2.58 ± 0.87 mV, EE (%): 92.79 ± 0.53 | Antimicrobial material proposed for the food industry | Wu et al., 2018c |
aCS: Chitosan; bD: hydrodynamic diameter (nm); cPIEA: post-immobilization enzyme activity; dZ: Z potential/charge; eEE: encapsulation efficiency; fT: film thickness; gSI: Swelling index; hRRC: relative release capacity; iTPP: tripolyphosphate; jND: not determined.
Conclusions and perspectives. Lysozymes are present in a variety of living organisms, and notably, they play an important role in the immunological system, digestion, and nutrition. To date, it is well known that lysozymes and lysozyme-like enzymes can inhibit bacterial growth and affect fungi and yeasts. The reported action mechanisms for inhibiting fungi and bacteria include aggregation on the cell surface and, subsequently, the activity of muramidase and/or isopeptidase, which compromise important proteins and polysaccharides present in the cell wall. However, the mechanism to inhibit the fungal and yeast polysaccharides is still unclear. Although the type-c lysozyme is the most common enzyme used for antimicrobial purposes, other types are exhibiting this activity; thus, all types of lysozymes and lysozyme-like enzymes should be carefully studied, particularly those from plants and insects, since they can affect not only bacteria but also phytopathogenic fungi and yeast. Based on the literature review, it has been proposed to develop micro and nanomaterials formulated with these antimicrobial enzymes and polymeric matrices and the latter with polycationic polymers such as chitosan. Since it is expected that this combination could be capable of acting synergically for food preservation, crop protection, and pharmaceutical formulations, moreover, it is crucial to study the implications of its application at the laboratory and at higher-levels, specifically, on those materials directed to the control of phytopathogenic fungi.











 texto en
texto en