Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.38 no.2 Texcoco Mai. 2020 Epub 27-Nov-2020
https://doi.org/10.18781/r.mex.fit.2001-6
Phytopathological notes
Isolation and identification of pathogens causing stem rot of the fig tree (Ficus carica)
1 Colegio de Postgraduados, Km 36.5, Carretera México-Texcoco, Montecillo, Texcoco, Estado de México, México, CP 56230.
In Mexico, the intensive production system of fig (Ficus carica) var. “Nezahualcoyotl” requires densities of 12,500 plants ha-1 for yields greater than 100 t ha-1. The asexual propagation of the fig through cuttlings in the nursery is not exempt from diseases. The objective of this investigation was to identify the causal agents of the rot of cuttings of the variety “Nezahualcoyotl” and to generate the corresponding severity scale. The pathogenicity of the isolates was corroborated through Koch’s postulates. The sequencing of the ITS1-ITS4 regions indicated that the isolates were highly related to Fusarium solani, Alternaria alternata and Pythium ultimum. A severity scale with five levels of damage induced by F. solani and A. alternata and three by P. ultimum is reported for the first time. Inoculation separately with F. solani and A. alternata promoted 100% cortex damage between 21-25 days after inoculation (DAI). The coinoculation of the three pathogens induced earlier (between 11-13 DAI) necrosis of the epidermis (85%) and the cortex (80%). The severity scale will be a valuable help in the quantification and monitoring of the rot of the fig tree stem.
Key words: Propagation; fig; necrosis; severity; Oomycetes; Ascomycetes.
En México, el sistema de producción intensivo de higo (Ficus carica) var. “Nezahualcoyotl” requiere de densidades de 12,500 plantas ha-1 para rendimientos superiores a 100 t ha-1. La propagación asexual de esta variedad mediante estacas no está exenta de enfermedades. El objetivo de esta investigación fue identificar los agentes causales de la pudrición de estacas de la variedad “Nezahualcoyotl” y generar la correspondiente escala de severidad. La patogenicidad de los aislamientos se corroboró a través de los postulados de Koch. La secuenciación de las regiones ITS1-ITS4 indicó que los aislamientos estuvieron altamente emparentados con Fusarium solani, Alternaria alternata y Pythium ultimum. Se reporta por primera vez una escala de severidad con cinco niveles de daño inducidos por F. solani y A. alternata y tres por P. ultimum. La inoculación por separado con F. solani y A. alternata promovió el 100% de daño de la corteza entre los 21-25 días después de la inoculación (DDI). La coinoculación de los tres patógenos indujo más tempranamente (entre los 11-13 DDI) la necrosis de la epidermis (85%) y de la corteza (80%). La escala de severidad será un valioso auxilio en la cuantificación y seguimiento de la pudrición del tallo de la higuera.
Palabras clave: Propagación; higo; necrosis; severidad; Oomicetos; Ascomicetos
Fig trees (Ficus carica) arrived in Mexico during the Spanish conquest in the 16th Century (Flaishman et al., 2008), and it was the common fig trees that adapted best, since they required no pollination for the development of syconia. However, with time, the original varieties underwent phenotypical variations, such as size, color and consistency. One of these new varieties was described and named the “Nezahualcóyotl” cultivar by García et al. (2013). This vegetative material is used for the intensive production, under cover, due to its quick growth, simple handling and high productivity, which helps obtain densities of 12,500 plants ha-1 with yields of over 100 t ha-1 (Mendoza et al., 2017). The implementation of this production system requires twenty times as many plants than conventional systems, and for them to be free of pests and diseases. The main way to disseminate fig trees is using a cuttling of a ligneous stem trimmed after harvest (Boliani et al., 2019). The plants produced in the greenhouse may display rotting of the stem and roots, related to the pathogens of the damping off complex (Fusarium, Rhizoctonia, Pythium and Phytophthora), which is favored by a relative humidity of 80%, a temperature of 25 °C and high densities (García et al., 2008).
In order to carry out a disease control plan, the priority is to identify the causal agents, as well as to create guides that help understand the symptoms and the progress of the disease. The severity scales are useful to standardize and quantify the disease, which will help implement management plans (Hernández and Sandoval, 2015). The aim of this investigation was to isolate and identify the causal agents of stem rot in fig trees, as well as to create scales for the evaluation of the severity of the causal agents to serve as guides in future epidemiological studies.
For the isolation of the causal agents, 50 individuals, distributed totally at random were chosen out of a plot of trees grown out of 20-day-old cuttlings, which displayed slow growth, chlorosis, wilting, fallen leaves and rot in stems and roots. The plants were taken from the propagation greenhouse in the Fitotecnia Experimental Field of the Universidad Autónoma Chapingo at 19° 29’ 30.8” latitude North, 98° 52’ 22.0” longitude West, and an altitude of 2,266 m. The plants were washed with running water and soap. Later, the epidermis was removed using a scalpel to expose the cortex, which showed reddish-brown lesions on the ends of the cuttling (Figure 1). Using a scalpel, we cut four sections on each cuttling on the edge of the lesions, 0.3 x 0.3 cm each side, and 0.1 cm thick (Leslie and Summerell, 2006), for a total of 200 sections. Four sections were taken for each stem, which were submerged while constantly shaking in a sodium hypochlorite solution (NaClO) at 0.5% for one minute. They were then rinsed three times in sterile distilled water and dried on sterile paper towels inside a laminar flow hood. Under aseptic conditions, the sections of tissue from each stem were placed in Potato-Dextrose-Agar, or PDA, medium plates (BD Bioxon®), and they were incubated at 28 °C for five days. Two types of fungi were isolated, along with one oomycete, all of which were purified using the hyphal tip method and monosporangia, and were morphologically identified by microcultivations of each isolation in PDA medium blocks, re-sown by puncture and incubated at room temperature. The samples were dyed using methylene blue, observed under an optical microscope at a magnification of 40X, and identified morphologically with the keys by Barnett and Hunter (1998).
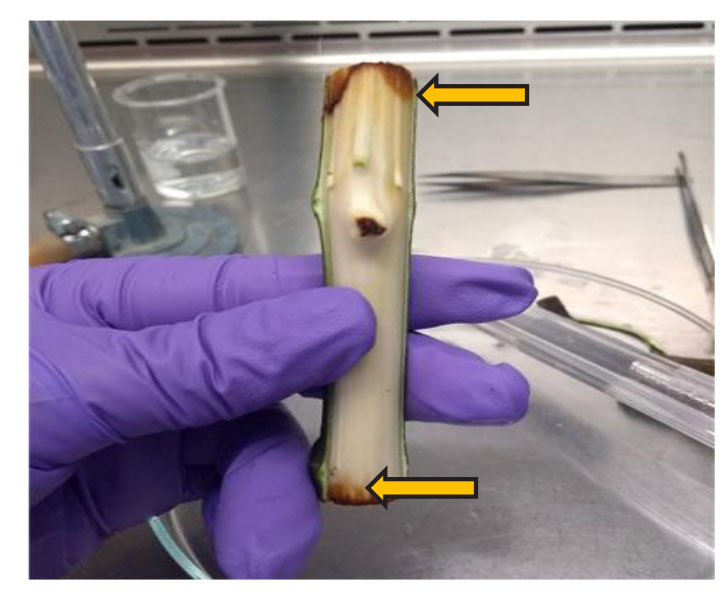
Figure 1. Reddish-brown lesions on the cortex (arrows) of a cuttling of F. carica inoculated with F. solani, A. alternata and P. ultimum.
Koch’s postulates were reproduced using the microbial isolations related to stem rot. Fresh fig tree cuttlings, 8 cm in length, were used, which were washed with running water and disinfested in a 1% NaOCl solution for two minutes. Next, the cuttlings were rinsed with sterile distilled water, and exposed to ethyl alcohol at 96% for one minute and rinsed again. The cuttlings were dried on sterile paper towels inside a laminar flow hood. The cuttlings were then placed in wet chambers, inoculated in triplicate with the isolated microorganisms, and incubated at 28 °C. After ten days, tissue samples were taken to re-isolate the inoculated microorganism and record the symptomatology of each one of the infected cuttlings. The wet chamber consisted of a transparent 240 mL cup with a lid, inside of which a fig cuttling was placed, held up with a styrofoam base. The inoculant consisted of three discs, 0.5 cm in diameter, with 7 days of growth, for individual inoculations, 1.5 discs of each medium in double inoculations, and one disc of each medium for triple inoculations. The discs were placed on a 5 x 5 cm sterile, damp paper towel and 7 x 7 piece of aluminum foil, attached to the base of the cuttling with Parafilm “M” ®. The bottom of the cup was covered with a 10 x 10 cm damp paper towel and the lid was sealed with Parafilm “M” ®.
For the molecular identification, the isolations were incubated at 27 °C for seven days, to then extract the DNA. By means of PCR, we amplified the internal transcribed regions ITS1 and ITS4 of fungi and oomycetes, using the primers ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC). For the fungi, we used an initial cycle of 94 °C for five minutes, followed by 35 cycles (94 °C, 30 seconds; 56 °C, 45 seconds and 72 °C, two minutes) and a final extension at 72 °C for five minutes. For the oomycetes, we used an initial 95 °C cycle lasting four minutes, 34 cycles (95 °C, one minute; 55 °C, one minute and 72 °C, two minutes) and a final extension step at 72 °C for 10 minutes (White et al., 1990). The amplified products were separated by electrophoresis in agarose gels at 0.8%. The sequences were assembled and edited using the program BioEdit, and they were compared with the sequences contained in the GeneBank database of the National Center of Biotechnology Information (NCBI) using BLAST to determine the possible species of each isolation.
On the other hand, fresh cuttlings, approximately eight centimeters long, were placed in wet chambers individually, as in the pathogenicity tests. The cuttlings were inoculated individually with the isolated pathogens, as well as with all the combinations, establishing fifteen repetitions per treatment, which resulted in a total of 120 experimental units. They were incubated at 28 °C and three cuttlings or repetitions of each treatment were chosen at random with time, in order to determine the levels of severity (scale of severity).
Finally, the percentage of rotting was evaluated. This was measures by measuring the area of the rot in the cuttling, both in the cortex and the epidermis, as well as the total area of the cortex and the epidermis, obtaining the proportion with the following formula: (A2 x 100)/A1, where A2 is the total area of the epidermis or of the cortex of the cuttling, and A1 is the area of the epidermis or of the cortex with rot. The measurements were carried out in triplicate, the average was calculated and damage percentage range was proposed for each level of severity with each treatment. The measurements were taken using the image processing software IMAGE J, version 1.50i, developed by the National Institute of Health (Rasband, 2018).
The isolations turned out to be highly related to F. solani (99% similarity) with a 95% frequency in diseased cuttlings, A. alternata (99% similarity) with a 72% frequency, and P. ultimum (100% similarity) with an 80% frequency. Koch’s postulates confirmed that F. solani, A. alternata and P. ultimum were the causal agents in the rotting of the fig cuttlings, since similar symptoms were observed to those found on the field. The re-isolation of the two fungi and oomycetes matched those inoculated initially at 95%.
Table 1 shows the scale of severity, including the percentage of damage caused by F. solani, A. alternata, and P. ultimum individually, as well as the damage induced by its combinations with time in the cortex and epidermis of the cuttling. In general, five visually different levels of damage were established. However, for P. ultimum, only three were established, since the progress of the disease was only detected 32 days after inoculation (DAI). To date, there are no reports of the presence of these three pathogens in fig cuttlings, although they are present in other crops with similar symptoms under greenhouse conditions.
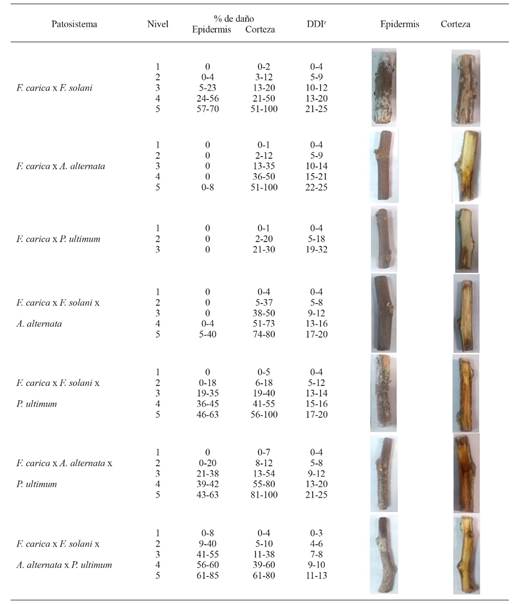
zDays after inoculation.
Table 1. Scale of severity showing the percentage of damage on Ficus carica cuttlings induced by Fusarium solani, Alternaria alternata and Pythium ultimum inoculated individually and in combination.
Fusarium solani attacks adult mango plantations, displaying symptoms of wilting in the stem apex and necrosis in vascular tissues (Khanzada et al., 2004). Likewise, in apple trees, it causes smut and defoliation (Wang et al., 2010). This fungus produces enzymes such as cutinases, cellulases, chitosanases, pectinases and others that degrade phytoalexins (Leslie and Summerell, 2006). Due to this mechanism, F. solani can easily affect soft and lignified tissues, such as the fig tree stems, which displayed their first symptoms of rotting five days after inoculation (DAI). The symptoms of the pathogenic species of the genus Fusarium are vascular wilting and the rotting of stems and roots (Torres, 2000). In this investigation, the highest percentages of rotting of the epidermis and cortex, the presence of mycelia on the surface of the cuttling, and the detachment of the epidermis took place in stage 5 of the scale of severity, showing 57-70 and 51-100% of damage on the epidermis and cortex, respectively, between 21 and 25 DAI.
On the other hand, the most important species of the genus Alternaria is A. alternata, which can appear as a pathogen, an opportunist or a saprophyte (Logrieco et al., 2009). In adult fig trees, damages caused by species of Alternaria have been reported, although they are always related to damages in leaves and syconia, as well as in apple, citrus, olive and tomato fruits (López et al., 2016). All the pathogenic species of Alternaria produce phytotoxins that are specific and non-specific to their hosts; among the non-specific toxins is the zinniol, which induces necrosis (Lou et al., 2013). The highest percentage of necrosis on the cortex of fig tree cuttlings, promoted by A. alternata was displayed on level 5 of the severity scale, with 51-100% of damage, between 22 and 25 DAI. However, the epidermis only displayed 8% rotting on level 5. The presence of gray mycelia was also observed on the outside of the cuttling. The separate inoculation with F. solani and A. alternata induced 100% of damage on the cortex between 22 and 25 DDI.
The oomycete P. ultimum is a member of the damping off complex, which causes the death of seedlings of various species of agricultural importance. This oomycete is one of the most aggressive species in its genus (Mavrodi et al., 2012). Pythium ultimum is related to the rotting of roots in adult plants, rootstocks and apple seedlings (Mazzola et al., 2002; Zhu et al., 2016). In this investigation, P. ultimum promoted 21-30% of the damage on the cortex 19-32 DAI. The presence of mycelia was also observed in the upper section of the cuttling, and it detached easily when touched. The epidermis presented no rotting or detaching.
The highest damage percentages appeared in a lower time range (17 to 20 DAI), when mixed inoculations were carried out with F. solani + A. alternata and F. solani + P. ultimum. The drastic appearance of necrosis on the epidermis (61-85%) and cortex (61-80%) took place between 11-13 DAI, when F. solani, A. alternata and P. ultimum were inoculated together. This could be explained by the synergic effect of the enzymes that each pathogen synthesizes, helping the disease to progress. The control cuttlings showed slight damage due to oxidation from the cut. On the field, the necrosis of the fig tree stem is related to the dissemination of spores and mycelia through water, wind or tools. Notwithstanding this, in the greenhouse, the high density may favor the dissemination of propagules and infestation, since the seedlings are near each other in the processes of propagation, climatization and transportation.
This work is the first time in which F. solani, A. alternata and P. ultimum are reported as causal agents of the rotting of fig tree cuttlings. The symptoms found in the cuttlings evaluated in vitro were similar to those presented in propagation greenhouse seedlings, thus confirming Koch’s postulates. The proposed scale of severity will help identify the degree of damage caused by F. solani, A. alternata and P. ultimum, whether individually or combined, in intensive fig production systems. This investigation leads to the need to validate the scales of severity in the fig propagation system in the greenhouse. In this way, when verifying their precision and accuracy, they may be used successfully in the evaluation of damages of this species. On the other hand, the identification of the pathogens F. solani, A. alternata and P. ultimum will help carry out an adequate phytosanitary control, and offer better-quality plants that allow an optimum establishment of intensive systems.
Acknowledgements
This contribution is part of an investigation for a Master in Science degree from the Soil Science program of the Colegio de Postgraduados, Campus Montecillo, which was economically funded by the National Science and Technology Council (Conacyt).
REFERENCES
Barnett, HL. and Hunter, BB. 1998. Illustrated genera of imperfect fungi. The American Phytopathological Society, Minnesota, USA. 200p. [ Links ]
Boliani, AC., Ferreira, AFA., Monteiro, LNH., Silva, MSACD. and Rombola, AD. 2019. Advances in propagation of Ficus carica L. Revista Brasileira de Fruticultura 41:1-13. doi: 10.1590/0100-29452019026 [ Links ]
Flaishman, MA., Rodov, V. and Stover, E. 2007. The fig: botany, horticulture, and breeding. Horticultural Reviews 34:113-196. doi: 10.1002/9780470380147.ch2 [ Links ]
García, V., Iriarte, A., Flores, S. y Lesino, G. 2008. Monitoreo higrotérmico de un edificio acondicionado para propagacion agámica de plantas. Avances en energías renovables y medio ambiente 12:29-36. Recuperado de https://www.mendoza-conicet.gob.ar/asades/modulos/averma/trabajos/2008/2008-t002-a005.pdf [ Links ]
García, RMT., Mendoza, CVM., Valadez, ME. y Muratalla, LA. 2013. Initial assessment of natural diversity in Mexican fig landraces. Genetics and Molecular Research 12:3931-3943. doi: 10.4238/2013.September.23.12 [ Links ]
Hernández, RL. y Sandoval, IJS. 2015. Escala diagramática de severidad para el complejo mancha de asfalto del maíz. Revista Mexicana de Fitopatología 33:95-103. Recuperado de http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0185-33092015000100095&lng=es&tlng=es. [ Links ]
Khanzada, MA., Lodhi, AM. and Shahzad, S. 2004. Pathogenicity of Lasiodiplodia theobromae and Fusarium solani on mango. Pakistan Journal of Botany 36:181-190. Recuperado de https://www.researchgate.net/profile/Saleem_Shahzad/publication/266069789_Pathogenicity_of_Lasiodiplodia_theobromae_and_Fusarium_solani_on_mango/links/543d23540cf2c432f742531c.pdf [ Links ]
Leslie, JF. and Summerell, BA. 2006. The Fusarium laboratory manual. Blackwell Publishing. USA. 388p. [ Links ]
Logrieco, A., Moretti, A. and Solfrizzo, M. 2009. Alternaria toxins and plant diseases: an overview of origin, occurrence and risks. World Mycotoxin Journal 2:129-140. doi: 10.3920/WMJ2009.1145 [ Links ]
López, P., Venema, D., de Rijk, T., de Kok, A., Scholten, JM., Mol, HG. and de Nijs, M. 2016. Occurrence of Alternaria toxins in food products in The Netherlands. Food Control 60:196-204. doi: 10.1016/j.foodcont.2015.07.032 [ Links ]
Lou, J., Fu, L., Peng, Y. and Zhou, L. 2013. Metabolites from Alternaria fungi and their bioactivities. Molecules 18:5891-5935. doi: 10.3390/molecules18055891 [ Links ]
Mavrodi, OV., Walter, N., Elateek, S., Taylor, CG. and Okubara, PA. 2012. Suppression of Rhizoctonia and Pythium root rot of wheat by new strains of Pseudomonas. Biological Control 62:93-102. doi: 10.1016/j.biocontrol.2012.03.013 [ Links ]
Mazzola, M., Andrews, PK., Reganold, JP. and Levesque, CA. 2002. Frequency, virulence, and metalaxyl sensitivity of Pythium spp. isolated from apple roots under conventional and organic production systems. Plant Disease 86:669-675. doi: 10.1094/PDIS.2002.86.6.669 [ Links ]
Mendoza, CVM., Vargas, CJM., Calderón, ZG., Mendoza, CMDC., and Santacruz, VA. 2017. Intensive production systems of fig (Ficus carica L.) under greenhouse conditions. Experimental Agriculture 53:339-350. doi: 10.1017/S0014479716000405 [ Links ]
Rasband, WS. 2018. ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997-2018. [ Links ]
Torres, GA. 2000. Algunos aspectos de los hongos del género Fusarium y de la especie Fusarium oxysporum. Agronomía Colombiana 17:11-16. Recuperado de http://www.bdigital.unal.edu.co/24385/ [ Links ]
Wang, PH., Chen, YS., Lin, MJ., Tsou, YJ. and Ko, WH. 2010. Severe decline of wax apple trees caused by Fusarium solani in northern Taiwan. Botanical Studies 51:75-80. Recuperado de http://hdl.handle.net/11455/68359 [ Links ]
White, TJ., Bruns, T., Lee, S. and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Pp:315-322. In: Innis, MA., Gelfand, DH., Sninsky, JJ. and White, TJ (eds.). PCR protocols: a guide to methods and applications. Academic Press. San Diego USA. 482p. doi: 10.1016/b978-0-12-372180-8.50042-1 [ Links ]
Zhu, Y., Shin, S. and Mazzola, M. 2016. Genotype responses of two apple rootstocks to infection by Pythium ultimum causing apple replant disease. Canadian Journal of Plant Pathology 38:483-491. doi: 10.1080/07060661.2016.1260640 [ Links ]
Received: January 24, 2020; Accepted: April 09, 2020











 texto em
texto em 


