Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.37 no.3 Texcoco Set. 2019 Epub 30-Set-2020
https://doi.org/10.18781/r.mex.fit.1905-1
Phytopathological notes
Host suitability of five populations of wild tomato (Solanum lycopersicum var. cerasiforme) for nematode Nacobbus aberrans sensu lato
1 CIIDIR-IPN Unidad Michoacán. Justo Sierra 28, Jiquilpan, Michoacán, México, C.P. 59510;
2 Universidad de La Ciénega del Estado de Michoacán de Ocampo. Avenida Universidad 3000, Lomas de la Universidad, Sahuayo, Michoacán, México, C.P. 59103;
3 Universidad de Sonora, Departamento de Agricultura y Ganadería. Carretera Bahía de Kino Km 21, Hermosillo, Sonora, México, C.P. 83340;
4 CONACYT-Instituto Politécnico Nacional, CIIDIR Unidad Michoacán. Justo Sierra 28, Jiquilpan, Michoacán, México, C. P. 59510.
Nacobbus aberrans sensu lato is one of the most important plant parasitic nematodes (PPN) in tomato crop. For the management of this PPN, it is important to study sustainable strategies such as genetic resistance, commonly found in crop wild relatives. In this regard, there was interest in knowing the response of local populations (Michoacán, Mexico) of wild tomato (Solanum lycopersicum var. cerasiforme) to the inoculation with second stage juveniles. Three experiments (E1, E2, and E3) were mounted. Five wild genotypes and one cultivated genotype (cv. Rio Grande) were evaluated. At 7 and 21 days after the inoculation (dai), the number of nematodes within the root was evaluated, and at the 45 and 60 dai root galling was recorded. In the E1 and E2 experiments, at 7 and 21 dai, the J2 and J3-J4 stages were found. In all three experiments, there was root galling, and at 60 dai egg production was evident. N. aberrans s.l. invaded the roots and completed its development and reproduction. These results reveal the suitability of the five Mexican populations of wild tomatoes as hosts of the nematode and suggest the need to explore other wild solanaceae as possible sources of resistance to the nematode.
Key words: phytoparasite; false root-knot nematode; tinguaraque; susceptibility
Nacobbus aberrans sensu lato es uno de los nematodos más importantes en el cultivo de jitomate. Para su manejo, es de importancia el estudio de estrategias sostenibles como la resistencia genética, comúnmente encontrada en parientes silvestres de los cultivos. Al respecto, surgió el interés por conocer la respuesta de poblaciones locales (Michoacán, México) de jitomate silvestre (Solanum lycopersicum var. cerasiforme) a la inoculación con juveniles de segundo estadio de N. aberrans s.l. Se montaron tres experimentos (E1, E2, y E3). Se evaluaron cinco genotipos silvestres y uno cultivado (cv. Río grande). A los 7 y 21 días posteriores a la inoculación (dpi) se evaluó el número de nematodos dentro de la raíz, y a los 45 y 60 dpi el agallamiento de raíces. En E1 y E2, a 7 y 21 dpi se encontraron los estadios J2 y J3-J4. En los tres experimentos ocurrió agallamiento, y a 60 dpi la producción de huevos fue evidente. N. aberrans s.l. invadió las raíces, y completó su desarrollo y reproducción. Estos resultados revelan la aptitud de las cinco poblaciones mexicanas de jitomate silvestre como hospedantes del nematodo, y sugieren la necesidad de explorar otras solanáceas silvestres como posibles fuentes de resistencia al nematodo.
Palabras clave: fitoparásito; falso agallador; tinguaraque; susceptibilidad
Tomato (Solanum lycopersicum L.) is one of the most economically and agri-food important vegetables (Blancard, 2012). Mexico ranks ninth in tomato production worldwide (FAOSTAT, 2017), but its production is compromised by diseases caused by fungi, bacteria, viruses and nematodes (Blancard, 2012). The most problematic nematodes that affect tomato crops are root-knot nematodes Meloidogyne spp. and Nacobbus aberrans sensu lato (Cristóbal-Alejo et al., 2006; Blancard, 2012). In Mexico, N. aberrans s.l. affects mainly chili pepper crops (Capsicum annuum L.), tomato (S. lycopersicum) and bean (Phaseolus vulgaris L.) (Manzanilla-López et al., 2002; Reid et al., 2003). The nematode causes root galling, which hinders the absorption of water and nutrients and leads to a reduced root system, chlorosis, stunting and wilt, and, like other nematodes, its management involves the use of synthetic nematicides (Manzanilla-López et al., 2002; Hallmann and Meressa, 2018).
Therefore, the study of ecological alternatives for management of plant parasitic nematodes is of interest in order to contribute to reducing the use of chemical nematicides, and thus their negative effects on human health and the environment. These alternatives include the use of biological control agents, organic amendments and genetic resistance, among others (Manzanilla-López et al., 2002; Hallmann and Meressa, 2018). The use of the genetic background of crop wild relatives (WR) in agricultural systems is well documented (Sánchez-Peña et al., 2006; Hajjar and Hodgkin, 2007). WRs include the progenitors and species that are more or less related; because of their high levels of genetic diversity, these species could be used as sources of resistance to pests and diseases (Hajjar and Hodgkin, 2007). Several tomato WRs have been reported to be resistant to plant pathogens (Grandillo et al., 2011); for example, the resistance to M. incognita, M. javanica and M. arenaria is conferred mainly by the Mi genes, a characteristic that was transferred to tomato from its wild relative S. peruvianum (L. peruvianum) (Yaghoobi et al., 1995). It has been demonstrated that Mi genes are not effective against N. aberrans s.l., and no reliable sources of resistance against this nematode in tomato have been found (Veremis et al., 1997; Cabrera et al., 2017). In Mexico, wild tomato (S. lycopersicum var. cerasiforme) grows under inhospitable conditions and is commonly considered a crop-weed. In Michoacán, wild tomatoes are locally known as “tinguaraques”, and some populations are tolerant to water stress and certain pests and diseases (Sánchez-Peña et al., 2006; Álvarez-Hernández et al., 2009). So far, no studies have been conducted on the development of N. aberrans s.l. in these genotypes in Michoacán. Therefore, the aim of this study was to know the response of five local populations of wild tomato and one cultivated variety to inoculation with second-stage juveniles (J2) of the nematode.
The seeds of wild tomato (WT) were obtained from tomatoes collected in the municipalities of Jiquilpan (19° 59’ 5’’ N, 102° 42’ 2’’ W) and Villamar: Los negritos (20° 03’ 28’’ N, 102° 36’ 29’’ W), El platanal (20° 03’ 43’’ N, 102° 35’ 55’’ W) and Emiliano Zapata (19° 58’ 47’’ N, 102°37’ 12’’ W) (located in the Ciénega de Chapala “Michoacán region”); the tomatoes were small in size according to the classification described by Álvarez-Hernández et al. (2009). The populations were named TGM-J, TGM-N, TGM-P, TGM-B and TGM-Z. The surface of the seeds was disinfected with 1% NaClO and the seeds germinated at 25±1 °C. As a reference of the susceptibility to N. aberrans s.l., cultivar Río Grande (cvRG) was included as the control. The seedlings were transplanted to pots containing sterilized sand and kept in a growth chamber at 27±1 °C and a photoperiod of 14 h light. Irrigation was applied every 24 h, and fertilization using NitrofoskaTM 12-12-12 (3.1 g per liter of water) every two weeks. For the inoculation of the plants with N. aberrans s.l., the inoculum was obtained from galled tomato roots (monoxenic culture) (population from Colegio de Postgraduados Campus Montecillo, State of Mexico, Mexico) (Villar-Luna et al., 2017). Egg extraction was performed according to Hussey and Barker (1973), and J2 was obtained by applying Baermann’s technique, that is, using Petri dishes and incubating the eggs in sterile water at 25±1 °C. The inoculation with J2 was carried out when the plants turned 25 days old. To count the nematodes within the roots, the roots were stained using the sodium hypochlorite-acid fuchsin method (Byrd et al., 1983), the number of nematodes per root (NNR) was recorded, and the juvenile and adult stages were observed under an optical microscope (10x) (Zeiss Primo Star, Germany). The galling rate was evaluated using a 0-5 scale: 0: roots without galls; 1: 1-20% galling; 2: 21-40%; 3: 41-60%; 4: 61-80%; and 5: 81-100% (Oka et al., 2009). To determine the number of eggs, they were extracted from each root, according to Hussey and Barker (1973), and counted under a stereoscopic microscope (Zeiss, Germany).
Three independent experiments were established using a completely random design. In experiments 1 and 2, the following treatments were evaluated: 1) WT (TGM-J) and 2) cvRG, both inoculated with N. aberrans s. l. The first experiment (E1) included thirteen plants of each genotype, which were grown in pots containing 75 cm3 of sand and inoculated (1000 J2/plant). Twenty-one days post inoculation (dpi), the NNR (n=5 plants) was evaluated, and at 45 dpi, the rate of root galling (n=8 plants) was recorded. The second experiment (E2) also included thirteen plants of each genotype, which were inoculated with 300 J2, and evaluated at 7 and 21 dpi (NNR) (n=4 plants), and at 60 dpi (number of galls and eggs) (n=5 plants). In E2, the plants were grown in pots containing 25 cm3 of sand, transplanted at 21 dpi for a second time to pots containing 75 cm3 of sand (to allow better root development). In experiment 3 (E3), the treatments included five genotypes: TGM-J, TGM-N, TGM-P, TGM-B, TGM-Z and cvRG; the plants were grown in 25 cm3 pots, the level of the inoculum was 500 J2 per plant, and galls per gram of root (n=5 plants) were evaluated at 60 dpi. Data of the number of nematodes, galls and eggs were converted to log10 (x + 1), subjected to an analysis of variance (ANOVA) and the means were compared by applying Tukey’s method (p≤ 0.05) using the SAS program version 9.0 (SAS Institute Inc., 2002).
Juveniles observed in roots of all the genotypes corresponded to stages J2, J3 and J4 at the different evaluation times (Figure 1). Even though there were numerical differences in E1 at 21 dpi, they were not statistically significant, and at this time, stages J3 and J4 were evident. In E2, J3 predominated at 7 dpi, and few J2 were observed; at 14 dpi, the juveniles corresponded to J3 and J4; and at 21 dpi, J4 predominated. In E1, at 21 dpi, the average percentage of nematodes within the roots was 5.6% in WT (TGM-J) and 6.7% in cvRG. In E2, at 7 dpi, the TGM-J was 20%, while in cvJRG, it was 24.3%; and at 21 dpi, it was 4.7% and 13.2%, respectively (Table 1). In the third experiment (E3), all the wild tomato populations showed typical N. aberrans s. l. galls in their roots (rosary-type galls). It must be noted that in some wild populations, the nematode caused greater galling than in cvRG, but they were not statistically different (Table 2). In E2, the second transplanting promoted better root development, but the number of galls did not increase at 60 dpi.
All the stages of N. aberrans s.l. were evident both in WT TGM-J and cvRG, and their characteristics were in agreement with the results described by Manzanilla-López et al. (2002), that is, the J2 had long and thin bodies, J3 and J4 were longer and wider (arranged in the shape of a “C” or rolled up), and the adult female had a spindle-to-globose shape (Figure 1D). As for the NNR, the reduced number at 7 dpi (E2) compared to the initial inoculum (300 J2) may be associated with the juveniles’ migratory habit , and it is possible that a certain proportion of individuals were outside of the root when measurements were taken, and that not all of them succeeded in invading the root. Before reaching the adult stage, N. aberrans s.l. nematodes are usually migratory (they enter and leave the root frequently), and this is a common behavior of J2, J3, J4 and the immature female, while the adult female has a sessile habit similar to that of Meloidogyne spp. (Manzanilla-López et al., 2002). Similarly, Godínez-Vidal et al. (2013) reported that during the compatible Capsicum annuum-N. aberrans interaction, at 7 dpi only 12.3% of the inoculated J2 were observed inside the roots. The same as in Meloidogyne spp., a certain proportion of individuals that enter the root are different, some are female and others are male; males are vermiform and leave the root, while females become wider and adopt a sessile stage (Manzanilla-López et al., 2002). This explains what happened in E2, where the NNR at 21 dpi was lower than at 7 dpi. Regarding root galling and egg production, all the WT populations in E2 and E3 were susceptible, because the nematode completed its life cycle, the same as in cvRG. The opposite occurs in resistant plants, since the nematode’s life cycle is usually interrupted at any of its juvenile stages. This blockage is conditioned by a hypersensitive response, a mechanism characterized by local necrosis at the infection site, which confines and eliminates the pathogen (Williamson and Kumar, 2006). The resistance to root-knot nematodes is characterized by the inability of the individuals to establish a specialized feeding site, females do not complete their development, and there is minimum formation of galls and eggs, which sometimes cannot even be observed (Williamson and Kumar, 2006). The data on galling and eggs in this study are similar to those reported by Veremis et al. (1997), who confronted an L. esculentum var. cerasiforme genotype against an Argentinian N. aberrans s.l. population; although the number of eggs and galls was lower than that on cv. Rutgers (susceptible), the genotype was considered susceptible. Different N. aberrans s. l. populations can induce a different response in their hosts and vice versa. For example, Toledo et al. (1993) revealed the great parasitic ability of N. aberrans s.l. in 10 hosts: tomato, chili, purslane (Portulaca oleracea), sugar beet (Beta vulgaris), chard (Beta vulgaris var. cicla), potato (Solanum. tuberosum), cucumber (Cucumis sativus), radish (Raphanus sativus), squash (Cucurbita pepo) and bean; six of them were susceptible to four population of the nematode. This also suggested that N. aberrans s.l. races are present in Mexico.
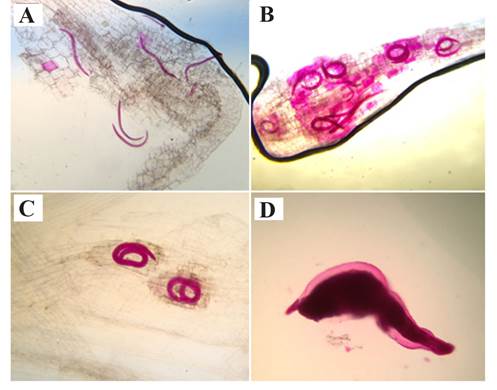
Figure 1 Stages of Nacobbus aberrans sensu lato observed in roots of Solanum lycopersicum var. cerasiforme and S. lycop ersicum cv. Río grande. A: juveniles of the second stage (J2); B: juveniles of the third stage (J3); C: juveniles of the fourth stage (J4); and D: adult female. Representative photos of roots stained using the sodium hypochlorite-acid fuchsin method.
Table 1 Juvenile stages, galls and eggs in tinguaraque roots (Solanum lycopersicum var. cerasiforme) inoculated with Nacobbus aberrans sensu lato.
| Genotipo | Exp | Nematodosz | Agallamiento | Huevos | ||
|---|---|---|---|---|---|---|
| 7 | 21 | 45z | 60y | 60y | ||
| TGM-J | 1 | nd | 56.40±4.93 | 1.75±0.16a | nd | nd |
| (1.75±0.04a) | ||||||
| RG | nd | 66.80±6.30 | 2.25±0.31a | nd | nd | |
| (1.82±0.04a) | ||||||
| TGM-J | 2 | 60.00±12.68 | 14.00±5.12 | nd | 2.46±0.37 | 219.21±21.75 |
| (1.75±0.10a) | (1.09±0.16a) | (0.53±0.05a) | (2.33±0.04a) | |||
| RG | 73.00±7.12 | 39.75±11.74 | nd | 0.68±0.12 | 137.52±28.77 | |
| (1.86±0.04a) | (1.56±0.11a) | (0.22±0.03b) | (2.10±0.10a) | |||
TGM-J: wild tomato; RG: cv. Río grande. 7, 21, 45 and 60: days post inoculation with Nacobbus aberrans sensu lato. †Number of nematodes (different stages) per root. yNumber of galls and eggs per g of root. zGall index according to a 0-5 scale, where 0: roots without galls; 1: 1-20% galling; 2: 21-40%; 3: 41-60%; 4: 61-80%; and 5: 81-100% (Oka et al., 2009). nd: not determined. The values represent the means ± standard error. Means with the same letter are not statistically different (Tukey, p˂0.05). The ANOVA was conducted using converted data [log10 (x + 1) ] and are shown in parentheses.
Table 2 Galls caused by Nacobbus aberrans sensu lato in roots of Solanum lycopersicum var. cerasiforme (tinguaraque).
| Tratamiento | Agallas/g de raíz |
|---|---|
| TGM-J | 98.38±7.64 (1.99±0.03a) |
| TGM-N | 65.18±12.53 (1.79±0.08a) |
| TGM-P | 90.42±18.76 (1.92±0.10a) |
| TGM-B | 57.22±5.79 (1.76±0.04a) |
| TGM-Z | 59.77±4.27 (1.78±0.03a) |
| RG | 58.32±2.02 (1.77±0.01a) |
The values represent the media ± standard error. Means with the same letter are not significantly different (Tukey, p˂0.05). RG: cv. Río Grande. The ANOVA was conducted using converted data [log10 (x + 1) ] and they are shown in parentheses.
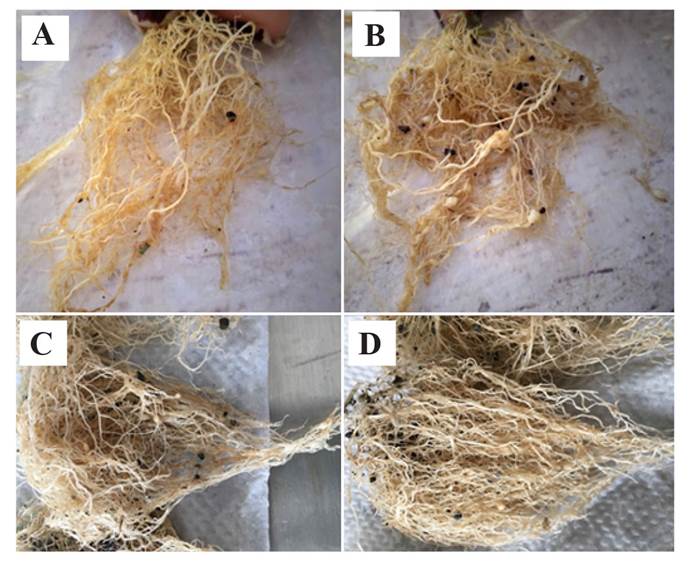
Figure 2 Galls caused by Nacobbus aberrans sensu lato in roots (Montecillo population) at 45 (A-B) and 60 (C-D) days post inoculation. A and C) Solanum lycopersicum var. cerasiforme; B and D) S. lycopersicum cv. Río grande.
The susceptibility of wild plants to phytoparasitic nematodes is not unusual. For example, Veremis et al. (1997) found that several wild accessions of L. chmielewskii, L. peruvianum, L. peruvianum var. glandulosum, L. hirsutum and L. pimpinellifolium were susceptible to N. aberrans s.l. A study conducted by Ruiz de Galarreta et al. (1998), who evaluated 90 wild species of Solanum (98 accessions) against Globodera pallida, also revealed that only 12 accessions were resistant. However, several of the susceptible ones showed resistance to the Phytophthora infestans oomycete and to four phytopathogenic viruses. Under another scenario, the wild relative of L. pimpinellifolium (=S. pimpinellifolium) was susceptible to M. javanica; this genotype had a galling rate similar to that of susceptible tomato lines but was catalogued as tolerant because its fruit production was not altered (Udo et al., 2008). The results reveal that local collections of S. lycopersicum var. cerasiforme are able to host the N. aberrans population used in this study. N. aberrans s.l. invaded the roots and completed its development and reproduction cycle. The above suggests the need to explore other wild native solanaceous plants as possible sources of resistance, because this information is currently scarce. We do not recommend including tinguaraque in N. aberrans s.l. management programs (in the region where samples were taken), for example, those that are used as rootstocks in soils infested with the nematode. In addition to its phytosanitary use, tinguaraque is a resource valued by the rural population in western Mexico, because it has food (in sauces) and medicinal uses for humans and animals. Therefore, it is essential to promote its conservation. However, when it grows as a weed in crop fields, it is at risk because herbicides are used and grasses are burned (Rodríguez-Guzmán et al., 2009).
Literatura citada
Álvarez-Hernández JC, Cortez-Madrigal H, García-Ruiz I, Ceja-Torres LF y Pérez-Domínguez JF. 2009. Incidencia de plagas en injertos de jitomate (Solanum lycopersicum) sobre parientes silvestres. Revista Colombiana de Entomología 35: 150-155. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-04882009000200007 [ Links ]
Blancard D. 2012. Tomato diseases, identification, biology and control: a colour handbook. Second edition. Manson Publishing Ltd. London, UK. 688 p. http://dx.doi.org/10.1201/b15145 [ Links ]
Byrd Jr DW, Kirkpatrick T and Barker KR. 1983. An improved technique for clearing and staining plant tissues for detection of nematodes. Journal of Nematology 15:142-143. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2618249/pdf/142.pdf [ Links ]
Cabrera VA, Dottori N and Doucet ME. 2017. Histopathology of roots of three tomato cultivars infected with two separate isolates of the false root-knot nematode Nacobbus aberrans. European Journal of Plant Pathology 148: 393-403. http://dx.doi.org/10.1007/s10658-016-1097-1 [ Links ]
Cristóbal-Alejo J, Mora-Aguilera G, Manzanilla-López RH, Marbán-Néndoza N, Sánchez-García P, Cid del Prado-Vera I and Evans K. 2006. Epidemiology and integrated control of Nacobbus aberrans on tomato in Mexico. Nematology 8: 727-737. http://dx.doi.org/10.1163/156854106778877929 [ Links ]
FAOSTAT. 2017. Food and Agriculture Organization of the United Nations, FAOSTAT Database. Available online at: http://www.fao.org/faostat/en/#data/QC/visualize [ Links ]
Godínez-Vidal D, Rocha-Sosa M, Sepúlveda-García EB, Lozoya-Gloria E, Rojas-Martínez RI, Guevara-Olvera L and Zavaleta-Mejía E. 2013. Tanscript accumulation of the mevalonate pathway genes and enzymatic activity of HMGCoA-r and EAS in chilli CM-334 infected by the false root-knot nematode Nacobbus aberrans. Plant and Soil 372: 339-348. http://dx.doi.org/10.1007/s11104-013-1743-8 [ Links ]
Grandillo S, Chetelat R, Knapp S, Spooner D, Peralta I, Cammareri M, Perez O, Termolino P, Tripodi P, Chiusano ML, Ercolano MR, Frusciante L, Monti L and Pignone D. 2011. Solanum sect. Lycopersicon. Pp: 129-215. In: Kole C (ed.). Wild crop relatives: genomic and breeding resources, vegetables. Springer Heidelberg Dordrecht London New York. 282 p. http://dx.doi.org/10.1007/978-3-642-20450-0 [ Links ]
Hajjar R and Hodgkin T. 2007. The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica 156:1-13. http://dx.doi.org/10.1007/s10681-007-9363-0 [ Links ]
Hallmann J and Meressa BH. 2018. Nematode parasites of vegetables. Pp: 346-410. In: Sikora RA, Coyne D, Hallmann J and Timper P. (eds.). Plant parasitic nematodes in subtropical and tropical agriculture. Third edition. CABI International. Oxfordshire, UK. 876 p. http://dx.doi.org/10.1079/9781786391247.0346 [ Links ]
Hussey RS and Barker KR. 1973. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. The Plant Disease Reporter 57: 1025-1028. https://babel.hathitrust.org/cgi/pt?id=uc1.31175001263642;view=1up;seq=491 [ Links ]
Manzanilla-López RH, Costilla MA, Doucet M, Franco J, Inserra RN, Lehman PS, Cid del Prado-Vera I, Souza RM, and Evans K. 2002. The genus Nacobbus Thorne & Allen, 1944 (Nematoda: Pratylenchidae): systematics, distribution, biology and management. Nematropica 32:149-227. http://journals.fcla.edu/nematropica/article/view/69655 [ Links ]
Oka Y, Shuker S and Tkachi N. 2009. Nematicidal efficacy of MCW‐2, a new nematicide of the fluoroalkenyl group, against the root‐knot nematode Meloidogyne javanica. Pest Management Science 65: 1082-1089. http://dx.doi.org/10.1002/ps.1796 [ Links ]
Reid A, Manzanilla-López RH and Hunt DJ. 2003. Nacobbus aberrans (Thorne, 1935) Thorne & Allen, 1944 (Nematoda: Pratylenchidae); a nascent species complex revealed by RFLP analysis and sequencing of the ITS-rDNA region. Nematology 5: 441-451. [ Links ]
Rodríguez-Guzmán E, Vargas-Canela D, Sánchez-González JJ, Lépiz-Ildefonso R, Rodríguez-Contreras A, Ruíz-Corral JA, Puente-Ovalle P y Miranda-Medrano R. 2009.-Etnobotánica de Solanum lycopersicum var. cerasiforme en el occidente de México. Naturaleza y Desarrollo 7:45-57. https://www.ciidiroaxaca.ipn.mx/revista/sites/www.ciidiroaxaca.ipn.mx.revista/files/pdf/vol7num2/NatyDes_Vol-7-2-Art4.pdf [ Links ]
Ruiz de Galarreta JI, Carrasco A, Salazar A, Barrena I, Iturritxa E, Marquinez R, Legorburu FJ and Ritter E. 1998. Wild Solanum species as resistance sources against different pathogens of potato. Potato Research 41: 57-68. http://dx.doi.org/10.1007/BF02360262 [ Links ]
Sánchez-Peña P, Oyama K, Núñez-Farfán J, Fornoni J, Hernández-Verdugo S, Márquez-Guzmán J and Garzón-Tiznado JA. 2006. Sources of resistance to whitefly (Bemisia spp.) in wild populations of Solanum lycopersicum var. cerasiforme (Dunal) spooner G.J. Anderson et R.K. Jansen in Northwestern Mexico. Genetic Resources and Crop Evolution 53: 711-719. http://dx.doi.org/10.1007/s10722-004-3943-9 [ Links ]
SAS Institute Inc. 2002. SAS Procedures Guide, Version 9.0 (Computer program). SAS Institute Inc., Cary, N.C. [ Links ]
Toledo RJC, Sosa-Moss C y Zavaleta-Mejía E. 1993. Gama de hospederos de cinco poblaciones mexicanas de Nacobbus aberrans. Nematropica 23: 105-108. http://journals.fcla.edu/nematropica/article/view/64066 [ Links ]
Udo IA, Uguru MI, Ogbuji RO and Ukeh DA. 2008. Sources of tolerance to root-knot nematode, Meloidogyne javanica, in cultivated and wild tomato species. Plant Pathology Journal 7: 40-44. http://dx.doi.org/10.3923/ppj.2008.40.44 [ Links ]
Veremis JC, Cap GB and Roberts PA. 1997. A search for resistance in Lycopersicon spp. to Nacobbus aberrans. Plant Disease 81: 217-221. http://dx.doi.org/10.1094/PDIS.1997.81.2.217 [ Links ]
Villar-Luna E, Rojas-Martínez RI, Reyes-Trejo B, Gómez-Rodríguez O and Zavaleta-Mejía E. 2017. Mevalonate pathway genes expressed in chilli CM334 inoculated with Phytophthora capsici and infected by Nacobbus aberrans and Meloidogyne enterolobii. European Journal of Plant Pathology 148:867-881. https://doi.org/10.1007/s10658-016-1142-0 [ Links ]
Williamson VM and Kumar A. 2006. Nematode resistance in plants: the battle underground. Trends in Genetics 22: 396-403. http://dx.doi.org/10.1016/j.tig.2006.05.003 [ Links ]
Yaghoobi J, Kaloshian I, Wen Y and Williamson VM. 1995. Mapping a new nematode resistance locus in Lycopersicon peruvianum. Theoretical and Applied Genetics 91: 457-464. http://dx.doi.org/10.1007/BF00222973 [ Links ]
Received: May 03, 2019; Accepted: July 03, 2019











 texto em
texto em 


