Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.37 no.3 Texcoco Set. 2019 Epub 30-Set-2020
https://doi.org/10.18781/r.mex.fit.1904-7
Phytopathological notes
Distribution, incidence and severity of the dieback (Lasiodiplodia spp.) in persa lime in Morelos, Mexico
1 Escuela Superior de Ciencias Naturales, UAGro, Avenida Universidad S/N, Ex Rancho El Shalako, Carretera Nacional Chilpancingo-Petaquillas, Guerrero, México. C.P. 39105;
2 Escuela de Estudios Superiores de Xalostoc (EESuX), UAEM, Av. Nicolás Bravo S/N, Parque industrial Cuautla, Ayala, Morelos;
3 Facultad de Ciencias Agropecuarias. UAEM. Avenida Universidad No. 1001, Colonia Chamilpa, Cuernavaca, Morelos, México. C.P. 62209;
4 CIB, UAEM, Avenida Universidad No. 1001, Colonia Chamilpa, Cuernavaca, Morelos, México. C.P. 62209;
5 INIFAP, C. E. - Zacatepec, Carretera Zacatepec-Galeana Km 0.5, Zacatepec, Morelos, México;
6 INIFAP, C. E. - Iguala. Carretera Iguala-Tuxpan Km 2.5, Tuxpan, Iguala de la Independencia, Guerrero, México C.P. 40000;
7 Centro Nacional de Investigación Disciplinaria en Conservación y Mejoramiento de Ecosistemas Forestales-INIFAP, Coyoacán. CDMX.
The distribution, incidence and severity damage caused by the dieback complex (Lasiodiplodia spp.) in Persian lime (Citrus latifolia) was determined in 46 orchards of six average years old, 25 trees per orchard in the municipalities of Amacuzac, Ayala, Coatlan del Rio, Cuautla, Jantetelco, Jonacatepec, Puente de Ixtla, Tepalcingo and Tlaltizapan of Zapata, Morelos State, Mexico. Incidence was evaluated according to the number of plants having symptoms of dieback. There were four levels of severity, where 0= healthy tree, 1= gum exudation, 2= cracking with exposure of internal tissue and 3= well-defined canker and branch dieback. Which were transformed to a percentage of means of a scale of three levels of severity, transforming to a percentage of infection using the Townsend and Heuberger equation. Data were analyzed trough the Kruskal Wallis test and Tukey (α=0.05) and p<0.0001 in incidence and severity. There was a high incidence of dieback on stems 78% of Persian lime trees. The incidence and severity of dieback of Persian trees on different orchards had a range of 31.8 to 100% and 30 to 100%, respectively. By the other hand, municipalities such as Amacuzac, Ayala, Coatlan del Rio and Tlaltizapan, had the lowest levels of incidence and severity of dieback.
Key words: Lasiodiplodia theobromae; Lasiodiplodia citricola; Lasiodiplodia pseudotheo-bromae; Citrus latifolia
Se determinó la distribución, incidencia y severidad de la muerte descendente (Lasiodiplodia spp.) en lima persa (Citrus latifolia) en 46 huertos de seis años de edad, 25 árboles por huerto en los municipios de Amacuzac (7 huertos), Ayala (4), Coatlán del Río (9), Cuautla (1), Jantetelco (5), Jonacatepec (1), Puente de Ixtla (5), Tepalcingo (3) y Tlaltizapán de Zapata (11), del estado de Morelos, México. La incidencia fue determinada con base al número de plantas enfermas y la severidad mediante escala de daño de cuatro clases: 0= árbol sano, 1= exudación de goma, 2= agrietamiento con exposición de tejidos internos y 3= cancro bien definido y muerte descendente de ramas. La severidad fue transformada a porcentaje de infección con la fórmula de Townsend y Heuberger, analizados por Kruskal Wallis y separados por la comparación de medias, prueba de Tukey (α= 0.05) y p< 0.0001 en incidencia y severidad. La enfermedad se registró en el 78% de los huertos, con incidencias de 31.8 a 100% y severidades de 30.0 al 100%. Los huertos con menor incidencia y severidad fueron los ubicados en Amacuzac, Ayala, Coatlán del Río y Tlaltizapán de Zapata.
Palabras clave: Lasiodiplodia theobromae; Lasiodiplodia citricola; Lasiodiplodia pseudotheobromae; Citrus latifolia
Mexico ranks second in Mexican lemon (Citrus aurantifolia) and Persian lime (Citrus latifolia) production and accounts for 21% of production worldwide with 627.4 thousand tons (FAOSFAT, 2018), from which 70% of the total production is used to supply the national market and the rest is exported mainly to United States. In 2016, Mexico produced 2’439,477 ton of Persian lime mostly in the states of Veracruz (717,014 t), Michoacan (619,612 t) and Oaxaca (263,448 t); Morelos is in twelfth place with 3,880 ton and currently has 612 ha sown to citrus in 17 municipalities, where lemon (32%) and Valencia orange (66%) prevail on the established area and yield 12.1 and 7.7 ton ha-1, respectively (SIAP, 2018).
However, Persian lime trees are affected by fungal diseases that infect roots, trunk, foliage and fruits, and cause production losses (Christensen, 2017). In Morelos, it was thought that the death of trees was caused by gummosis (Phytophthora parasitica), since it had been reported in the states of Colima and Tabasco, where symptoms of gum secretion and rot of the foot caused the death of ungrafted and grafted rootstock citrus trees and, consequently, the elimination of orchards (Acosta-Pérez et al., 2012; Vidales 1982). However, there are several fungi genera of the Botryosphaeriaceae family that are considered to be very aggressive endophyte pathogens that often kill most of their host after causing physical damage, usually when under stress; another symptoms are foliar spots, fruit rot, dieback, gummosis, perennial canker and, finally, the death of economically important species of woody perennial crops and ornamental plants, as well as species of native and introduced forest trees (Mohali et al., 2007; Slippers and Wingfield, 2007).
Recent studies cite species of Lasiodiplodia genus in the tropic that cause diseases in important crops such as cocoa (Theobroma cacao), avocado (Persea americana), papaya (Carica papaya), natural rubber (Hevea brasiliensis), chirimoya (Annona cherimola), peach (Prunus persica), sugarcane (Saccharum officinarum), grape (Vitis vinifera) and citrus (Picos-Muñoz et al., 2015). Valle-De la Paz et al. (2019) reported the presence of at least three species of Lasiodiplodia (L. theobromae, L. citricola and L. pseudotheobromae) genus that cause gummosis, dieback of branches and the death of Persian lime trees in Morelos. However, the distribution and impact of these pathogens in lime commercial crops in Morelos is unknown. Therefore, the objective of this study was to determine the distribution, the incidence and the severity of dieback caused by the complex of Lasiodiplodia spp. in Persian lime orchards in Morelos.
Samples were collected in 46 Persian lime orchards from September 2014 to August 2015 (Figure 1). In order to identify the species, according to Valle-De la Paz et al. (2019), 46 samples were taken from primary and secondary branches, as well as from trunks showing disease symptoms; the 15 monosporic isolates obtained from the samples were re-sown in V8-Agar medium. The morphological identification at the genus level was achieved using the keys and taxonomic descriptions of Phillips et al. (2013).
The molecular identification was performed using genomic DNA (ADNg) from mycelium following the AP method described by Sambrook and Russell (2012). The primers used were ITS5 and ITS4 from ribosomal genes (ADNr). The results of the sample analysis were corroborated through traditional PCR according to the protocol described by Ahrens and Seemüller (1992), with modifications to the components, according to Sambrook and Russell (2012). The quality was evaluated through horizontal electrophoresis in 1% agarose gel (Ultrapure, Gibco, USA) and the bands were visualized using a transilluminator (Gel Doc 2000, BIO RAD®, USA). The concentration of DNA was quantified using a NanoDrop 2000 ((Thermo Scientific®). The DNA fragments amplified by PCR were purified with the Wizard (Promega®, USA) kit, following the manufacturer’s protocol. The purified PCR product was sequenced in both directions by Macrogen Inc. (Seoul, South Korea). The sequences were assembled and edited using the CAP option (Contig Assembly Program) in the BioEdit software v7.0.9.1. The fungi sequences were compared and deposited in the GenBank’s database. The phylogenetic trees were built using data from the ITS5 and ITS4 ends and analyzed with MEGA (Molecular Evolutionary Genetics Analysis) software version 7.0.14 (Tamura et al., 2007), aligned using ClustalW 1.8.1 (Thompson et al., 1994) and compared to sequences of homologous genes in the database of the National Center for Biotechnology Information. After analyzing the congruence among data sets, an analysis of maximum parsimony (MP) was conducted, and phylogenetic analyses were done by using the Phylogenetic Analysis Using Parsimony (PAUP) software version 4.0b10 (Swofford, 2003). The phylogenetic trees were found using the heuristic search function with 1,000 random addition replicates, tree bisection and reconstruction (TBR) selected as branch-swapping algorithm, and the missing spaces or data were considered complete deletions (Kimura, 1980; Hillis and Bull, 1993). The HQ231345 sequence of Phoma tracheiphila from the GenBank was used as a taxon external to the group.
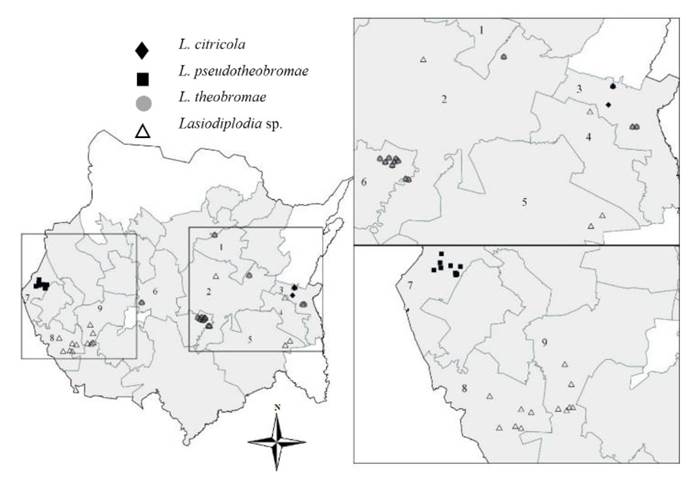
Figure 1 Distribution of Lasiodiplodia species in Persian lime (Citrus latifolia) orchards in Morelos, Mexico. Municipalities where samples were collected = 1: Cuautla; 2: Ayala; 3: Jantetelco; 4: Jonacatepec; 5: Tepalcingo; 6: Tlaltizapan de Zapata; 7: Coatlan del Rio; 8: Amacuzac; 9: Puente de Ixtla. White polygons do not include a citrus area.
The incidence was evaluated following the methodology proposed by Acosta-Pérez et al. (2012), that is, by selecting five rows from each orchard, 4 to 21 trees per row, and a total of 21 to 50 trees per orchard. The percentage of incidence was determined by dividing the number of infected plants by the total of plants evaluated and then multiplied by 100. The disease severity was evaluated using the scale proposed by Orozco-Santos (1995) and modified by Valle-De la Paz, which consists of four classes, where 0= healthy tree, 1= presence of gum exudation on the main trunk or on the primary and secondary branches, 2= visible cracks, exposure of internal tissue, on the main trunk or on the primary and secondary branches, and 3= presence of well-defined canker and dieback of branches (Figure 2). The severity was converted to percentage of infection (% PI) using the formula of Townsend and Heuberger (1943). The resulting data were subjected to tests of normality (Shapiro-Wilk) and homogeneity of variance (Levene and Bartlett), but since the principles were not fulfilled, a nonparametric statistics analysis was done using the Kruskall Wallis test and a mean comparison using Tukey’s test (α= 0.05) and p< 0.0001.
The symptoms observed in Persian lime trees in Morelos were stem cracks, gum exudation (Figure 2 B), cankers at the stem base (Figure 2 E), visible cracking with internal tissue exposure (Figure 2 C), canopy yellowing, dieback of branches, descending necrosis and black spots on the branches (Figure 2 D), which correspond to the pathogen’s fructiferous bodies (pycnidia) (Figure 3 E and F). The disease advanced to the base of the annual branch and invaded the secondary branches, a fact that is in agreement with the results obtained by Khanzada et al. (2004), who reported that new branches of infected plants start to wilt, and then the leaves become brown, their margins fold up, dry and fall off. Under severe conditions, the branches dry one after another in sequence until the entire tree dies.
Some of the symptoms are similar to those caused by P. parasitica, particularly gum secretion and the formation of cankers (Acosta-Pérez et al., 2012; Vidales, 1982), while Lasiodiplodia spp. causes dieback of branches, formation of pycnidia, as well as browning of the vascular tissue of dead branches (Figure 2). Valle-De la Paz et al. (2019), based on morphological traits, identified the Lasiodiplodia sp. genus. The molecular analysis determined groups of four clades: one for 11 isolates of the Lasiodiplodia sp. genus, three clades for species, one isolate aligned for Lasiodiplodia citricola, another for L. pseudotheobromae and two for L. theobromae (Figure 3).

Figure 2 Symptoms and levels of damage caused by dieback of Persian lime trees in Morelos. A) 0: Healthy tree; B) 1: Presence of gum exudation on the main trunk or on the primary and secondary branches; C) 2: Visible cracks, exposure of internal tissue, on the main trunk or on the primary and secondary branches; D and E) 3: Dieback of branches and well-defined canker.
Figure 1 shows the distribution of Lasiodiplodia species by municipality where the samples were collected: Cuautla (Lasiodiplodia sp., and L. theobromae), Tlaltizapan de Zapata (Lasiodiplodia sp. and L. theobromae) and Jantetelco (Lasiodiplodia sp., L. citricola and L. theobromae). L. pseudotheobromae was found only in Coatlan del Rio, Lasiodiplodia sp. in Ayala, Jonacatepec, Tepalcingo, Amacuzac and Puente de Ixtla. L. theobromae was the prevailing species (28.57%), followed by L. pseudotheobromae (16.07%) and L. citricola (3.57%). Lasiodiplodia sp. was found in 51.78% of the sampled orchards, which indicates that new species are likely present.
The mean tests of dieback incidence were separated in 18 groups with statistical differences among them. In the group A were the orchards with the highest percentage of incidence (96.7-100%), located in the municipalities of Jonacatepec (1 orchard), Coatlan del Rio (2 orchards), Tlaltizapan de Zapata (2 orchards) and Cuautla (1 orchard). The following 10 groups included the orchards with intermediate-high incidence (55.4-88.1%), located in the municipalities of Jantetelco (2 orchards), Coatlan del Rio (4 orchards), Amacuzac (3 orchards), Tlaltizapan de Zapata (3 orchards), Puente de Ixtla (4 orchards), Tepalcingo (3 orchards) and Ayala (1 orchard). In the group EDHIGCF were the orchards with intermediate incidence (48.1-49.2%), located in the municipalities of Amacuzac (1 orchard), Puente de Ixtla (1 orchard) and Jantetelco (2 orchards). Then there were five groups of orchards with low-to-intermediate incidence (44.3-23.8%), located in the municipalities of Coatlan del Rio (2 orchards), Jantetelco (1 orchard), Tlaltizapan de Zapata (3 orchards) and Ayala (1 orchard). Finally, in the group I were the orchards with the lowest disease incidence (11.9-25.4%), located in the municipalities of Tlaltizapan de Zapata (3 orchards), Ayala (2 orchards), Amacuzac (3 orchards) and Coatlan del Rio (1 orchard) (Figure 6).
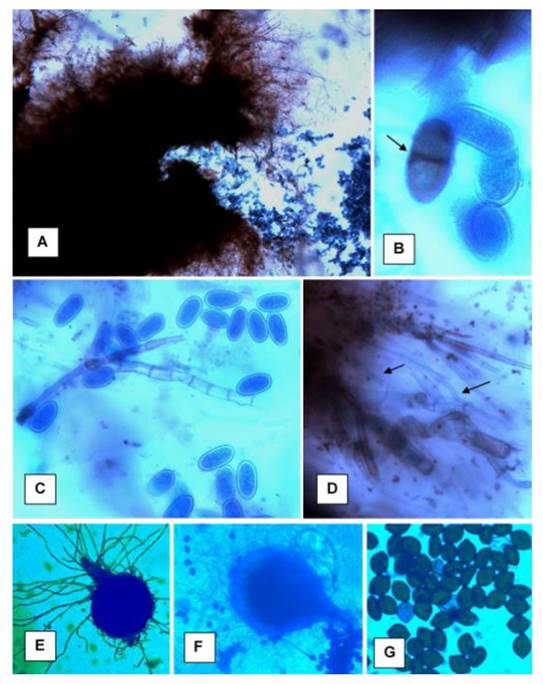
Figure 3 Lasiodiplodia theobromae, L. pseudotheobromae and L. citricola structures in a PDA medium. A) L. theobromae conidium; B) L. theobromae mature didymospore and immature amerospores; C) L. pseudotheobromae immature conidia (amerospores); D) L. pseudotheobromae paraphyses; E and F) L. citricola pycnidia and conidia; G) L. citricola conidia. Photographs taken under an optical microscope (A, F and E) 10 X; B, C, D and G) 100 X.
The percentage of incidence ranged from 49 to 100% in 37 out of the 46 orchards evaluated, from which 78% showed a level of incidence ranging from 31.8 to 100% (Figure 6), which means that the disease was observed in all the Persian lime producing areas in the state of Morelos, where factors that favor the disease were observed, including age of the plant, agronomic management, pruning and use of fertilizers, among others. However, it is important to highlight that the municipalities of Coatlan del Rio and Tlaltizapan de Zapata are located in southern-western Morelos, integrated by the extension of the foothills area where the weather is warmer. The center of the location along with the southern mountain accounts for 60% of the state surface, with 22-26°C annual average temperature, 34°C maximum temperature and summer and winter rains up to 900 mm (INEGI, 2018).
The municipality with a lower percentage of disease incidence was Coatlan del Rio (11.97%), located in southern-western Morelos, and area adjacent to Guerrero and the State of Mexico, which is characterized by a prevailing sub-humid weather with summer and winter rains A (W). There are also humid subtropical and hot tropical microclimates with indefinite winter periods. The average rainfall is 1,000 annual millimeters, 34 °C maximum temperature, 24 °C average and 14 °C minimum (Enciclopedias Municipios y Delegaciones de México, 2018). The meteorological conditions have an impact on infection and disease development. In this regard, Picos-Muñoz et al. (2015) indicated that relative humidity and high temperatures favor the development of the fungus. The authors also stated that the fungus can cause important economic losses, especially in fruit orchards. It has also been reported that a source of spreading are infected plants and pruning debris left in the field, and that high temperatures and moisture provided by irrigation also favor the disease, because the latter promotes the release of spores from pycnidia that then accumulate in the atmosphere surrounding the crop and the soil (Muhammad, 2009).
Dieback of fruit trees susceptible to the disease can cause the interaction of the pathogen with water stress, clay soils, low organic matter, a large amount of active limestone, inadequate fertilization, excess irrigation, high moisture caused by excessive shade, sunburn, wounds from natural causes or caused by the use of crop cutting tools, pruning and compacted alkaline soils that hinder root development, nutrition and airing (Agustí, 2003; Ko et al., 2004).
The mean tests of dieback severity were separated in 30 groups with statistical differences among them (Figure 6). The highest level of severity (100%) was found in orchards located in the municipalities of Tlaltizapan de Zapata (1 orchard) and Coatlan del Rio (1 orchard), which were significantly different from all the orchards from the group A. The following 14 groups included the municipalities with 55.5-95%: Tepalcingo (1 orchard), Puente de Ixtla (3 orchards), Ayala (1 orchard), Coatlan del Rio (5 orchards), Tlaltizapan de Zapata (3 orchards), Amacuzac (3 orchards), Jonacatepec (1 orchard), Jantetelco (2 orchards) and Cuautla (1 orchard) (Figure 4). Then was the group KEJMIHLGN with 50.5 mean severity in an orchard in the municipality of Tepalcingo (1), followed by 13 groups with 9.8-43.3% of infection in the municipalities of Jantetelco (2 orchards), Puente de Ixtla (2 orchards), Coatlan del Rio (2 orchards), Amacuzac (4 orchards), Tlaltizapan de Zapata (5 orchards) and Amayuca (1 orchard) (Figure 5). Finally, the group Q included two orchards in the municipality of Tlaltizapan de Zapata, which were significantly different with a level of disease severity of 3.7-5.08%. The above-mentioned groups indicate that the level of infection severity ranged from 30.0 to 100% in more than 71% of the evaluated orchards (Figure 6).
Rodríguez (2010) points out that the fungus activity is favored by temperatures of 24 ±26 °C. While Almaguer et al. (2015) agrees that Lasiodiplodia species are common in the tropics and cause several diseases at crop stages, for example, postharvest, L. theobromae affects mainly fruit trees in sites where the rain and the wind are the factors that determine spore spreading within a cropping area.
The results of this study corroborate that the highest level of damage produced by Lasiodiplodia spp. was observed in the municipalities located in southeast Morelos (Figure 1), a region characterized by a semi-warm weather with 800 mm rainfall and 24°C annual average temperature, where the soils are abundantly irrigated with underground water, a factor that can be decisive for the fast spreading of the pathogen, as in the case of other pathogens in clay soils. Prior to this study, Acosta-Pérez et al. (2012) pointed out that there was a new disease in C. latifolia plantations with external symptoms similar to those of gummosis and that producers were mistakenly managing it as such. The disease was found in 44% of C. latifolia plantations with an average incidence of 10.2% and 1.7-25.0% interval.
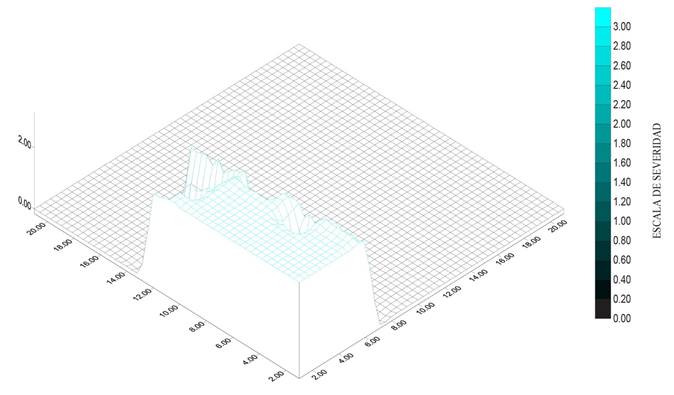
Figure 4 Severity (2.4) of die back in Persian lime (Citrus latifolia) in an orchard in Coatlan del Rio, Morelos.
The decrease in citrus area has been attributed to damage caused by Phythophthora, among other factors (Acosta-Pérez et al., 2012; Sosa et al., 2015; Vidales, 1982). However, the preliminary results of this study show a growing effect with damage up to level 3, canker and dieback of branches, which are attributed to several Lasiodiplodia species. The intensity of dieback varies in the state of Morelos and within the same municipalities, which shows that Morelos has the adequate temperature and moisture conditions, which are ideal for the disease, in addition to an inefficient management control by the producers, and that these factors promote the development and spread of the disease.
The conclusion is that dieback is a disease associated with several Lasiodiplodia species, such as Lasiodiplodia citricola, L. pseudotheobromae and L. theobromae, which cause serious damage to Persian lime trees given the high levels of incidence and severity in citrus orchards in the state of Morelos. It was found in 100% of the orchards evaluated and this shows the importance of the disease in reducing the crop profitability.
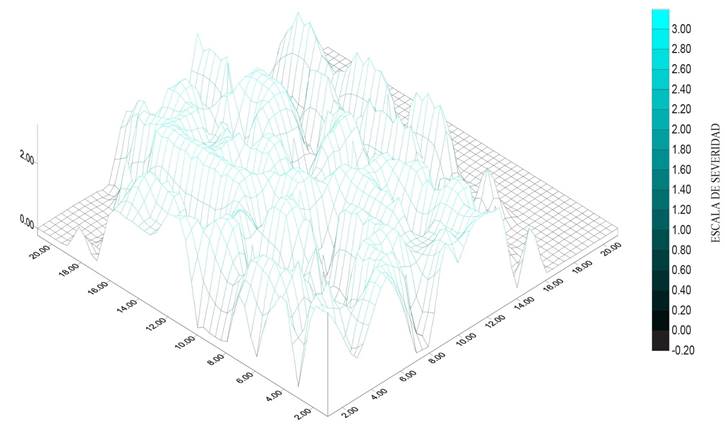
Figure 5 Severity (1.6) of die back in Persian lime (Citrus latifolia) in an orchard in the municipality of Puente de Ixtla, Morelos.
Literatura citada
Acosta-Pérez JA, Ortiz-García CF, Zaldívar-Cruz JM, Rodríguez-Cuevas M, Bautista-Muñoz CC y Castillo ACC. 2012. Identificación del agente causal e importancia de la gomosis en la zona citrícola de Huimanguillo, Tabasco, México. Universidad y Ciencia 28(3): 245-258. http://www.scielo.org.mx/pdf/uc/v28n3/v28n3a4.pdf [ Links ]
Agustí M. 2003. Citricultura. Mundi-Prensa. Madrid, España. 422 p. [ Links ]
Ahrens U. and Seemüller E. 1992. Detection de DNA of plant pathogenic mycoplasma like organisms by polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 82: 828-832. https://www.apsnet.org/publications/phytopathology/backissues/Documents/1992Articles/Phyto82n08_828.pdf [ Links ]
Almaguer CM, Sánchez EKC and Díaz VL. 2015. Lasiodiplodia theobromae in the atmosphere of Havana. Revista Cubana de Ciencias Biológicas. 4 (2):130-134. http://www.rccb.uh.cu/index.php/RCCB/article/view/138 [ Links ]
Christensen J. 2017. Common Diseases of Lime Trees. https://www.gardeningchannel.com/common-diseases-of-lime-trees/. [ Links ]
FAOSFAT. 2018. Citrus Fruit Fresh and Processed. Statistical Bulletin 2016. http://www.fao.org/economic/est/est-commodities/citricos/es/. [ Links ]
Hillis DM and Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42:182-192. DOI:10.1093/sysbio/42.2.182 [ Links ]
INEGI. 2018. Clima morelos. www.cuentame.inegi.org.mx/monografias/informacion/mor/ territorio/clima.aspx?tema=me&e=17. territorio/clima.aspx?tema=me&e=17. [ Links ]
Khanzada MA, Lodhi AM and Shahzad S. 2004. Mango dieback and gummosis in Sindh, Pakistan caused by Lasiodiplodia theobromae. http://www.plantmanagementnetwork.org/pub/php/ diagnosticguide/2004/mango/. Plant Health Progress. DOI:10.1094/PHP-2004-0302-01-DG. [ Links ]
Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 16: 111-120. DOI:10.1007/BF01731581 [ Links ]
Ko WH, Wang IT and Ann PJ. 2004. Lasiodiplodia theobromae as a causal agent of Kumquat dieback in Taiwan. Plant Dis. 88: 1383. https://www.ncbi.nlm.nih.gov/pubmed/30795207 [ Links ]
Mohali S, Slippers B and Wingfield, MJ. 2007. Identification of Botryosphaeriaceae species from Eucalyptus, Acacia and Pinus in Venezuela. Fungal Diversity 25: 143-65. http://www.fungaldiversity.org/fdp/sfdp/25-7.pdf [ Links ]
Muhammad, S, Zafar, I, Ahmad, S and Muhammad, A. 2009. Association of Lasiodiplodia theobromae with different decline disorders in mango (Mangifera indica L.) Pak. J. Bot. 41(1):359-368. [ Links ]
Orozco-Santos M. 1995. Enfermedades presentes y potenciales de los cítricos en México. Universidad Autónoma Chapingo. Chapingo, Edo. de México. 150 p. [ Links ]
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, and Crous PW. 2013. The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology. 76: 51-167. DOI: 10.3114/sim0021 [ Links ]
Picos-Muñoz PA, García-Estrada RS, León-Félix J, Sañudo-Barajas A y Allende-Molar R. 2015. Lasiodiplodia theobromae en cultivos agrícolas de México: Taxonomía, hospedantes, diversidad y control. Revista Mexicana de Fitopatología. Vol. 33, núm. 1, pp. 54-74. http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S018533092015000100054&lng=es&nrm=iso [ Links ]
Rodríguez GE. 2010. Lasiodiplodia theobromae: fitopatógeno de mango (Mangifera indica) y palto (Persea americana). Lima: Manufacturas Gráficas S.A.C. [ Links ]
Sambrook J and Russell DW. 2012. Molecular cloning. A laboratory manual. Third Edition. 1. 1.32-1.34. Cold Spring Harbour Laboratory Press. New York. https://www.sigmaaldrich.com/catalog/product/sigma/m8265?lang=es®ion=MX [ Links ]
Servicio de Información Agroalimentaria y Pesquera (SIAP). 2018. Producción Anual. Cierre de la producción agrícola por cultivo. http://www.siap.gob.mx/index.php?option=com_ content&view=article&id=10&Itemid=15. [ Links ]
Slippers B and Wingfield MJ. 2007. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal biology reviews 21 (2007) 90-106. DOI:10.1016/j.fbr.2007.06.002. [ Links ]
Sosa A, Ruíz G, Muro J y Gordillo G. 2015. Control de gomosis de los cítricos (Phytophthora parasitica) en mandarina cv. royal en el Petacal, Jalisco, México. XVII Congreso Latinoamericano de Fitopatología 2015, Nutrilite-Amway. DOI:10.13140/RG.2.1.3909.8008 [ Links ]
Swofford DL. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods) Version 4. Sunderland, Massachusetts: Sinauer Associates. DOI: 10.1111/j.0014-3820.2002.tb00191.x [ Links ]
Tamura K, Dudley J, Nei M and Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 24:1596-1599. https://www.ncbi.nlm.nih.gov/pubmed/17488738 [ Links ]
Thompson JD, Higgins DG and Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 22 (22), 4673-4680. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC308517/ [ Links ]
Townsend GR. and Heuberger JV. 1943. Methods for estimating losses caused by diseases in fungicide experiments. Plant Disease Report. 24: 340-343. [ Links ]
Valle-De la Paz M, Guillén-Sánchez D, Gijón- Hernández AR, Alía- Tejacal I, López- Martínez V, Juárez- López P, Martínez- Fernández V, Hernández- Arenas M y Ariza- Flores F. 2019. Species of Lasiodiplodia in lima ‘Persa’ (Citrus latifolia Tanaka) in Morelos, Mexico. Revista Bio Ciencias 6 e595. DOI: 10.15741/revbio.06.01.35 [ Links ]
Vidales FJA. 1982. Etiología de la Gomosis de los Cítricos en Tecomán, Colima y búsqueda de fuentes de resistencia. Tesis de Maestría. Chapingo, México. [ Links ]
Received: April 14, 2019; Accepted: August 13, 2019











 texto em
texto em 



