Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.37 n.1 Texcoco Jan. 2019 Epub Aug 21, 2020
https://doi.org/10.18781/r.mex.fit.1812-3
Phytopathological notes
The Brazilian Huanglongbing disease-associated phytoplasma is present in Citrus spp in Hidalgo state, Mexico
1 Posgrado en Fitosanidad, Colegio de Postgraduados. Km 36.5 Carretera México-Texcoco, CP. 56230 Texcoco, Estado de México;
2 Departamento de Parasitología Agrícola, Universidad Autónoma Chapingo. Km 38.5 Carretera México-Texcoco, Estado de México, CP. 56230. México;
3 SENASICA-SAGARPA. Centro Nacional de Referencia Fitosanitaria. Carretera Federal México-Pachuca Km 37.5, Tecámac, 55740, Estado de México;
4 Comité Estatal de Sanidad Vegetal de Hidalgo Avenida 2, Manzana F, Lote 11, No. 800, Parque de Poblamiento, 42032 Pachuca de Soto, Hidalgo
This work had the purpose of detecting the presence of phytoplasmas associated to HLB-like symptoms, a disease caused by CLas, to improve the sampling and detection carryout in official phytosanitary actions in the Mexican citriculture. Base on alerts of negative results to CLas in citrus trees with putative symptoms of HLB, directed samplings were conducted in commercial orchards and country backyards in Hidalgo, Colima, Puebla and Veracruz in 2012, 2013 and 2018. The total DNA was analyzed by PCR with the primers P1 / P7 nested with R16F2 / R16R2 and D7f2 / D7r2. CLas infection was assessed with A2 / J5 primers. The presence of the Brazilian Huanglongbing disease-associated phytoplasma, member of the 16Sr IX group, associated with C. latifolia (NCBI, MK282760) and C. aurantifolia (NCBI, MK282761) was confirmed inducing similar but contrasting symptoms to those caused by CLas. These hosts are reported for the first time in addition to the original report on C. sinensis in Brazil. Bidens odorata and Cajanus cajan are also reported as probable alternate weed hosts as well as the presence of specimens of Graphocephala and Acinopterus (Cicadellidae), genera recognized with vector species of 16Sr III-A and Aster Yellow phytoplasma groups, respectively.
Key words: Nested-PCR; Mollicutes; HLB; vector
Este trabajo tuvo la finalidad de investigar la presencia de fitoplasmas asociados a síntomas similares al HLB, enfermedad causada por CLas, con el propósito de optimizar los procesos de muestreo y diagnóstico en el marco de acciones fitosanitarias oficiales en la citricultura Mexicana. A partir de alertas de resultados negativos a CLas en árboles de Citrus spp con síntomas putativos de HLB se realizaron muestreos dirigidos en huertos comerciales y traspatios en Hidalgo, Colima, Puebla y Veracruz en 2012, 2013 y 2018. El ADN total se analizó por PCR con los iniciadores P1/P7 anidados con R16F2/R16R2 y D7f2/D7r2. La infección de CLas se determinó con los iniciadores A2/J5. Se confirmó en México la presencia del Brazilian Huanglongbing disease-associated phytoplasma, miembro del grupo 16Sr IX, asociado a C. latifolia (NCBI, MK282760) y C. aurantifolia (NCBI, MK282761) induciendo síntomas similares pero contrastantes a los causados por CLas. Estos hospederos se reportan por primera vez en adición al original reporte en C. sinensis en Brasil. También se reporta a Bidens odorata y Cajanus cajan como probables hospederos arvenses alternos y la presencia de especímenes de Graphocephala y Acinopterus (Cicadellidae), géneros reconocidos con especies vectoras de fitoplasmas de los grupos 16Sr III-A y Aster Yellow, respectivamente.
Palabras clave: PCR-anidado; Mollicutes; HLB; vector
Upon the imminent entry in 2008 of Candidatus Liberibacter asiaticus (CLas) in Mexico, SENASICA developed an Emergency Plan that derived in a campaign against HLB on citrus crops, supported by plant and vector detection of the bacterial agent CLas (SIRVEF, 2018; Flores-Sánchez et al., 2017; Mora-Aguilera et al, 2014). However, the reliability of the molecular diagnosis must include CLas and other organisms capable of inducing symptoms similar to HLB, consisting of chlorotic and asymmetric spots, slight vein whitening and leaf yellowing (Esquivel-Chávez et al, 2012). In Brazil and China in 2008 and 2009, and more recently, in 2018, phytoplasmas belonged to groups 16Sr I and IX were reported in Citrus sinensis causing symptoms similar to HLB (Wulff et al., 2018; Chen et al., 2009; Teixeira et al., 2008). Due to the importance of sampling in planning and operation of the campaign against HLB, and to the need for massive and reliable molecular diagnoses, the present investigation had the aim of detecting the occurrence of phytoplasmas in Citrus spp using nested PCR and sequencing with the purpose of establishing the potential implication of these organisms in the detection of CLas on different citrus species and identifying alternate hosts and potential vector insects to eventually be integrated onto risk models and control programs.
Base on phytosanitary alerts of the Plant Health Committee of the State of Hidalgo, in which Citrus trees with typical CLas symptoms had a negative diagnosis, in July of 2013, 15 country backyards and commercial orchards were explored in the municipalities of Atlapexco, Huejutla, Orizatlán, Xochiatipan and Jaltocan. Samples were taken from 16 Citrus sinensis, C. latifolia, C. aurantifolia and C. limetta trees with foliar symptoms consisting of asymmetric chlorotic spots, whitening and corky veins (Table 1). A compound sample was taken for every tree, from tissue collected for each cardinal direction and the center of the canopy. For each direction, a 10-15 cm shoot was selected with 2-5 mature leaves of the previous vegetative cycle. In addition, leaves, branches and stems were collected from 13 weeds with suspected symptoms of infection by phytoplasma consisting of chlorosis, shortening of internodes, proliferation of sprouts, phyllody and/or virescence. The plant tissue samples were stored in ice boxes and taken to the lab, where they were kept at 4 °C for 2-3 days until processing. The insects associated to sampled trees and weeds were collected using a butterfly net and kept in alcohol at 70% for their later identification at UACH and confirmation in the entomology lab of the National Plant Health Reference Center, DGSV. The weed samples that turned out positive for phytoplasmas were taxonomically identified in the herbarium of the Colegio de Postgraduados, Campus Montecillo. Other regions and crops selected by the COLPOS-LANREF research group included C. aurantifolia in Armeria, Colima in 2012 with 10 trees; C. sinensis and C. latifolia in Acateno, Puebla; Papantla, Martínez de la Torre, Tihutlán and Álamo, Veracruz in 2018 with a total of 49 samples obtained from trees with typical HLB symptoms yet negative to CLas (Table 1).
The total DNA extraction was carried out using DNaesy® Plant Mini Kit (Qiagen) from 0.1 g of plant tissue consistent in petioles, central vein and pieces of twig stems with a diameter of 1-0.5 cm. Phytoplasm was detected using nested PCR with primers related to the gene 16S of the rRNA (Ahrens & Semüller, 1992). The first PCR reaction required the use of universal primers P1/P7 (Berges et al., 2000; Ahrens & Semüller, 1992). The second PCR was carried out independently with two pairs of primers: R16F2/R16R2, universal to phytoplasmas (Lee et al., 1993), and with D7f2/D7r2 specific to the Brazilian Huanglongbing disease-associated phytoplasma (BHDA-Phytoplasma) (Teixeira et al., 2008). For the first PCR, the reaction volume consisted in 25 µL composed of: 1X GoTaq buffer, 1.5 mM of MgCl2, 200 mM dNTP’s, 20 pmol of each primer, 2 units of DNA Taq polymerase (Promega) and 2 µL of total DNA. The thermal program consisted in the denaturalization for 4 min at 94 °C, followed by 35 cycles at 92 °C for 45 s, 58 °C for 45 s and 72 °C for 90 s and a final extension at 72 °C for 7 min. For the second PCR, the reaction was carried out with 25 µL in total, which included: 1X GoTaq buffer, 1.5 mM of MgCl2, 200 mM dNTP’s, 20 pmol of each primer, 2 units of DNA Taq polymerase (Promega) and 5 µL produced by the first PCR diluted 1:10. The thermal program for the reaction with primers R16F2/R16R2 consisted in the denaturalization at 94 °C for 5 min followed by 35 cycles: 94 °C for 30 s, 53 °C for 50 s, 72 °C for 80 s and a final extension at 72 °C for 10 min. For the reaction with primers D7f2/D7r2, a denaturalization was carried out at 94 °C for 3 min and 40 cycles at 92 °C for 45 s, 62 °C for 45 s, 72 °C for 90 s and a final extension at 72 °C for 7 min.
Table 1. Species of citrus plants and weeds positive to CLas, the Brazilian Huanglongbing disease-associated phytoplasma, and to a putative phytoplasma of the Aster yellow group. Main symptom observed in samples from commercial orchards and country backyards in four Mexican states in 2012, 2013 and 2018.
| Estado | Especie Botánica | Muestras Positivas | Principal Síntoma | xBHDA phytoplasma | yAY phytoplasma | zHLB |
|---|---|---|---|---|---|---|
| Hidalgo | Citrus latifolia | 1/6 | Aclaramiento nervaduras | + | - | 0/6 |
| C. aurantifolia | 1/3 | Moteados | + | - | 0/3 | |
| C. limetta | 0/5 | Variegado | - | - | 0/5 | |
| C. sinensis | 0/2 | Clorosis | - | - | 0/2 | |
| Bidens odorata | 1/1 | Filodia | + | - | 0/1 | |
| Cajanus cajan | 1/1 | Escoba de bruja | + | - | 0/1 | |
| Catharanthus roseus | 2/2 | Filodia y virescencia | - | + | NA | |
| Commelina difusa | 0/1 | Clorosis | - | - | NA | |
| Cuscuta sp | 0/1 | Clorosis | - | - | NA | |
| Murraya paniculata | 0/1 | Clorosis | - | - | 0/1 | |
| Otras especies | 0/6 | Clorosis | - | - | NA | |
| Colima | C. aurantifolia | 7/10 | Moteado | - | - | 7/10 |
| Puebla | C. paradisi | 1/1 | Moteado | - | - | 1/1 |
| Veracruz | C. sinensis | 0/17 | Moteado | - | - | 0/17 |
| C. reticulata | 0/12 | Moteado | - | - | 0/12 | |
| C. latifolia | 0/9 | Moteado | - | - | 0/9 | |
XBrazilian Huanglongbing disease-associated phytoplasma, iniciadores P1/P7 y D7f2/D7r2 / Brazilian Huanglongbing disease-associated phytoplasma, primers P1/P7 and D7f2/D7r2
Y Putativo fitoplasma perteneciente al Aster yellow group, iniciadores P1/P7 y R16F2/R16R2 / Putative phytoplasma belonging to the Aster yellow group, primers P1/P7 and R16F2/R16R2
ZCandidatus Liberibacter asiaticus, iniciadores A2/J5 / Candidatus Liberibacter asiaticus, primers A2/J5.
The citrus samples were also analyzed using PCR to detect CLas, using primers A2/J5. The reaction volume consisted of 25 µL: 1X of GoTaq buffer, 1.5 mM of MgCl2, 200 mM dNTP’s, 20 pmol of each primer and 2 units of DNA Taq polymerase. The thermal program consisted in the denaturalization at 94 °C for 4 min followed by 35 cycles at 92 °C for 45 s, 58 °C for 45 s, 72 °C for 90 s and a final extension at 72 °C for 7 min. In all cases, the amplified fragments were analyzed by electrophoresis using agarose gel at 1.5% and viewed with a UV light transilluminator after staining with ethidium bromide. Amplicons that were positive for phytoplasmas were sequenced in the molecular biology laboratory of the National Plant Health Reference Center (DGSV, CNRF). The partial sequences of the gene 16S rRNA obtained with primers D7f2/ D7r2 were aligned with those reported in the National Center for Biotechnology Information (NCBI) and accessions were registered in that system. A phylogenetic analysis was carried out with the sequences found in C. latifolia and C. aurantifolia and those reported by other researchers (Wulff et al., 2018; Arratia et al., 2015; Chen et al., 2009). Seq Scanner v2 was previously used to purify the sequenced, followed by BioEdit to edit the alignment and obtain the consensus sequences in a FASTA format. Finally, MEGA v7 was used to generate the phylogenetic tree.
Only citrus and weed samples from Hidalgo were positive for phytoplasmas, but not for CLas. The nested PCR with the primers P1/P7 and D7f2/D7r2 amplified a fragment of 857, corresponding to BHDA-Phytoplasma, belonging to the Pigeon pea witches’-broom phytoplasma group 16Sr IX (99% homology). The positive samples were from C. latifolia (1/6), C. aurantifolia (1/3) and weeds identified as Bidens odorata and Cajanus cajan (2/13), although they had homologies between 80 and 89% (Table 1, Figure 1). The sequences obtained from C. latifolia and C. aurantifolia were registered in the NCBI with accession numbers MK282760 and MK282761, respectively. Using primers P1/P7 and R16F2/R16R2, a fragment of 1200 pb was amplified in 2 samples of Catharanthus roseus, possibly belonging to a phytoplasma of Aster Yellow group (Torres et al., 2004). In samples from other states, CLas was found, but no phytoplasmas. Of C. aurantifolia samples from Colima, 70% (7/10) were positive for CLas, whereas in the Acateno-Álamo citrus corridor, only 1/39 was positive, despite the citrus fruits presented symptoms of spotted chlorosis, similar to that caused by the bacteria (Esquivel-Chavez et al., 2012). These results indicate the restricted presence of BHDA-Phytoplasma, and possibly, of the group Aster Yellow in weeds (Torres et al., 2004), although not related in mixed infections with CLas. On the other hand, in Sinaloa, Nayarit and Colima, Arratia and collaborators (2015) found Candidatus Phytoplasma asteris, a new taxon included in group 16SrI (Lee et al., 2004), causing similar symptoms to HLB in C. aurantifolia, C. latifolia and C. sinensis trees in independent infections, or mixed with CLas (20/86 samples). Although no weeds or potential vector insects were included in that investigation, the frequency of this phytoplasm, the citrus host range, and the presence of mixed infections in the Pacific cost suggests the need to include this pathogen in future epidemiological studies, considering that group 16SrI is one of the most prevalent with over 100 hosts of worldwide economic importance (Lee et al, 2004).
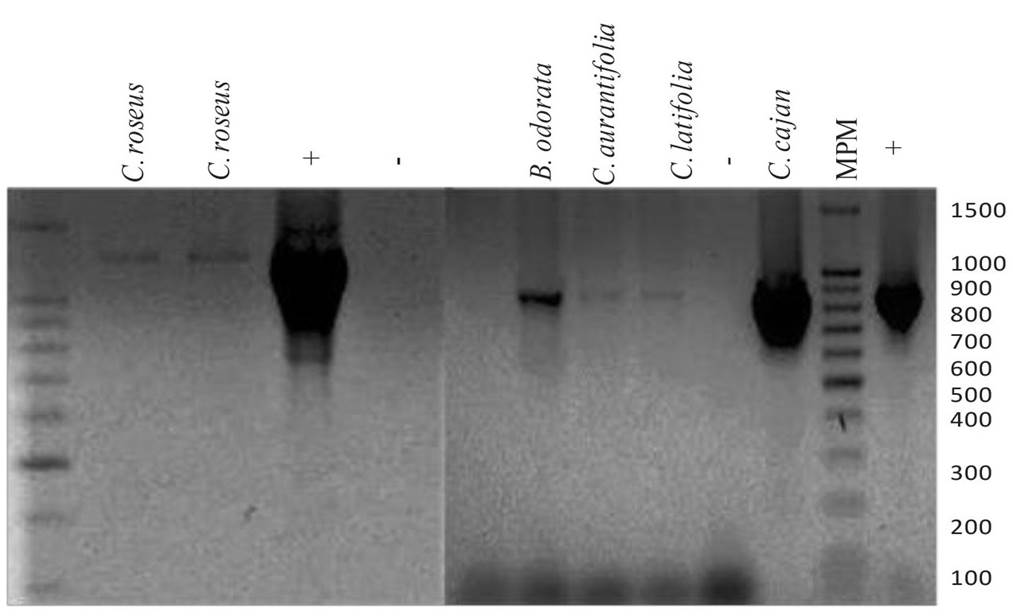
Figure 1 Visualization in agarose gel of DNA fragments amplified using nested PCR from tissue samples from Hidalgo, Mexico. Fragments of 1200 pb obtained using primers P1/P7 and R16F2/R16R2 of samples of Catharanthus roseus. Fragments of 857 pb obtained with primers P1/P7 and D7f2/D7r2 of samples of Citrus aurantifolia and C. latifolia, Bidens odorata and Cajanus cajan. Molecular weight marker (MWM-100 Pb), (+) Positive control and (-) Negative control.
The leaves symptoms in C. latifolia consisted of vein yellowing and thickening, angular chlorotic spots, diffuse mottling spots. An irregular chlorotic spotting was observed in C. aurantifolia along with a moderate thickening of nervations (Figure 2). In Brazil, the symptoms caused by BHDA-Phytoplasma in C. sinensis were indistinguishable from CLas (Teixeira et al., 2009); meanwhile, in Mexico, the symptoms induced by this phytoplasm in sour citrus are similar but not the same as those reported for HLB (Esquivel-Chávez et al., 2012; Robles et al., 2010). B. odorata displayed phyllody, reddish colors, the reduction of foliar areas, shortening of internodes, proliferation and plant shortening (Figure 3). In general, these symptoms have been widely related to infections by phytoplasmas (Bertaccini and Duduk, 2009). This weed, which appeared in the same orchard where a C. latifolia tree tested positive, had a 80% predominance indicating its potential as inoculum reservoir. The positive sample of C. cajan was found in a country backyard of Citrus limetta. The witches’-broom symptom, which consists in the proliferation of sprouts, internodes shortening, a general growth reduction and leaves chlorosis were observed in 50% of the plant’s canopy (Figure 3). Similar symptoms caused by the same phytoplasma have been reported in Brazil for Crotalaria juncea, a fabaceae weed associated to citrus (Marques et al., 2010). This is the first report of B. odorata and C. cajan as alternate hosts of the BHDA-Phytoplasma in Mexico.
Of the total insects collected on citrus orchards in Hidalgo, 77 specimens were identified, belonging to the families Cicadellidae, Membracidae and Liviidade, with the potential to transmit mollicutes and bacteria (Weintraub and Beanland, 2006). Six species belonged to the Cicadellidae family (Young, 1977; Medler, 1960; Beamer, R.H., 1944): Chlorogonalia coeruleovittata (7/61), Apogonalia stali (4/61), Graphocephala sp (7/61), Tylozygus fasciatus (8/61), Agrosoma syklis (9/61), Acinopterus sp (5/61). Only one colony was found (20/61) with immature and adult states of Membrasis mexicana (Membracide) in C.cajan. Out of the D. citri (Liviidae), Clas vector, 17/77 specimens were collected (Figure 4). Out of these species, members of the genera Graphocephala and Acinopterus have been reported as potential vectors of phytoplasmas of 16Sr III-A and Aster Yellow groups, respectively (Weintraub and Beanland, 2006). In the future, it will be important to identify at species level the specimens of these genera found in this investigation, as well as the revision of other reports of leafhoppers related to citrus in Mexico. For example, Blanco (2014), reported 21 species in the Yucatan Peninsula, though none were reported with the ability to transmit phytoplasmas (Weintraub and Beanland, 2006).

Figure 2 Symptoms of positive samples to the Brazilian Huanglongbing disease-associated phytoplasma. A-D: Leaves symp toms in Citrus latifolia consisting in yellowing and vein thickening, angular chlorotic spots, diffuse mottling spots and corky veins. E and F: Symptoms in C. aurantifolia characterized by irregular chlorotic spots.

Figure 3 A-C: Symptoms in Cajanus cajan positive for Brazilian Huanglongbing disease-associated phytoplasma (BHDA-phytoplasma); (A) Leaf tips chlorosis, (B) Proliferation of stem fasciation. (C) Membrasis mexicana (Membracidae) in proliferation of stems. E-G: Symptoms on Bidens odorata positive for BHDA-phytoplasma; (E and F) Inflores cence phyllody, (G) Reddish of leaves. I-K: Symptoms in Catharathus roseus putative to a phytoplasma of the Aster yellow group; (I) Flower phyllody, (J and K) Flowers virescence. D-L: Symptoms of witches’ broom in C. cajan (D), B. odorata (H) and C. roseus (L).
The phylogenetic analysis of partial sequences of the 16S rRNA gene obtained in this investigation and those published for phytoplamsas that induce similar or indistinguishable symptoms from those caused by CLas on citrus shows a clear genetic discrimination between groups and possible subgroups (Figure 5).
The group 16Sr IX Pigeon pea witches-broom phytoplasma seems to be the most homogenous and it includes the samples from this investigation and from Brazil (Teixeira et al., 2010; Teixeira et al., 2008). On the other hand, the group 16Sr I Aster Yellow Phytoplasma (AY180957), defined three subgroups with sequences from Sinaloa, Colima and Nayarit (AB858474.1, AB858473.1 and AB858472.1, respectively) reported as member of the new taxon Candidatus phytoplasma asteri subgroup S (Sinaloa and Colima) and B (Nayarit) (Arratia et al., 2015), as well as a sequence of C. sinensis from China (Chen et al., 2009) with a similar distance to the Aster Yellow-type sequence. Although the group related to the 16Sr III X-Disease is preliminary because the sequences of the phytoplasma recently reported in Brazil are not yet published in NCBI (Wulff et al., 2018), the use of sequences referred to in that publication help establish that it is relatively closer to group 16Sr IX.
These results demonstrate the presence of the Brazilian Huanglongbing disease-associated phytoplasma (BHDA-Phytoplasma), which belongs to group 16Sr IX, related to C. latifolia and C. aurantifolia in Mexico inducing similar symptoms, no different to those caused by CLas. These hosts are reported for the first time in addition to their original report in C. sinensis in Brazil (Teixeira et al., 2009). On the other hand B. odorata and C. cajan are reported as possible alternate hosts of the BHDA-Phytoplasma in Mexico. This phytoplasma is added to that reported by Arratia et al., 2015 for the Mexican Pacific region belonging to the taxon Candidatus phytoplasma asteri of the 16Sr I group. The presence of these phytoplasmas indicate the need to develop robust diagnosis systems for CLas, the pathogen with the seemingly highest parasitic and epidemiological aptitude, but which can develop symptoms that can be mistaken for at least three types of phytoplasmas (members of 16SrI, 16SrIII and 16Sr IX). Epidemiological studies, including the role of potentially alternate hosts and vectors, must be carried out in Mexico in order to establish the risk factors and symbiotic implications between parasitic organisms of the citrus biome to obtain an effective phytosanitary management program.
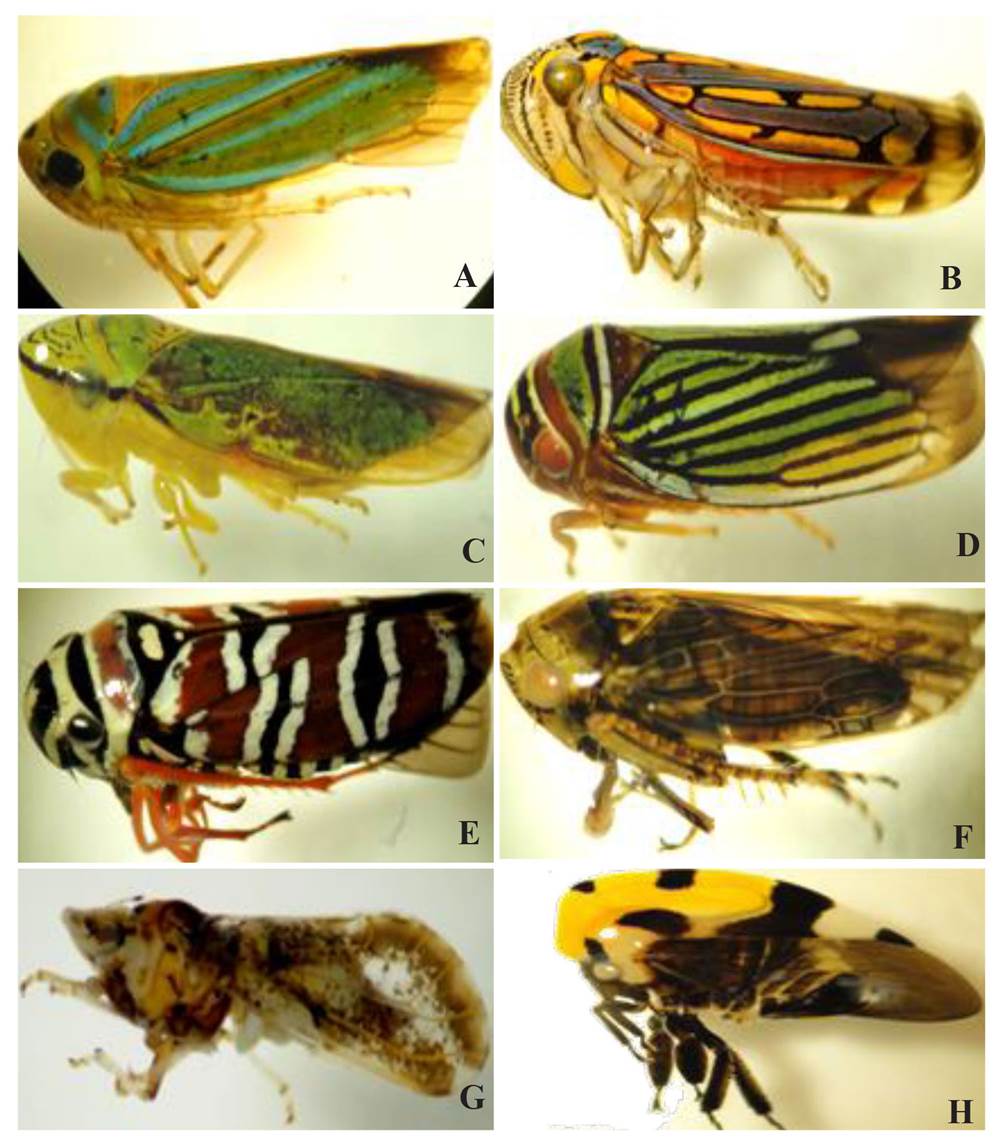
Figure 4 Insects collected in orchards with citrus trees or weeds positive to the Brazilian Huanglongbing disease-associated phytoplasma and to a putative phytoplasma of the Aster yellow group. Cicadellidae: (A) Chlorogonalia coeruleovittata, (B) Apogonalia stali, (C) Graphocephala sp, (D) Tylozygus fasciatus, (E) Agrosoma syklis, (F) Acinopterus sp.; Liviidae: (G) Diaphorina citri; Membracidae: (H) Membrasis mexicana.
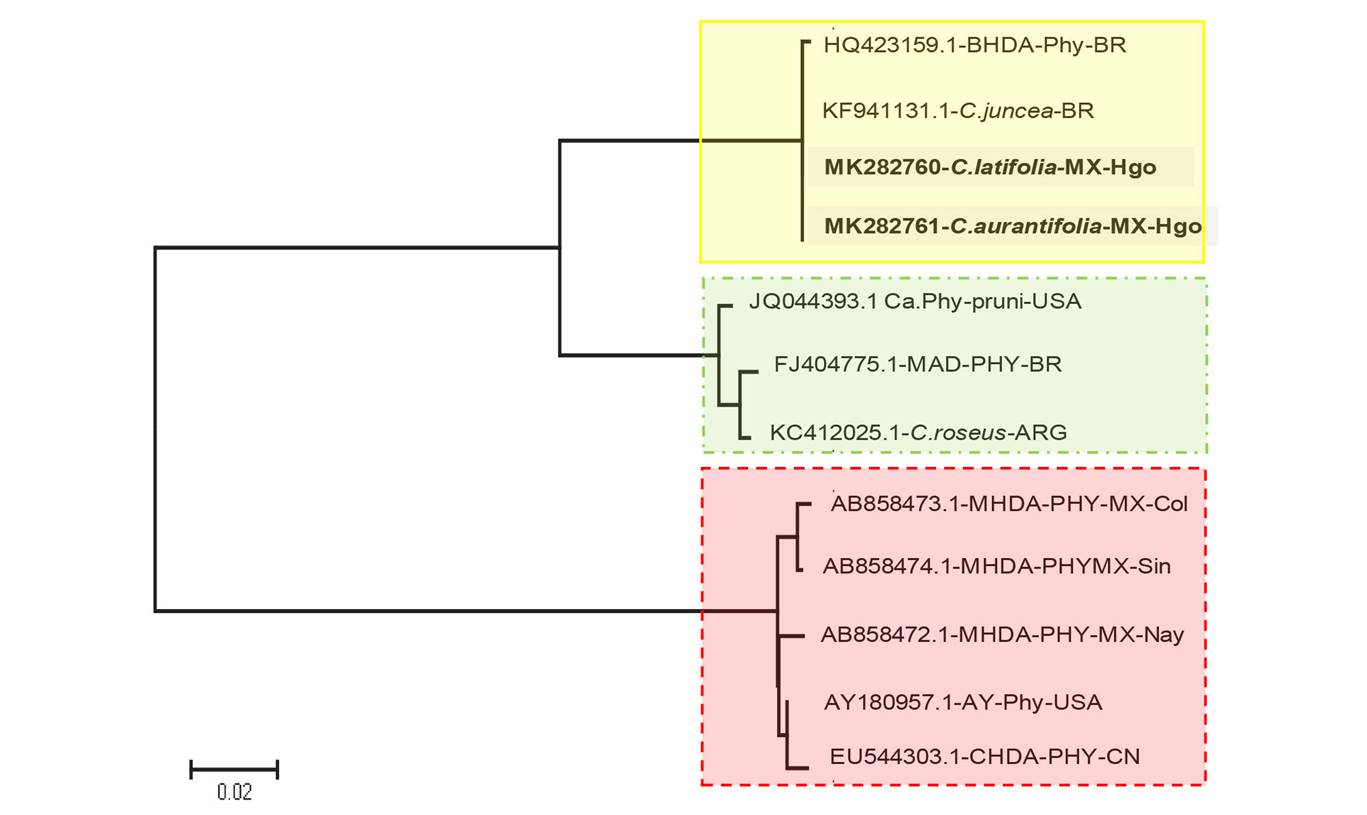
Figure 5 Phylogenetic tree constructed using the Maximum likelihood method, based on the General Time Reversible nucleotide substitution model (GTR+I) with 500 bootstraps. Three main branches are made up differentially: 1). 16Sr IX with samples of C.sinensis and Crotalaria juncea from Brazil, and C. latifolia and C. aurantifolia from Hidalgo, Mexico found in this investigation (yellow box and solid line). 2). 16Sr III with the recent report from Brazil in C. sinensis (green box and dotted line), and 3). 16Sr I made up of Citrus spp samples reported byArratia et al., 2015(red box and dotted line).
Literatura citada
Ahrens U and Seemüller E. 1992. Detection of DNA of plant pathogenic mycoplasmalike organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 82:828-832. https://www.apsnet.org/publications/phytopathology/backissues/Documents/1992Articles/Phyto82n08_828.PDF [ Links ]
Arratia CA, Santos CA, Fernández HM, Chávez ME, Flores ZJL, Camacho BG, Méndez LE, Leyva LJ. 2015. ‘Candidatus Phytoplasma asteris’ is associated with citrus “huanglongbing” disease in Mexico. Phytopathogenic Mollicutes http://dx.doi.org/10.5958/2249-4677.2015.00039.0. [ Links ]
Beamer RH. 1944. A new species of Acinopterus from California (Homoptera: Cicadellidae). Journal of Kansas Entomology Society 17: 21-22. http://direct.biostor.org/issn/0022-8567 [ Links ]
Berges R, Rott M, and Seemüller E. 2000. Range of phytoplasma concentrations in various plant hosts as determined by competitive polymerase chain reaction. Phytopathology 90:1145-1152. http://dx.doi.org/10.1094/PHYTO.2000.90.10.1145 [ Links ]
Bertaccini, A. and Duduk B. 2009. Phytoplasma and phytoplasma diseases: a review of recent research. Phytopathologia Mediterranea 48: 355-378. DOI: http://dx.doi.org/10.14601/Phytopathol_Mediterr-3300 [ Links ]
Blanco RE. 2014. Cicadelidos (Hemiptera:Cicadellide) Asociados a Cítricos en la Península de Yucatán. Tesis MC Colegio de Postgaduados. Montecillo, México. 87p. http://www.remeri.org.mx/tesis/INDIXE-TESIS.jsp?search=colpos&ind=1326&step=200&order=3&asc=0 [ Links ]
Chen J, Pu X, Deng X, Liu S, Li H and Civerolo E. 2009. A phytoplasma related to Candidatus Phytoplasma asteri detected in citrus showing HLB (Yellow shoot disease) symptoms in Guangdong, P. R. China. Phytopathology 99:236-242. http://dx.doi.org/10.1094/PHYTO-99-3-0236 [ Links ]
Esquivel-Chávez F, Valdovinos-Ponce G, Mora-Aguilera G, Gómez-Jaimes R, Velázquez-Monreal J., Manzanilla-Ramírez MA, Flores-Sánchez JF, López-Arroyo JI. 2012. Análisis histológico foliar de cítricos agrios y naranja dulce con síntomas ocasionados por Candidatus Liberibacter asiaticus. Agrociencia 46: 769-782. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952012000800003&lng=es&nrm=iso [ Links ]
Flores-Sánchez J, Mora-Aguilera, G, Loeza KE, López AJ, Gutiérrez EMA, Velázquez MJJ, Domínguez MS, Bassanezi R B, Acevedo SG and Robles GP. 2017. Diffusion model for describing the regional spread of huanglongbing from first reported outbreaks and basing an area wide disease management strategy. Plant Disease 101:1119-1127 http://dx.doi.org/10.1094/PDIS-04-16-0418-RE [ Links ]
Lee IM, Hammond RW, Davis RE and Gundersen DE. 1993. Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology 83:834-842. http://www.apsnet.org/publications/phytopathology/backissues/Documents/1993Articles/Phyto83n08_834.PDF [ Links ]
Lee IM, Gundersen Rindal DE, Davis RE, Bottner KD, Marcone C and Seemüller E. 2004. ‘Candidatus Phytoplasma asteris’, a novel phytoplasma taxon associated with aster yellows and related diseases. International Journal of Systematic and Evolutionary Microbiology 54: 1037-1048. DOI 10.1099/ijs.0.02843-0 [ Links ]
Marques RN, Teixeira DC, Martins EC, Leite APR, Sanches AL, Yamamoto PT and Lopes JRS. 2010. Detection of the phytoplasma associated with citrus huanglongbing symptoms in the leafhopper Scaphytopius marginelineatus. Citrus Research and Technology 31:40. http://bdpi.usp.br/single.php?_id=002243478. [ Links ]
Medler JT. 1960. Agrosoma, a new genus for Tettigoniapulchella Guerin and related species (Homoptera: Cicadellidae). Annals of the Entomological Society of America 53: 18-26. https://doi.org/10.1093/aesa/53.1.18 [ Links ]
Mora-Aguilera G, Robles GP, López AJI, Flores SJ, Acevedo SG, Domínguez MS, Gutiérrez EA, Loeza KE y González GR. 2014. Situación actual y perspectivas del manejo del HLB de los Cítricos. Revista Mexicana de Fitopatología 32: 108-119. http://rmf.smf.org.mx/Vol3222014/AR/32-2_03.pdf [ Links ]
Robles G, M. M, Velázquez MJJ, Manzanilla RMA, Orozco SM, Flores VR y U Medina UVM. 2010. Síntomas del Huanglongbing en limón mexicano. Primeras observaciones. Pp: 141-149. In: Memorias del 1er Simp. Nal. sobre Investigación para el Manejo del Psílido Asiático de los Críticos y el Huanglongbing en México. Monterrey, Nuevo León [ Links ]
SIRVEF. 2018. Sistema Integral de Referencia para la Vigilancia Epidemiologica Fitosanitaria. https://prod.senasica.gob.mx/SIRVEF/ [ Links ]
Teixeira DC, Wulff NA, Martins EC, Kitajima EW, Bassanezi R, Ayres AJ, Eveillard S, Saillard C and Bové JM. 2008. A phytoplasma closely related to the Pigeon pea witches’-broom phytoplasma 16srIX is associated with citrus huanglongbing symptoms in the state of São Paulo, Brazil. Phytopathology 98:977-984. http://dx.doi.org/10.1094/PHYTO-98-9-0977 [ Links ]
Teixeira DC, Wulff NA, Martins EC and Bove JM. 2010. Phytoplasma associated with citrus Huanglongbing in Brazil. National Center for Biotechnology Information (NCBI). https://www.ncbi.nlm.nih.gov/nuccore/312435222 [ Links ]
Torres L, Galdeano E, Docampo D and Conci L. 2004. Characterization of an Aster yellows phytoplasma associated with Catharanthus little leaf in Argentina. Journal of Plant Pathology 8(63) 209-214. http://www.sipav.org/main/jpp/volumes/0304/030404.pdf [ Links ]
Wulff AN, Fassini GC, Marques VV, Martins CE, Coletti BDA, Teixeira DC, Sanches MM, Bové J. 2018. Molecular characterization and detection of 16SrIII group phytoplasma associated with Huanglongbing symptoms. https://doi.org/10.1094/PHYTO-03-18-0081-R [ Links ]
Weintraub PG and Beanland L. 2006. Insect vectors of phytoplasmas. Annual Review of Entomology 51: 91-111. https://doi.org/10.1146/annurev.ento.51.110104.151039 [ Links ]
Young DA. 1977. Taxonomic study of the Cicadellinae (Homoptera: Cicadellidae). Part 2. New World Cicadellid and the genus Cicadella. Technical Bulletin of the North Carolina Agricultural Experiment Station. 239. 1135 pp. https://eurekamag.com/research/006/580/006580776.php [ Links ]
Received: October 04, 2018; Accepted: December 27, 2018











 text in
text in 


