Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.37 n.1 Texcoco Jan. 2019 Epub Aug 21, 2020
https://doi.org/10.18781/r.mex.fit.1810-2
Phytopathological notes
Fusarium sambucinum Fuckel causal agent of fruit rot of manzano chilli pepper (Capsicum pubescens) in Mexico
1 Colegio de Postgraduados, Campus Montecillo, Km. 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Estado de México, 56230, México.
Manzano pepper, like other fruits, is affected by postharvest pathogens that affect quality. In 2016, sunken lesions with whitish mycelium were observed in manzano pepper of samples from the Estado de Mexico. The objective was to identify the causal agent of the symptom described in fruits, by measurements of the morphological, molecular characterization, and pathogenicity tests. Pathogenicity tests were performed on wounded and unwounded fruits, as well as morphological and molecular characterization. The results indicate that the causal agent of the sinking symptoms was Fusarium sambucinum. The isolates were planted in PDA, Clavel-Agar, and SNA culture media, where they formed orange sporodochia, hyaline macroconidia with the papillated basal cell and the terminal cell in foot shape: 24.8-35.3 μm long and 3.67-4.46 μm wide, from three to five septa, this was confirmed by the sequencing analysis of the tef 1α gene (translation elongation factor 1α). The comparison between means (Tukey) detected significant differences in the method of inoculation but not between isolations on the fruit. This is the first report in Mexico of Fusarium sambucinum as the causal agent of sunken lesions with whitish mycelium in manzano peppers.
Key words: fungi; postharvest; pepper rot; fusariosis; PDA; and tef 1 α.
El chile manzano al igual que otros frutos, es afectado por patógenos postcosecha que afectan la calidad. En el 2016 se observaron lesiones hundidas con micelio blanquecino en frutos de chile manzano procedentes del Estado de México. El objetivo fue identificar al agente causal del síntoma descrito en frutos, mediante la caracterización morfológica, molecular y pruebas de patogenicidad. Las pruebas de patogenicidad se realizaron en frutos con herida y sin herida, así como la caracterización morfológica y molecular. Los resultados indican que el agente causal de los síntomas del hundimiento fue Fusarium sambucinum. Los aislados se sembraron en medios de cultivo PDA, Clavel-Agar y SNA, en donde formaron esporodoquios de color naranja, macroconidias hialinas con la célula basal papilada y la célula terminal en forma de pie: 24.8-35.3 μm de largo y 3.67-4.46 μm de ancho, de tres a cinco septos, esto fue confirmado con el análisis de secuenciación del gen tef 1α (translation elongation factor 1α). La comparación entre medias (Tukey) detectó diferencias significativas en el método de inoculación, pero no entre aislamientos sobre el fruto. Es el primer reporte en México de Fusarium sambucinum como agente causal de lesiones hundidas con micelio blanquecino en frutos de chile manzano.
Palabras clave: hongos; postcosecha; pudrición chile; fusariosis; PDA y tef 1α
The manzano chili pepper (Capsicum pubescens R and P) is an agricultural product with high possibilities of worldwide commercialization due to its demand for consumption as a vegetable or for its chemical properties to obtain sub products of capsaicin (Pérez-Grajales et al., 2004). Manzano peppers were introduced into Mexico at the beginning of the 20th Century, and it is the only type of chili pepper grown between 1700 and 2500 masl; it grows as a large bush, it can reach heights of up to 3 meters, the fruit is spherical and is green until its stage of maturity, when it turns red, yellow and orange. The main production areas of manzano peppers are located in the states of Michoacán, Puebla, the State of Mexico and Veracruz, and to a lower extent, in Chiapas and Oaxaca (Espinosa-Torres and Villa-Galindo, 2008). Its harvesting period is in the months of April and May. Its consumption is irreplaceable for the areas of the Sierra Norte in Puebla, Toluca, and Michoacan (mainly Morelia), where it is a part of people’s dietary habits. Its harvest yield in the open is between 9 and 14 t/ha, and in a greenhouse, between 40 and 70 t (Espinosa-Torres and Ramírez-Abarca, 2016). However, the crop can be affected by pathogens. Some of the diseases that affect the crop include wilting of the manzano pepper, caused by diverse pathogens such as Botrytis, Fusarium spp., and Phytium, etc., which cause significant losses in production in several parts of the world, including Mexico. Fusarium sambucinum [teleomorph Gibberella pulicaris (Fr.) Sacc (Leslie and Sumerell, 2006)] is one of the fungal species that cause dry rotting or black rotting in potatoes (Solanum tuberosum L.), affecting both in the field or in storage (Hanson et al., 1996; Peters et al., 2008). F. sambucinum has been reported in Europe, the United States, and recently, in China, as affecting fruits in storage and producing micotoxins such as trichothecenes (El-Banna et al., 1984; Altomare et al., 1995; Du et al., 2012). The correct identification of a plant pathogen is essential to propose the most effective alternatives for its management (Agrios, 2005). In this context, the aim was to determine the causal agent of sunken lesions in manzano pepper fruits using morhpological and molecular characteization with a factor of elongation (EF-1∞) and pathogenicity tests in fruits.
In November of 2015, samples were taken of manzano pepper fruits from the municipality of Coatepec de Harinas, State of Mexico, where fruits were gathered with symptoms of rotting and sunken lesions. The samples were placed in labeled plastic bags (Ziploc®) in a cooler and taken to the postharvest laboratory of the Instituto de Fitosanidad of the Colegio de Postgraduados.
To isolate the causal agent, we disinfested 1 cm fragments of the area of advancement of the rotting caused by fungi, and for this, cuts were made on the tissues of 40 fruits and placed them in a sodium hypochlorite solution at 0.2% for 2 min. The were then washed with sterile distilled water twice, left to dry on sterile paper towels and placed in Petri dishes with 20 mL of PDA, which were left in an incubator at 27+2 °C, under light for 24 h. After sporulating after six days of growth, monosporic cultures were performed and moved to Petri dished with a PDA culture medium. The conservation of isolations was carried out with the transfer of 10 mycelial discs (5 mm in diameter) to 2 mL cryogenic tubes with 1.5 mL of glycerine at 20% (v/v) stored at -80 °C and in test tubes with mineral oils.
The morphological characterization was carried out from their asexual reproduction structures. For this process, the isolations were transferred to PDA, SNA and Clavel-Agar culture mediums with three repetitions for each one of the fungal colonies; they were then incubated at 25±2 °C under conditions of continuous light. The mycelial growth was measured every 48 h until the fungus completely filled the dish, where the color of the colony was evaluated along with the type of mycelium, growth of the mycelium and spores (conidia, chlamydospores and sporodochia). After 15 days of incubation, preparations were made in glycerine at 50% with the purpose of recognizing the structures of the fungi using a compound microscope (Velab Ve-B6, México). Fifty macroconidia were observed and their size, color and number of septa were recorded using the program Motic Images plus 2.0 (Group Co., Ltd). The morphological identification of the fungi at the level of genus was carried out using Barnett and Hunter’s specialized code (1998), and for species, Leslie and Summerell’s (2006).
Once the pure isolations were taken from the fungi, we began the molecular identification with the extraction of genomic DNA from nine isolations and morphologically identified as Fusarium; for this, we followed the protocol indicated in the Plant DNeasy® Minikit extraction kit (Quiagen). A 0.03 g mycelium sample was placed in Eppendorf tubes, tenderizing with a hypodermic needle. We then added 400 μL of Buffer AP1 and 4 μL of RNase y se agitó. The samples were incubated in a double boiler for 10 min at 65 °C, we stirred by inverting them every 2 min, and later added 120 μL of the Buffer AP2 to the sample, and incubated for 5 min in ice. The mixture was placed in the QIAshredder Mini spin column and centrifuged for 2 min at 14000 rpm. The supernatant was then removed and placed in a new Eppendorf tube, to which 675 μL of the Buffer AP3/E were added. The mixture was placed in a DNeasy Mini spin tube and centrifuged at 8000 rpm for 1 min. The column was replaced with another supplementary one of the protocol and 500 μL off the Buffer AW were added in the membrane of the Mini spin DNeasy and centrifuged at 8000 rpm for 1 min; the column was changed once again with the filtrate, 500 μL of the Buffer AW were added to the membrane and centrifuged at 14000 rpm for 2 min. Finally, the DNeasy Mini spin column was transferred to a new Eppendorf tube and 100 μL of the Buffer AE were added. It was incubated for 5 min at room temperature and centrifuged at 8000 rpm for 1 min. The DNA extraction products were sent to the Macrogen® Inc. laboratory (Korea) to be purified, amplified, and sequenced. The sequences obtained were processes in the program BioEdit v7.0.9. The PCR Elongation factor (EF-1∞) tests were carried out with the following conditions: initial denaturalization for 5 min at 94 °C, 35 cycles of 45 s at 94 °C, 45 s at 63 °C, 1 min at 72 °C and a final extension of 10 min at 72 °C (O´Donnell et al., 1998).
The pathogenicity tests were carried out on 80 healthy manzano pepper fruits in a stage of physiological maturity, which were inoculated with nine isolations, identified morphologically as Fusarium sp. The fruits were disinfected using a 0.5 % sodium hypochlorite solution for 5 min, they were submerged in sterile distilled water for 10 min and placed on sterilized paper towels (Kimberly-Clark) until completely dry. Four fruits were inoculated and placed in Styrofoam trays with paper towels, moistened with sterile distilled water placed inside plastic, to provide humidity. The trays with the fruits were placed in a growth chamber under incubation conditions of 27+2 °C. Inoculation was carried out in fruits with lesions (punctured with a needle) and without lesions, and on top of that we placed a disc, 5 mm in diameter, that contained the mycelial growth of the fungus of each one of the seven-day-old isolations. As a control, we used peppers without wounds, inoculated with PDA discs without fungal mycelia. We measured the diameter of the lesions in the inoculated and uninoculated peppers with the isolations of Fusarium sp. every 48 h. The pathogenicity tests were carried out twice for each treatment and the control. For additional information on the size of the lesion, we carried out a Tukey comparison of averages using the program SAS V.9.1 for Windows.
From the morphological identification, we obtained nine isolations of the material processed from the manzano chili pepper fruits that showed an average growth rate of of 2 cm in diameter in a PDA culture medium. Each isolation displayed different colors in the PDA culture medium, and therefore, three groups were formed, each one of which were given a code. The first group displayed a mycelial growth colored whitish-pink, which turned pink with time in the culture medium (codes FS24, FS26 and FS27). The second group displayed a cream colored white growth, with a fuzzy, abundant cotton-like appearance (codes FS25, FS28 and FS32). The third group presented an initially white growth, which turned red with time (codes FS30, FS31 and FS33). These results coincide with studies reported by Baturo Ciesniewska et al. (2015), who observed different pigments in the culture medium, caused by Fusarium sambucinum, gathered from Solanum tuberosum culture. It was also observed that the nine isolations that grew in the PDA medium formed structures called macroconidia, and in the SNA culture, there was an abundant formation of macroconidia along with orange colored sporodochia. Only fungal mycelia formed in the Clavel-Agar culture medium, and this behavior was general for the nine isolations. Regarding the shape of the macroconidia, they were sickle-shaped hyaline with thin walls, and generally uniform in size, presenting a papillary basal cell in the shape of a foot. The average size of the conidia was 24.8-35.3 μm in length and 3.67-4.46 μm in width; three to five septa were found per conidium (Figure 1), there was no microconidia or formation of chlamydospores. With these morphological characteristics, the nine isolations were identified as Fusarium sambucinum. All characteristics coincided with those described by Leslie and Summerell (2006) for F. sambucinum. Unlike F. torulosum and F. venetanum, which produce chlamydospores, F. sambucinum does not. F. culmorum can only be distinguished for the molecular marker with a Factor of elongation (EF-1∞) which confirmed F. sambucinum.
The sequences of the nine isolations were compared with the sequences from the GenBank database, showing a 99% identity with F. sambucinum in chain with the polymerase with a Factor of elongation (EF-1∞). The consensus sequence was named CHF24 and it was obtained by aligning the progress and regression of the sequence, and it was deposited in the Gen Bank with accession number (KX632088.1). This confirmed the results of the morphological characterization.
The nine F. sambucinum isolations used to inoculate the manzano chilli pepper fruits were 100% pathogenic; in the treatments without lesions, only 20% of the inoculated fruits presented symptoms, whereas the control pepper fruits were asymptomatic. In pepper fruits with lesions, we noticed sunken circular lesions 96 h after inoculation (hai); also, surface mycelia developed at 144 hai and sporulation of conidia 192 hai (Figure 2). The pathogenic fungus was re-isolated from the manzano pepper fruits infected with each isolation of F. sambucinum, to confirm Koch’s postulates; the same morphological growth characteristics were observed for the fungus, both in the re-isolations and in the original inoculations. In the comparison of averages of the treatments with lesions, no significant differences were observed between the diameter of the lesion in the nine isolations evaluated (p >0.05) (Table 1).
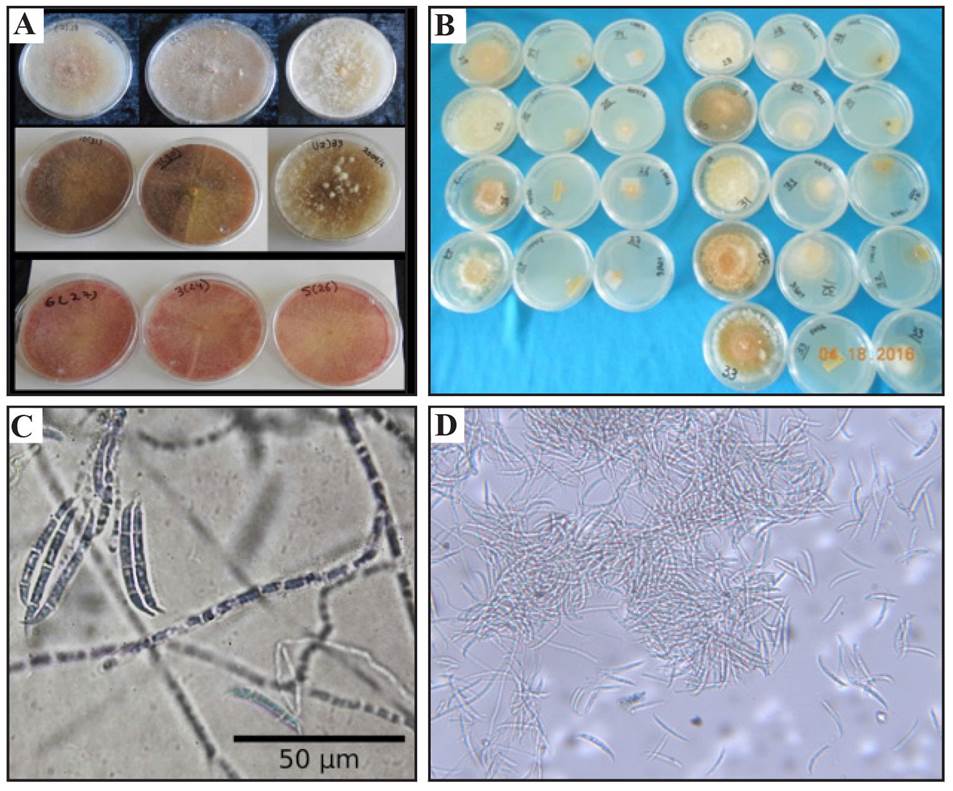
Figure 1 Cultural and morphological characterization of F. sambucinum (A). Cultures in eight-day-old PDA medium. (B) Seven-day-old macroconidia produced in a PDA 40X medium. (C) Culture growth in cultures in SNA and Clavel- Agar culture media. (D) Sporodochia produced in SNA 10 X medium.
This study observed that the nine F. sambucinum isolations are pathogenic. Regarding the two inoculation methods with and without lesions, we observed that in the first method 100% of the fruits inoculated displayed symptoms such as circular lesions, sunken with a milky white mycelium and fungal sporulation; meanwhile, in the pepper fruits inoculated without lesions, we observed that only 20% presented the characteristic symptoms mentioned above in manzano peppers. These results were similar to those reported by other authors (Nelson et al., 1981; Secor and Salas, 2001; Peters et al., 2008; Gachango et al., 2012; Baturo-Ciesniewska et al., 2015), who found that the pathogen causes dry rotting in Solanum tuberosum tubers with artificial lesions.

Figure 2 Symptoms of sinking observed 96 hai in manzano chili peppers inoculated with F. sambucinum discs (A). Presence of cloudy white mycelium at 144 hai (B and C). Presence of sporulation of conidia at 192 hai; these were with le sions (D). Micelia and sporulation of F. sambucinum in fruits with artificial lesions (192 hai) (E).
Table 1. Diameter of lesion induced by F. sambucinum in manzano pepper fruits with artificial lesions.
| Aislados | Diámetro de la lesión (mm) |
|---|---|
| FS24 | 18.05aZ |
| FS25 | 18.85 a |
| FS26 | 16.98 a |
| FS27 | 12.03 a |
| FS28 | 14.96 a |
| FS30 | 14.81 a |
| FS31 | 17.19 a |
| FS32 | 21.07 a |
| FS33 | 14.32 a |
ZValores con la misma letra, dentro de la columna son estadísticamente iguales (Tukey, α=0.05) / Values with the same letter within the column are statistically equal (Tukey, α=0.05).
In addition, we observed that the nine isolations grown in PDA formed three groups of mycelium colors, out of which two groups coincide with those reported by Baturo-Ciesniewska et al. (2015), who grouped the isolations into the colors pink, salmon and bright cream. For this study, cultures were presented colored creamy white, pink and reddish maroon.
Regarding morphological characteristics, we observed that the F. sambucinum isolations planted in PDA formed macroconidia, and in SNA, they formed abundant sporodochia and orange-colored macroconidia . No microconidia were present, nor were there chlamydospores; these characteristics coincide with those reported by Leslie and Summerell (2006).
The sequences obtained from the DNA of the nine isolations displayed a 99% similarity with other F. sambucinum sequences in the Gen Bank database, with accession number KP710620 and KP674193, which were reported as the causal agent of dry rotting in potato tubers (Stefancyk et al., 2016). In this investigation, all isolations were identified as F. sambucinum and one of the sequences carried out (accession number KX632088.1) was deposited in the Gen Bank; however, it is necessary to take samples from different areas of the country in which manzano chilli peppers are grown, in order to know the distribution of the pathogen in Mexico.
In conclusion, the results of this work show that morphological identification, molecular characterization and pathogenicity tests on the causal agent of the symptoms of sunken lesions and milky white mycelia on the manzano pepper is Fusarium sambucinum and the lesions favor the penetration of the pathogen in postharvest.
This is the first study carried out in Mexico on Fusarium sambucinum in postharvest manzano chilli peppers.
Literatura citada
Agrios N. 2005. Plant Pathology. 5th edn. San Diego, California. USA. Elsevier Academic Press. 922p. [ Links ]
Altomare CA, Logrieco A, Bottalico GM, Moretti and Evidente A 1995. Production of type A trichothecenes and enniatin B by Fusarium sambucinum Fuckel sensu lato. Mycopathology 129:177-181 Disponible en línea: https://link.springer.com/content/pdf/10.1007%2FBF01103344.pdf [ Links ]
Barnett LH and Hunter BB. 1998. Illustrated genera of imperfect fungi. The American Phytopathologycal Society. St. Paul, Minnesota, USA. 218 p. [ Links ]
Baturo-Ciesniewska A, Lenc L, Grabowski A and Lukanowski A. 2015. Characteristics of Polish isolates of Fusarium sambucinum: Molecular identification, pathogenicity, diversity and reaction to control agents. American Journal of Potato 92: 49-61.http://dx.doi.org/10.1007/s12230-014-9410-z [ Links ]
Du M, Ren, X, Sun Q, Wang Y and Zhang R. 2012. Characterization of Fusarium spp. causing potato dry rot in China and susceptibility evaluation of Chinese potato germplasm to the pathogen. Potato Research, 55:175-184. http://dx.doi.org/10.1007/s11540-012-9217-6 [ Links ]
El-Banna AA, Scott PM, Lau PY, Sakuma T, Platt HW and Campbell V. 1984. Formation of trichothecenes by Fusarium solani var. coeruleum and Fusarium sambucinum in potatoes. Appl. Environ. Microbiol. 47:1169-1171 Disponible en línea: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC240098/pdf/aem00162-0289.pdf [ Links ]
Espinosa-Torres, LE y Villa-Galindo, A. 2008. Regiones productoras de chile manzano. Rev. Ext. Campo. 7-8: 8-12 [ Links ]
Espinosa-Torres LE, y Ramírez-AO. 2016. Rentabilidad de chile manzo (Capsicum pubescens R&P) producido en invernadero en Texcoco, Estado de México. Revista Mexicana de Ciencias Agrícolas 7(2):325-335 Disponible en línea: http://www.scielo.org.mx/pdf/remexca/v7n2/2007-0934-remexca-7-02-00325.pdf [ Links ]
Gachango E, Hanson LE, Rojas A, Hao J J and Kirk WW. 2012. Fusarium spp. causing dry rot of seed potato tubers in Michigan and their sensitivity to fungicides. Plant Disease, 96:1767-1774. https://dx.doi.org/10.1094/PDIS-11-11-0932-RE [ Links ]
Hanson LE, Schwager SJ and Loria R. 1996. Sensitivity to thiabendazole in Fusariumspecies associated with dry rot of potato. Phytopathology, 86:378-384. http://dx.doi.org/10.1094/Phyto-86-378. [ Links ]
Leslie JF, and Summerell BA. 2006. The Fusarium laboratory manual. United States of America; Blackwell Publishing, Oxford, UK. p 388. [ Links ]
Nelson PE, Toussoun TA, and Cook, RJ. 1981. Fusarium: diseases, biology and taxonomy. USA: The Pennsylvania State University Press. 474 p. [ Links ]
O´Donnell K, Kistler CK, Cigelnik E and Ploetz R.C. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: Corcordant evidence from nuclear and mitochondrial gene genealogies. Proc, Natl. Acad. Sci. 95:2044-2049 Disponible en línea: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC19243/pdf/pq002044.pdf [ Links ]
Pérez-Grajales M, Gónzalez-Hernández VA, Mendoza-Castillo MC and Peña-Valdivia C 2004. Physiological characterization of Manzano hot pepper (Capsicum pubescens R and P) Landraces. J. Amer. Soc. Hort. 129(1):88-92 Disponible en línea: http://journal.ashspublications.org/content/129/1/88.full.pdf+html [ Links ]
Peters RD, MacLeod C, Seifert KA, Martin RA, Hale LR, Grau CR, and MacInnis S. 2008. Pathogenicity to potato tubers of Fusarium spp. isolated from potato, cereal and forage crops. American Journal of Potato Research, 85(5):367-374. http://dx.doi./10.1007/s12230-008-9037-z [ Links ]
SAS Versión .9.1 for Windows [ Links ]
Secor GA, and Salas B. 2001. Fusarium dry rot and Fusarium wilt. In: Stevenson WR, Loria GDF, and Weingartner DP (eds). Compendium of potato diseases, 2nd ed., St. Paul, MN, USA: APS Press. 23-25 pp. [ Links ]
Stefanczyk E, Sobkowiak S, Brylinska M, and Sliwka J. 2016. Diversity of Fusarium spp. associated with dry rot of potato tubers in Poland. European Journal Plant Pathol. 145(4): 871-884. http://dx.doi.org/10.1007/s10658-016-0875-0 [ Links ]
Received: October 02, 2018; Accepted: December 12, 2018











 text in
text in 


