Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.37 no.1 Texcoco Jan. 2019 Epub 21-Ago-2020
https://doi.org/10.18781/r.mex.fit.1807-5
Scientific articles
Evaluation of synthetic hexaploid wheats for resistance to Wheat Blast disease
1 Departamento de Investigación, Cámara Paraguaya de Exportadores y Comercializadores de Cereales y Oleaginosas, Central, Avenida Brasilia 840, Asunción, Paraguay;
b Facultad de Politécnica, Universidad Nacional de Asunción, Mcal. Estigarribia Km 11,5. Central, San Lorenzo, Paraguay.
Wheat Blast disease is caused by the fungus Pyricularia oryzae pathotype Triticum. The disease has been endemic to tropical South American region for over 30 years until 2016, when it occurred in Bangladesh, opening the possibility of its expansion to other regions. Considering the limited availability of known sources of resistance in wheat and the synthetic hexaploid wheats are known to be a source of resistance to multiple type of stresses, the objective of this research was to evaluate a collection of synthetic hexaploid wheats for their resistance to Wheat Blast. The experiments were carried out in the Hernando Bertoni Research Center, Paraguayan Institute of Agricultural Technology, Paraguay. Test spikes were inoculated, concentration of 5.104 conidios.mL-1. The reaction was evaluated 15 days after inoculation and observed for next 15 days to calculate the disease-progress. Sixty-four synthetic hexaploid wheats, provided by the International Maize and Wheat Improvement Center, Mexico, were subjected to infections by isolate (P14ATae039) of P. oryzae, 18 materials were selected based on their maximum values of reaction, to be reevaluated with two different isolates P14ATae039 and P14YTae031. TS29, TS49 synthetic wheat were identified as new sources of resistance to wheat blast and TS73 wheat that is moderately susceptible, but of different genetic origin. To our best knowledge, this is the first report of synthetic hexaploid wheats selected as new sources of genetic resistance to wheat blast disease.
Key words: Fungal disease; resistance to pathogen; Aegilops tauschii; Triticum durum
La enfermedad Brusone en trigo es causada por el hongo Pyricularia oryzae patotipo Triticum. Desde hace 30 años, esta enfermedad ha sido endémica de Sudamérica hasta su aparición en Bangladés en el año 2016, abriendo la posibilidad de su expansión a otras regiones. Considerando poca disponibilidad de las fuentes de resistencia conocida en trigo y conociendo la resistencia de los trigos sintéticos hexaploides (TSHs) a distintos tipos de estrés, el objetivo de este trabajo fue evaluar una colección por su resistencia a la Piricularia. Los ensayos fueron realizados en el Centro de Investigación Hernando Bertoni, Instituto Paraguayo de Tecnología Agraria, Paraguay. Las infecciones fueron realizadas en espigas, concentración 5.104 conidios.mL-1. La reacción se evaluó a los 15 días después de la inoculación y observada durante los próximos 15 días para calcular el avance de la enfermedad. Sesenta y cuatro TSHs proporcionados por el Centro Internacional de Mejoramiento de Maíz y Trigo, México, fueron sometidos a infecciones por un aislado (P14ATae039) de P. oryzae., donde 18 materiales fueron seleccionados por su valor máximo de reacción, para una reevaluación con dos aislados P14ATae039 y P14YTae031. Los trigos TS29, TS49 fueron identificados como nuevas fuentes de resistencia a Brusone, al igual que TS73 que es moderadamente susceptible, pero de distinto origen genético. Para nuestro conocimiento este es el primer reporte de selección de los trigos sintéticos hexaploides como nuevas fuentes de resistencia genética para Brusone de trigo.
Palabras clave: Enfermedad fúngica; resistencia a patógeno; Aegilops tauschii; Triticum durum
The Wheat Blast disease is considered one of the emerging diseases in the world, and is caused by the fungus Pyricularia oryzae, (synonym Magnaporthe oryzae Catt.) (Couch and Kohn, 2002). Its specificity in wheat was attributed to a subpopulation named Triticum pathotype (MoT) by Cruz and Valent (2017).
This disease has been endemic to the tropical region of South America, covering the plains of Bolivia, central and south-central Brazil, and Paraguay (Kohli et al., 2011), up to its first report in Bangladesh in 2016 (Malaker et al., 2016), which shows its possibility of expansion to other similar regions. Considering that chemical control is not very efficient and under favorable conditions, losses may be of up to 80% of the production (Kohli et al. 2011), the search for resistance to this pathogen is crucial to ensure the regional and global production of wheat.
The most efficient resistance to wheat blast reported recently is related to the translocation of the 2NS/2AS, from the Triticum ventricosum (Tausch) Cess, (Cruz et al. 2016). In a recent study, Chávez and Kohli (2018) identified only two of the commercial Paraguayan wheat varieties with resistance and moderate resistance, displaying the urgency of broadening its genetic base.
Considering the possibility of finding greater genetic variability in the species allied to wheat, Urushima and Kato (1994) explored Aegilops species and found resistance in two species: Ae. tauschii and Ae. umballulata. The Synthetic Hexaploid Wheats (SHWs) are a result of crosses between durum wheats (Triticum durum L., 2n=4x=28, AABB) and the allied species, Ae. tauschii (2n=2x=14, DD) (Ogbonnaya et al., 2013). Several of the SHWs have proven their potential as an important source of alleles for the improvement of agronomic traits, such as grain size and shape (Masood et al., 2016; Okamoto et al., 2013; Rasheed et al., 2014), bread quality (Lu et al., 2005), resistance to abiotic (Sohail et al., 2011) and biotic stresses (Gul Kazi et al., 2012; Jighly et al., 2016). Taking this into account, the aim of this work was to evaluate a group of SHWs according to their reaction to the wheat blast disease under controlled conditions of infection.
Materials and methods
Trials were carried out in the Hernando Bertoni Research Center, Paraguayan Institute of Agricultural Technology, (CIHB-IPTA), Caacupé, Paraguay.
The isolates used, from the wheat spikes, were morphologically identified following Klaubauf et al., (2014) and confirmed with the specific molecular marker MoT3 (Pieck et al., 2016). Previous evaluations in materials of known reactions of susceptibility and resistance (Caninde 11 and Milan) confirm their capability for infection.
Inoculum were obtained following the method suggested by Marangoni et al. (2013). The pieces of filter paper, 0,5 cm in diameter with preserved isolates, were planted in Petri dishes with Oat meal Agar (OA), and incubated for 12 days at 25 °C with light periods of 12 hours. Later, in order to force sporulation, the mycelia were crushed on the dish and incubated under constant light for 72 hours.
Forced inoculations were carried out on a minimum of 6 and a maximum of 10 spikes per genotype in the phenological stage of anthesis, using a 1/5 HP 58 PSI Oilless Airbrush Compressor Kit, at a concentration of 5.104conidia.mL-1. Approximately 0,3 mL of inoculum was used for each spike. The inoculated plants were kept under controlled temperature (28±2 °C) and humidity conditions (85±5 %) for a period of 72 hours for both trials.
SHWs are materials with a broad variability in terms of their reactions to different diseases. Considering differential reactions in P. oryzae, even between old and new isolates reported by Cruz et al., (2016), the maximum value of reaction (MVR) was used to classify the SHWs and guarantee the reproducibility of the test. The option of pondering the material according to the MVR over the total of spikes evaluated helps discard biases due to fortuitous escapes to the infection (that is, susceptible plants with no reaction). This classification criterion is robust for the selection of materials according to their response to the infection, since only the genotypes with a low MVR display resistance.
Two independent evaluations were carried out on synthetic wheats. In a first evaluation, 64 genotypes of SHWs (Table 1), provided by the International Maize and Wheat Improvement Center (CIMMYT), were inoculated with a monosporic isolate (P14ATae039) of the fungus P. Oryzae.
In a second evaluation, based on the maximum values of reaction (MVR) for wheat blast on the spike, 18 materials were selected for a second evaluation. The chosen genotypes displayed MVR between resistant and moderately resistant, and as a form of susceptibility control, other susceptible reaction materials were included. In order to cover a broader range of pathogenic susceptibility, two virulent isolates, P14ATae039 and P14YTae031, were used in the second inoculation.
The reaction to the disease was evaluated 15 days after inoculation (DAI), using the scale proposed by Chavez et al. (2017), modified for the MVR as follows: 0 = No infection, 1 = Up to 10% of the spike necrotized (resistant), 2 = Up to 40% of the spike necrotized (moderately resistant), 3 = Up to 60% of the spike necrotized (moderately susceptible), and 4 = 100% of the spike necrotized (susceptible). After this period, the spikes of seven genotypes that displayed resistance or moderate resistance in the first reading, along with a susceptible control, were kept in the conditions described above, to observe the development of the spike infection for both isolates for a 30-day period. For the progress of the disease, we used a totally randomized design; each spike represents an experimental unit in which the reaction was observed at 8, 15, 22 and 30 DAI. The calculation of the value of the area under the disease progress curve was carried out using the function AUDPC, implemented in the package agricolae for R version 3.4.2. Analyses of the AUDPC underwent an ANOVA and the comparisons between averages were performed by Test: LSD Fisher Alfa=0,05.
Results and discussion
The data of the first infection and classification of the 64 SHWs based on their maximum values of reaction to an isolate (P14ATae039) are shown in Table 2.
Based on the first evaluation of synthetic wheats, over 10% of the SHWs were classified as Resistant (MVR 0-1), 34% were classified as moderately resistant or moderately susceptible (MVR between 2 and 3), and over 55% as susceptible (MVR 4).
Table 1 List of synthetic hexaploid wheats provided by the International Maize and Wheat Improvement Center (CIMMYT).
| Código | Pedigree |
|---|---|
| TS2 | DOY1/AE. SQUARROSA (188) |
| TS3 | ALTAR84/AE.SQUARROSA (193) |
| TS4 | ALTAR84/AE.SQUARROSA (198) |
| TS15 | ALTAR84/AE.SQUARROSA (219) |
| TS22 | CROC1/AE. SQUARROSA (224) |
| TS23 | ACO89/AE. SQUARROSA (309) |
| TS28 | YAV-3/SCO//J069/CRA/3/YAV79/4/AE.SQUARROSA (498) |
| TS29 | DOY1/AE. SQUARROSA (511) |
| TS32 | 68.111/RGB-U//WARD/3/FGO/4/RABI/5/AE.SQUARROSA (629) |
| TS33 | FGO/USA2111//AE.SQUARROSA (658) |
| TS34 | CROC-1/AE. SQUARROSA (725) |
| TS35 | 68.111/RGB-U//WARD RESEL/3/STIL/4/AE.SQUARROSA (781) |
| TS36 | 68.111/RGB-U//WARD RESEL/3/STIL/4/AE.SQUARROSA (783) |
| TS37 | YAR/AE.SQUARROSA (783) |
| TS39 | 68.111/RGB-U//WARD/3/FGO/4/RABI/5/AE.SQUARROSA (878) |
| TS43 | 68.111/RGB-U//WARD/3/FGO/4/RABI/5/AE.SQUARROSA (890) |
| TS49 | LCK59.61/Ae. SQUARROSA (313) |
| TS50 | LCK61/Ae. SQUARROSA (324) |
| TS53 | GAN/AE.SQUARROSA (408) |
| TS54 | SCA/AE.SQUARROSA (518) |
| TS55 | YAR/AE.SQUARROSA (518) |
| TS57 | SNIPE/YAV79//DACK/TEAL/3/A.SQUARROSA (629) |
| TS58 | D67.2/PARANA66270//AE.SQUARROSA (633) |
| TS59 | D67.2/PARANA//AE.SQUARROSA (659) |
| TS60 | SNIPE/YAV79//DACK/TEA/3/AE.SQUARROSA (700) |
| TS61 | TRN/AE.SQUARROSA (700) |
| TS62 | SNIPE/YAV79//DACK/TEA/3/AE.SQUARROSA (877) |
| TS63 | GAN/AE.SQUARROSA (897) |
| TS64 | YAV-2/TEZ//AE.SQUARROSA (895) |
| TS65 | ARLIN/AE.SQUARROSA (283) |
| TS67 | RASCON/AE. SQUARROSA (312) |
| TS68 | SCOT/MEXI-1//AE. SQUARROSA (314) |
| TS69 | DOY1/AE. SQUARROSA (333) |
| TS70 | DOY1/AE.SQUARROSA (428) |
| TS71 | DOY1/AE.SQUARROSA (458) |
| TS72 | GREEN/AE.SQUARROSA (458) |
| TS73 | SCA/AE.SQUARROSA (409) |
| TS74 | CP18/GDIZ/3/GOOD//ALB/CRA/4/AE.SQUARROSA (409) |
| TS76 | ALTAR84/AE.SQUARROSA (502) |
| TS77 | CROC-1/AE. SQUARROSA (517) |
| TS78 | ZETA/AE. SQUARROSA (1024) |
| TS79 | ZETA/AE. SQUARROSA (1027) |
| TS80 | DOY1/AE.SQUARROSA (1030) |
| TS82 | CROC1/AE. SQUARROSA (210) |
| TS86 | GAN/AE. SQUARROSA (236) |
| TS87 | SORA/AE. SQUARROSA (323) |
| TS88 | D66.2/PARANA66270//AE.SQUARROSA (308) |
| TS89 | LCK59.61/Ae. SQUARROSA (693) |
| TS90 | ZETA/AE. SQUARROSA (1025) |
| TS91 | DOY1/AE.SQUARROSA (1027) |
| TS92 | ZETA/AE. SQUARROSA (386) |
| TS94 | ZETA/AE. SQUARROSA (533) |
| TS95 | CP18/GDIZ/3/GOOD//ALB/CRA/4/AE.SQUARROSA (1018) |
| TS97 | ZETA/AE. SQUARROSA (1038) |
| TS98 | ZETA/AE. SQUARROSA (1053) |
| TS99 | CROC1/AE. SQUARROSA (212) |
| TS100 | ZETA/AE.SQUARROSA (368) |
| TS101 | ARLIN-1/AE.SQUARROSA (430) |
| TS102 | D67.2/PARANA66270//AE.SQUARROSA (1015) |
| TS103 | GAN/AE.SCUARROSA (206) |
| TS104 | ARLIN-1/AE.SQUARROSA (335) |
| TS105 | GAN/AE.SQUARROSA (335) |
| TS106 | 68.111/RGB-U//WARD RESEL/3/STIL/4/AE.SQUARROSA (385) |
| TS109 | DOY1/AE.SQUARROSA (534) |
Like in Gul Kazi et al. (2012) and Das et al. (2015), the reactions to fungal diseases using characteristics have been differentiated in this study. The correct phenotyping of these materials is crucial for the selection of the SHWs that can be used as parents in the breeding programs and/or in different studies of molecular characterization and genic expression, differential to the interaction of wheat and P. oryzae.
The MVR of the preselected 18 SHWs, with two isolates (P14ATae039 and P14YTae031), is graphed in Figure 1.
The data shown in Figure 1 indicates that only one of the genotypes (TS29) was resistant to both isolates; seven were classified as intermediates (TS77, TS53, TS101, TS78, TS32, TS22 and TS63), since they presented the resistant to moderately resistant reaction to one of the isolates, but moderately resistant to moderately susceptible for the other. This interaction trait was reported by Chávez and Kohli (2018), where moderately resistant varieties for one isolates showed a moderately susceptible for the other. This type of reaction specificity may probably be explained by the genetic flow between isolates from different hosts, which gives the isolates a virulence variation (Gladieux et al., 2018).
Table 2. Classification of the 64 SHW genotypes infected with P14ATae039, according to their maximum value of reaction to the Pyricularia. Caacupé, 2018.
| ESCALA DE REACCIÓN | ||||||
|---|---|---|---|---|---|---|
| Resistente | Moderadamente | Moderadamente Susceptible | Susceptibles | |||
| (R) | Resistente (MR) | (M) | (S) | |||
| (0-1) | (2) | (3) | (4) | |||
| TS101z | TS4z | TS32z | TS87 | TS50 | TS70z | TS100 |
| TS34 | TS22z | TS64 | TS103 | TS54z | TS71 | TS102 |
| TS53z | TS28 | TS67 | TS2 | TS55 | TS72z | |
| TS73 | TS29z | TS68 | TS3 | TS57 | TS76 | |
| TS77z | TS33z | TS74z | TS15 | TS58 | TS82 | |
| TS78z | TS49z | TS79 | TS23 | TS59 | TS86 | |
| TS105z | TS63z | TS80 | TS35 | TS60 | TS88 | |
| TS89 | TS92 | TS36 | TS61 | TS91 | ||
| TS90z | TS95 | TS37 | TS62 | TS94 | ||
| TS98 | TS104 | TS39z | TS65 | TS97 | ||
| TS109 | TS106 | TS43 | TS69 | TS99 | ||
zTSHs seleccionados para una segunda evaluación / SHWs selected for a second evaluation.
The data displayed in Figure 2 shows the differences in the average progress of the disease amongst the genotypes identified as resistant or moderately resistant. Only three of the genotypes, TS49, TS53 and TS77, kept their resistance or moderate resistance for the 30-day period. The materials TS29, TS73 and TS101 changed their reaction from moderate resistance to moderate susceptibility, while the synthetic TS78 became susceptible at the end of the 30 days. The SHW TS105, used as a control, was susceptible throughout its development. Considering the differential reactions presented by the materials to the different isolates, the progress of the disease was analyzed in a second instance and is shown in Figures 3a and 3b. The results show a significant interaction between the synthetic wheats evaluated and the two isolates of P. oryzae, which confirms the results obtained by Cruz et al., (2016), in which the Overland and RonL cultivars were resistant to an isolate, but susceptible to the other.
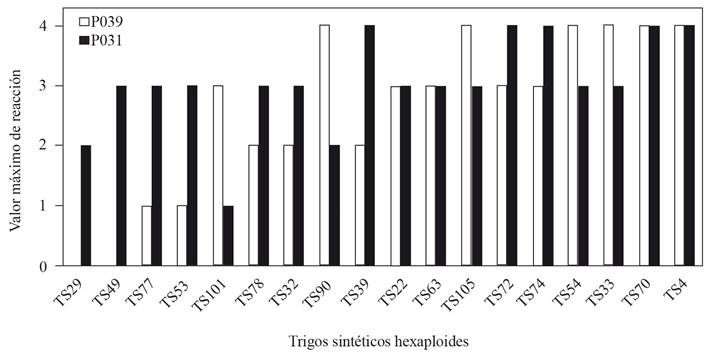
Figure 1 Maximum values of reaction of 18 SHWs to the forced infections of two isolates of P. oryzae. Reactions to the isolate P14ATae039 (P039) and reactions to the isolate P14YTae031 (P031). Caacupé, 2018.
Wheats viz. TS29 and TS49 were relatively stable in maintaining their resistance or moderate resistance, and other viz. TS73, TS78 and TS105 were moderately susceptible or susceptible to both isolates. However, the remaining SHWs viz. TSHs TS53, TS77 and TS101, have a significantly different reaction between the two isolates (Figure 1).
Synthetic wheat TS101 was resistant to isolate P031, yet susceptible to P039; wheats TS53 and TS77 were between moderately susceptible and susceptible to P031, yet resistant to P039 (Figure 3).
This result is consistent with those obtained by Chávez et al. (2018), who observed an interaction of the varieties with different isolates of P. oryzae. This interaction of genotypes with different isolates extends to a greater number of isolates under study (data not reported here).

Figure 2 The average disease progress at 8, 15, 22 and 30 DAI in eight selected synthetic hexaploid wheat genotypes. Caacupé, 2018.
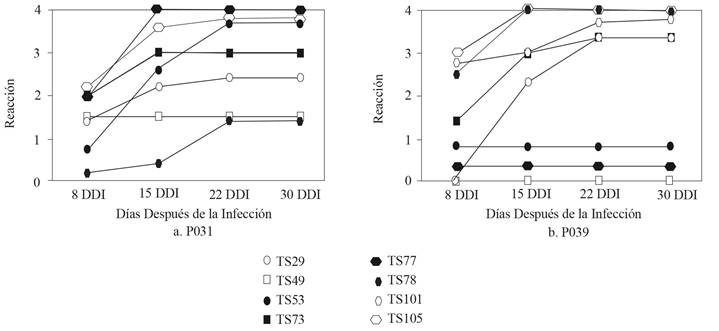
Figure 3 Evolution of the reaction to the infection and its development against the isolate P14YTae031 (P031) (a) and P14ATae039 (P039) (b) in eight synthetic hexaploid wheats at 8, 15, 22 and 30 DAI. Caacupé, 2018.
In order to study the progress of the disease in the preselected wheats, the area under the disease progress curve was calculated for both isolates, Table 3.
If we analyze the data presented in Table 3, on average, and considering the wheat by isolate interaction, the progress of the disease for both isolates is significantly different. In a similar study on four isolates of P. oryzae,Lule et al. (2014) did not find significant differences in the AUDPC in rice when interacting with its host, but they did when comparing isolates.
The data of the area under the disease curve confirm the classification of the materials based on the maximum value of infection, without considering the materials with differential reactions. Only synthetic wheats TS29 and TS49 changed their classification based on the maximum value of infection and area under the disease curve.
The TS29, classified as moderately resistant due to its maximum value of infection, was considered moderately susceptible, since it presented a greater progress of the disease after the evaluation of 15 DAI.
The situation with the TS49 was the complete opposite of the reaction of the TS29, since it had a much slower development to reach a lower AUDPC. This selection strategy using AUDPC for this pathogen was also used by Prabhu et al. (2003) to classify the reactions of six elite lines of rice in a breeding program. Jeger and Viljanen-Rollinson (2001) agree that the progress of the disease is a more accurate and precise estimate for the selection of materials for a breeding program or genetic studies, helping identify the most stable materials in time.
Considering the genetic variability within the different collections of Ae tauschii crossed to obtain the SHWs, this type of reactions is to be expected. Similar results were reported by Guz Kazi et al., 2012 and Masood et al. (2016) in their studies on the reaction to diseases as well as in agronomical traits and in quality in general.
Table 3. Area under the disease progress curve in eight synthetic hexaploid wheats. Caacupé, 2018.
| Trigos Sintéticos Hexaploidesx | Área bajo la Curva del Progreso de la Enfermedad (ABCPE)y | |
|---|---|---|
| P14YTae031 | P14ATae039 | |
| TS49 | 33ab | 0 a |
| TS77 | 81c | 7.3 a |
| TS53 | 70.3 c | 16.5 a |
| TS29 | 59.9 abc | 54.7 b |
| TS73 | 66.7 bc | 64 bc |
| TS78 | 63.2 abc | 73.8 bc |
| TS101 | 32.7 a | 82.8 bc |
| TS105 | 75.1 c | 84.5 c |
| Promedio por aisladoz | 60.3 a | 47.9 b |
xTest: LSD Fisher Alfa=0,05. Medias con una letra común no son significativamente diferentes (p>0,05) / Test: LSD Fisher Alfa=0,05. Averages with a common letter are not significantly different (p > 0,05).
yValor promedio de ABCPE por TSHs / Average value of AUDPC by SHWs.
zValor promedio de ABCPE con interacción por aislados / Average value of AUDPC with interaction by isolations.
In a study related to the complete wheat genome, Jighly et al., (2016) mapped regions known for the resistance to multiple fungal pathogens in a collection of SHWs. Aside from identifying the presence of combinations previously reported in bread wheats, they were able to observe interactions for various diseases individually. Their assertion that the use of markers for the introduction of multiple resistances to diseases from SHWs can be an important source for the creation of elite varieties is very valid for the case in question, where sources of resistance are very limited.
It is worth mentioning that no molecular markers have yet been identified for the resistance to P. oryzae that validate their presence in the SHWs. Although TS29 and TS49 can be considered parents for the breeding programs and can be combined with each other, genotypes such as TS49 are the most valuable to provide a higher level of resistance and low progress of the disease until the end of the infection.
Conclusion
Taking advantage of the wide variability that exists in the hexaploid synthetic wheats, new sources of resistance to wheat blast, from different genetic origins were identified. These genetic resources represent a move forward in the search for more variability for resistance to P. oryzae in wheat, which is currently very narrow and may serve as a source to transfer its resistance to future varieties worldwide.
Acknowledgements
To the International Maize and Wheat Improvement Center, CIMMYT, Mexico, for providing the collection of hexaploid synthetic wheats, and to the Paraguayan Agricultural Technology Institute, for their facilities in the Hernando Bertoni Research Center, Caacupé, Paraguay.
REFERENCES
Chávez, A., Cazal, C. and Kohli, M. M. 2017. Difference in the reaction to Pyricularia oryzae of wheat materials in the vegetative and reproductive stages. Investigación Agraria, 19(1), 56-63. https://doi.org/10.18004/investig.agrar.2017.junio.56-63 [ Links ]
Chávez, A. R. and Kohli, M. M. 2018. Patogenicidad de Magnaporthe oryzae en variedades y líneas de trigo cultivadas en Paraguay. Revista Mexicana de Fitopatología, 36(2), 1-11. https://doi.org/10.18781/R.MEX.FIT.1712-3 [ Links ]
Couch, B. C. and Kohn, L. M. 2002. A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia, 94(4), 683-693. https://doi.org/10.2307/3761719 [ Links ]
Cruz, C. D., Peterson, W., Bockus, W. W., Kankanala, P., Dubcovsky, J., Jordan, K. W., Akhunov, E., Chumley, F., Baldelomar, F., Valent, B. (2016). The 2NS Translocation from Aegilops ventricosa Confers Resistance to the Triticum Pathotype of Magnaporthe oryzae. Crop Science, 56(3), 990-1000. https://doi.org/10.2135/cropsci2015.07.0410 [ Links ]
Cruz, C. D., and Valent, B. (2017). Wheat blast disease: danger on the move. Tropical Plant Pathology, 42(3), 210-222. https://doi.org/10.1007/s40858-017-0159-z [ Links ]
Das, M. K., Bai, G., Mujeeb-Kazi, A. and Rajaram, S. 2015. Genetic diversity among synthetic hexaploid wheat accessions (Triticum aestivum) with resistance to several fungal diseases. Genetic Resources and Crop Evolution, 63(8), 1285-1296. https://doi.org/10.1007/s10722-015-0312-9 [ Links ]
Gladieux, P., Condon, B., Ravel, S., Soanes, D., Maciel, J. L. N., Nhani, A., Chen, Li., Terauchi, R., Lebrun, M., Tharreau, D., Mitchell, T., Pedley, K., Valent, B., Talbot, N., Farman, M.,Fournier, E. 2018. Gene Flow between Divergent Cereal- and Grass-Specific Lineages of the Rice Blast Fungus Magnaporthe oryzae. American Society for microbiology, 9(1), e01219-17. https://doi.org/10.1128/mBio.01219-17 [ Links ]
Gul Kazi, A., Awais, R., Tariq, M. and Mujeeb-Kazi, A. 2012. Molecular and morphological diversity with biotic stress resistances of high 1000-grain weight synthetic hexaploid wheats. Pakistan Journal of Botany, 44(3), 1021-1028. Retrieved from file:///C:/Users/user/Downloads/Gul-Kazietal2012PJB.pdf [ Links ]
Jeger, M. J. and Viljanen-Rollinson, S. L. H. 2001. The use of the area under the disease-progress curve (AUDPC) to assess quantitative disease resistance in crop cultivars. Theoretical and Applied Genetics, 102(1), 32-40. https://doi.org/10.1007/s001220051615 [ Links ]
Jighly, A., Alagu, M., Makdis, F., Singh, M., Singh, S., Emebiri, L. C., & Ogbonnaya, F. C. 2016. Genomic regions conferring resistance to multiple fungal pathogens in synthetic hexaploid wheat. Molecular Breeding, 36(9). https://doi.org/10.1007/s11032-016-0541-4 [ Links ]
Klaubauf, S., Tharreau, D., Fournier, E., Groenewald, J. Z., Crous, P. W., de Vries, R. P., Lebrun, M.-H. (2014). Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Studies in Mycology, 79, 85-120. https://doi.org/10.1016/j.simyco.2014.09.004 [ Links ]
Kohli, M., Mehta, Y. R., Guzman, E., de Viedma, L. and Cubilla, L. E. 2011. Pyricularia blast-a threat to wheat cultivation. Czech Journal of Genetics and Plant Breeding, 47(SPEC. ISSUE 1), 130-134. https://doi.org/DOI: 10.17221/3267-CJGPB [ Links ]
Lu, C. M., Yang, W. Y., Zhang, W. J. and Lu, B. R. 2005. Identification of SNPs and development of allelic specific PCR markers for high molecular weight glutenin subunit D tx1.5 from Aegilops tauschii through sequence characterization. Journal of Cereal Science, 41(1), 13-18. https://doi.org/10.1016/j.jcs.2004.05.006 [ Links ]
Lule, D., de Villiers, S., Fetene, M., Bogale, T., Alemu, T., Geremew, G., Gashaw, G., Tesfaye, K. 2014. Pathogenicity and yield loss assessment caused by Magnaporthe oryzae isolates in cultivated and wild relatives of finger millet (Eleusine coracana). Indian Journal of Agricultural Research, 48(4), 258-268. https://doi.org/10.5958/0976-058X.2014.00659.3 [ Links ]
Malaker, P. K., Barma, N. C. D., Tiwari, T. P., Collis, W. J., Duveiller, E., Singh, P. K., Joshi, A., Singh, R., Braun, H., Peterson, G., Pedley, K., Farman, M., Valent, B. 2016. First Report of Wheat Blast Caused by Magnaporthe oryzae Pathotype triticum in Bangladesh. Plant Disease, PDIS-05-16-0666-PDN. https://doi.org/10.1094/PDIS-05-16-0666-PDN [ Links ]
Marangoni, M. S., Nunes, M. P., Fonseca, J. N., & Mehta, Y. R. 2013. Pyricularia blast on white oats - a new threat to wheat cultivation. Tropical Plant Pathology, 38(3), 198-202. https://doi.org/10.1590/S1982-56762013005000004 [ Links ]
Masood, R., Ali, N., Jamil, M., Bibi, K., Rudd, J. C. and Mujeeb-Kazi, A. 2016. Novel genetic diversity of the alien D-genome synthetic hexaploid wheat (2n=6x=42, Aabbdd) germplasm for various phenology traits. Pakistan Journal of Botany, 48(5), 2017-2024. https://doi.org/10/2016; 48(5):2017-2024 [ Links ]
Ogbonnaya, F. C., Abdalla, O., Mujeeb-Kazi, A., Kazi, A. G., Xu, S. S., Gosman, N., Lagudah, Evans S., Bonnett, D., Sorrells, M., Tsujimoto, H. 2013. Synthetic hexaploids: Harnessing species of the primary gene pool for wheat improvement. Plant Breeding. Reviews 37 (First Edition). https://doi.org/10.1002/9781118497869.ch2 [ Links ]
Okamoto, Y., Nguyen, A. T., Yoshioka, M., Iehisa, J. C. M. and Takumi, S. 2013. Identification of quantitative trait loci controlling grain size and shape in the D genome of synthetic hexaploid wheat lines. Breeding Science, 63(4), 423-429. https://doi.org/10.1270/jsbbs.63.423 [ Links ]
Pieck, M. L., Ruck, A., Farman, M., Peterson, G. L., Stack, J. P., Valent, B., & Pedley, K. F. 2016. Genomics-Based Marker Discovery and Diagnostic Assay Development for Wheat Blast. Plant Disease, PDIS-04-16-0500-RE. https://doi.org/10.1094/PDIS-04-16-0500-RE [ Links ]
Prabhu, A. S., De Castro, E. D. M., De Araújo, L. G. and Berni, R. F. 2003. Resistance spectra of six elite breeding lines of upland rice to Pyricularia grisea. Pesquisa Agropecuaria Brasileira, 38(2), 203-210. https://doi.org/10.1590/S0100-204X2003000200006 [ Links ]
Rasheed, A., Xia, X., Ogbonnaya, F., Mahmood, T., Zhang, Z., Mujeeb-Kazi, A. and He, Z. 2014. Genome-wide association for grain morphology in synthetic hexaploid wheats using digital imaging analysis. BMC Plant Biology, 14(1), 128. https://doi.org/10.1186/1471-2229-14-128 [ Links ]
Sohail, Q., Inoue, T., Tanaka, H., Eltayeb, A. E., Matsuoka, Y. and Tsujimoto, H. 2011. Applicability of Aegilops tauschii drought tolerance traits to breeding of hexaploid wheat. Breeding Science, 61(4), 347-357. https://doi.org/10.1270/jsbbs.61.347 [ Links ]
Received: July 31, 2018; Accepted: October 19, 2018











 texto em
texto em 


