Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.36 n.3 Texcoco Oct./Dec. 2018
https://doi.org/10.18781/r.mex.fit.1803-2
Phytopathological notes
Evaluation of nanoformulations on in vitro development of fungal phytopathogens
1 CONACYT-Instituto Politécnico Nacional. Centro de Desarrollo de Productos Bióticos. Carretera Yautepec-Jojutla, Km. 6.8, San Isidro, CEPROBI 8, Yautepec, C.P. 62731, Morelos. México
2 Instituto Politécnico Nacional. Centro de Desarrollo de Productos Bióticos. Carretera Yautepec-Jojutla, Km 6.8, San Isidro, CEPROBI 8, Yautepec, C.P. 62731, Morelos. México
3 Instituto Tecnológico de Zacatepec. Calzada Tecnológico 27, Plan de Ayala, Zacatepec de Hidalgo, C.P. 62780, Morelos. México.
For controlling phytopathogenic microorganisms, there exist new proposals of nanomaterials including those from chitosan and essential oils, which may be applied alone or in coating nanoformulations. Six fungal isolates were subjected to different nanoparticles treatments in order to evaluate their effect on mycelial growth and spore germination. It was observed a total growth inhibition (100%) in most fungi when they were incubated on nutrient media amended with nanoparticles of chitosan loaded with thyme essential oil at 5%, applied either alone or in nanostructured coatings. As for the spore germination tests, a notable inhibition (50 % and 100%) was observed at 1% concentration of thyme essential oil depending on the isolate. When fungi were grown on individually treatments, germination was delayed by 6 h but completely stopped when incubated on coated nanoformulations. Compared to the lime essential oil, it was the thyme essential oil which provided a remarkable control of the tested fungi.
Key words: nanomaterials; chitosan; essential oils
En el control de microorganismos fitopatógenos, existen nuevas propuestas de nanomateriales que incluyen aquellas con quitosano y aceites esenciales, los cuales puedes aplicarse solo o en nanoformulaciones. Seis aislamientos fúngicos se sometieron a diferentes tratamientos para evaluar su efecto en el crecimiento micelial y germinación de las esporas. Se observó una inhibición total (100%) en la mayoría de los hongos cuando se incubaron en medio nutritivo adicionado con nanopartículas de quitosano con aceite esencial de tomillo al 5%; solamente o en cubiertas nanoestructuradas. Dependiendo del aislamiento, hubo una inhibición notable (50% y 100%) en la germinación con el aceite esencial de tomillo 1%. Cuando los hongos crecieron en los tratamientos individuales, la germinación se retrasó 6 h pero se inhibió completamente cuando se incubaron en las nanoformulaciones. Comparado con el aceite esencial de limón, fue el aceite esencial de tomillo el que proporcionó un control remarcable en los hongos evaluados.
Palabras clave: nanomateriales; quitosano; aceites esenciales
As antimicrobial agents, acting individually or combined, chitosan and essential oils (EO) have demonstrated their fungicidal performance against various postharvest fungi (Sivakumar and Bautista-Baños, 2014). As another alternative control measure, nanotechnology has opened new avenues through the use of nanoparticles that due to their large surface area relative to the mass, they can be highly reactive. According to Foladori (2009) the strength of nanotechnology lies mainly in making more efficient and multifunctional products, thereby saving raw materials. Chitosan nanoparticles individually or in combinations with other compounds, eventually can be integrated into coating formulations that may contain antimicrobial compounds such as EO (Sotelo et al., 2015; Sotelo-Boyás et al., 2017). In previous studies, Saharan et al. (2013) reported a better inhibitory effect by applying the chitosan nanoparticles at concentration of 0.1%, on Alternaria alternata, Macrophomina phaseolina and Rhizoctonia solani. Yien et al. (2012) also reported an inhibitory effect for the low and high molecular weight chitosan nanoparticles in a range of minimum concentrations from 0.86-1.2 mg mL-1 and 0.5-1.2 mg mL-1, respectively on Fusarium solani, while Beyki et al. (2014) conducted a study on the effect against Aspergillus flavus using an 800 ppm concentration of peppermint (Mentha piperita) essential oil encapsulated in chitosan nanogels with cinnamic acid. The aim of this research was to evaluate the fungal growth of various pathogenic fungi treated with chitosan nanoparticles, based chitosan-essential oil nanoparticles and chitosan-essential oil coating nanoformulations.
The studied isolates were A. alternata obtained from infected figs, Colletotrichum gloeosporioides and C. fragariae from fruit of papaya and soursop and custard apple, respectively, and Rhizopus stolonifer from papaya and strawberry. The lime and white thyme essential oils were supplied by Essential Oils-Essencefleur (therapeutic degree) and canola oil from a trademark. Chitosan (Medium molecular weight, deacetylation = 89%) was obtained from Sigma-Aldrich. Chitosan and lime and thyme essential oils nanoparticles, were synthetized as follows: chitosan solutions at concentrations of 0.05% (w/v) were dissolved in glacial acetic acid (1% v/v) (Fermont Chemicals Inc.) and distilled water. 2.5 mL of chitosan solution was added to lime and thyme at concentrations of 1, 3, 5 % (w/v) previously dissolved in methanol (40 mL) (Fermont Chemicals Inc.) by using a peristaltic pump (Bio-Rad, EP-1 Econo Pump) under moderate stirring. The obtained solution was placed in a rotary evaporator (Rotary Evaporator RE 300, BM 500 Water Bath, Yamato CF 300) at 40 °C and 50 rpm. The final volume of nanoparticles was 2 mL. The independent treatments were: chitosan nanoparticles at 0.05% (ChNp 0.05%), chitosan-lime essential oil nanoparticles at 1%, (Ch-LEO-Np 1%), 3% (Ch-LEO-Np 3%) and 5% Ch-LEO-Np 5%), chitosan-thyme essential oil nanoparticles at 1% (Ch-TEO-Np 1%), 3% (Ch-LEO-Np 3%) and 5% (Ch-TEO-Np 5%), and controls [control-Potato Dextrose Agar (C-PDA) and methanol (Meth) and canola oil at 0.1 % (CO 0.1 %)]. The nanostructured coatings were: 98.6% chitosan + 1% Ch-TEO-Np 5% (COAT1), 89.6% chitosan + 10% Ch-TEO-Np 5% (COAT2), 49.6% chitosan + 50% Ch-TEO-Np 5% (COAT3), 46.6% chitosan + 53% Ch-TEO-Np 5% (COAT4), 44.6% chitosan + 55% Ch-TEO-Np 5% (COAT5) and control-PDA (COAT6) (Correa-Pacheco et al., 2017). All formulations contained glycerol at 0.3% (J.T.Baker®) and canola oil at 0.1%. Treatments (0.5 ml) were uniformly dispersed in the PDA culture medium. Disks of 5 mm in diameter of each strain (4-14 days old) were placed separately in the center of the Petri dishes containing the treatment and incubated at 20-25 ºC until fungi reached its maximum development (4-14 days). Radial mycelial growth of the fungi was measured (cm) in 5 Petri dishes (8 cm of diameter) per treatment at the end of incubation period, with a Truper vernier caliper, which was 14 days for A. alternata, 10 for all Colletotrichum species and four days for R. stolonifer. The results were expressed as percentage inhibition of mycelial growth. An additional experiment was carried out to evaluate the germination process during a given 0, 6, 8 and 10 h incubation periods. For this, spore aliquots of 30 ml of a 105 concentration were placed onto 6 PDA discs containing the individual treatment or the coating formulation. Germination was stopped by adding lactophenol-safranin. Observations were carried out using a Nikon ALPHAPHOT-2YS2-H optical microscope with a 40X objective. Data for each incubation period was expressed as percentage germination. Treatments were arranged in a completely randomized design. Mean and standard deviations were also calculated. Data of the final mycelial growth and germination were subjected to ANOVA and means comparison by Tukey test at p ≤ 0.05 at the end of the incubation period. For both variables, the square root transformation was carried out to fulfil the ANOVA assumptions of homogeneity and data normal distribution. The statistical software used was Sigmaplot v.13.
The final mycelial inhibition and germination showed significant differences (p≤0.05) among individual treatments and nanoformulations. Overall, the isolates response, showed growth differences and spore survival according to the treatment applied and isolate. In this study, in all tested fungi, mycelial growth was considerably affected by the application of chitosan-thyme essential oil nanoparticles at 1, 3 and 5 % (Ch-TEO-Np 1%, Ch-TEO-Np 3% and Ch-TEO-Np 5%). In addition, at the last two concentrations; 3% and 5%, inhibition reached 100% and therefore, there were no spore formation (Table 1). As for the tested nanostructured coatings, mycelial growth inhibition in all isolates was evident in those where the percentage of nanoparticles of chitosan-thyme essential oil 5%, increased by 50, 53 and 55% (COAT3, COAT4 and COAT5). Overall as the thyme essential oil increased, the fungal inhibition increased, being the most sensitive those treated with COAT3 on C. fragariae, followed by both isolates of R. stolonifer. (Table 2). For all isolates, it was observed that the number of germinated spores increased as the incubation time increased. With respect to the untreated isolates, there was not a defined pattern with the remaining treatments. However, in C. fragariae the process of germination was able to be delayed until the 6th h of incubation in most treatments (Figure 1). For C. gloeosporioides (soursop) and R. stolonifer (papaya) most treatments promoted their germination after 6 h of incubation. As for the nanostructured coatings, at the highest percentage of nanoparticles (55%), a total inhibition was obtained in the isolates A. alternata, both C. gloeosporioides and R. stolonifer and C. fragariae during the whole 10 h incubation period (Figure 2).
Table 1 Summary of the effect of chitosan nanoparticles and chitosan-essential oils nanoparticles on mycelial inhibition at the end of the incubation period.
| Individual treatments |
Mycelial inhibition (%) | |||||
|
A. alternata (fig) |
C. gloeosporioides (papaya) |
C. gloeosporioides (soursop) |
C. fragariae (custard apple) |
R. stolonifer (papaya) |
R. stolonifer (strawberry) |
|
| ChNp 0.05% | 0a* | 0a* | 0a* | 1.6a* | 0a* | 0a* |
| Ch-LEO-Np 1% | 0a | 24.5c | 0a | 1.6a | 0a | 0a |
| Ch-LEO-Np 3% | 0a | 28.1c | 0a | 3.4b | 0a | 0a |
| Ch-LEO-Np 5% | 2.4a | 50.0d | 10.8b | 6.5b | 33.5b | 2.4a |
| Ch-TEO-Np 1% | 40.3b | 45.3d | 54.4c | 52.7c | 74.0c | 71.7c |
| Ch-TEO-Np 3% | 100c | 100e | 100d | 100d | 100d | 100d |
| Ch-TEO-Np 5% | 100c | 100e | 100d | 100d | 100d | 100d |
| C-PDA | 0a | 0a | 0a | 0a | 0a | 0a |
| Meth | 0a | 5.2b | 1.7a | 0a | 0a | 0a |
| CO 0.1% | 0a | 35.6cd | 0a | 4.7b | 0a | 0a |
*Means followed by the same letter are not significant different (p ≤ 0.05) determined by Tukey’s multiple test. p values after square root transformation.
Treatment labels: Chitosan nanoparticles 0.05% (ChNp 0.05%); Chitosan-lime essential oil nanoparticles 1%, (Ch-LEO-Np 1%); 3 % (Ch-LEO-Np 3%), 5% (Ch-LEO-Np 5%); Chitosan-thyme essential oil nanoparticles 1% (Ch-TEO-Np 1 %), 3% (Ch-TEO-Np 3 %), 5 % (Ch-TEO-Np 5%); Control-Potato-Dextrose-Agar (C-PDA); methanol (Meth) and canola oil 0.1% (CO 0.1%).
Table 2 Summary of the effect of coating nanoformulations based on chitosan-essential oils on mycelial inhibition at the end of the incubation period
| Treatments | Mycelial inhibition (%) | |||||
|
A. alternata (fig) |
C. gloeosporioides (papaya) |
C. gloeosporioides (soursop) |
C. fragariae (custard apple) |
R. stolonifer (papaya) |
R. stolonifer (strawberry) |
|
| COAT1 | 0a* | 0a* | 0a* | 0a* | 0a* | 0a* |
| COAT2 | 0a | 0a | 0a | 0a | 0a | 0a |
| COAT3 | 19.2b | 17.7b | 30.0b | 85.0b | 72.5b | 62.0b |
| COAT4 | 70.2c | 65.0c | 92.0c | 100c | 100c | 62.4b |
| COAT5 | 100d | 100d | 100d | 100c | 100c | 100c |
| COAT6 | 0a | 0a | 0a | 0a | 0a | 0a |
*Means followed by the same letter are not significant different (p ≤ 0.05) determined by Tukey’s multiple test. p values after square root transform.
Treatment labels: 98.6% chitosan + 1% Ch-TEO-Np 5% (COAT1); 89.6% chitosan + 10% Ch-TEO-Np 5% (COAT2); 49.6% chitosan + 50% Ch-TEO-Np 5% (COAT3); 46.6% chitosan + 53% Ch-TEO-Np 5% (COAT4); 44.6% chitosan + 55% Ch-TEO-Np 5% (COAT5) and control (COAT6).

Figure 1 Germination process of conidial fungi during a 10 h incubation period of a) A. alternata (fig), b) C. gloeosporioides (papaya), c) C. gloeosporioides (soursop), d) C. fragariae (custard apple), e) R. stolonifer (papaya) and f) R. stolonifera (strawberry) and subjected to the following treatments: chitosan nanoparticles at 0.05% (Ch-NPs 0.05%), chitosan-lime essential oil nanoparticles at 1% (Ch-LEO-NPs 1%), 3% (Ch-LEO-NPs 3%) and 5% (Ch-LEO-NPs 5%), chitosanthyme essential oil nanoparticles at 1% (Ch-TEO-NPs 1%), 3% (Ch-TEO-NPs 3%) and 5% (Ch-TEO-NPs 5%), PDA, methanol (Meth) and canola oil at 0.1% (CO 0.1%).
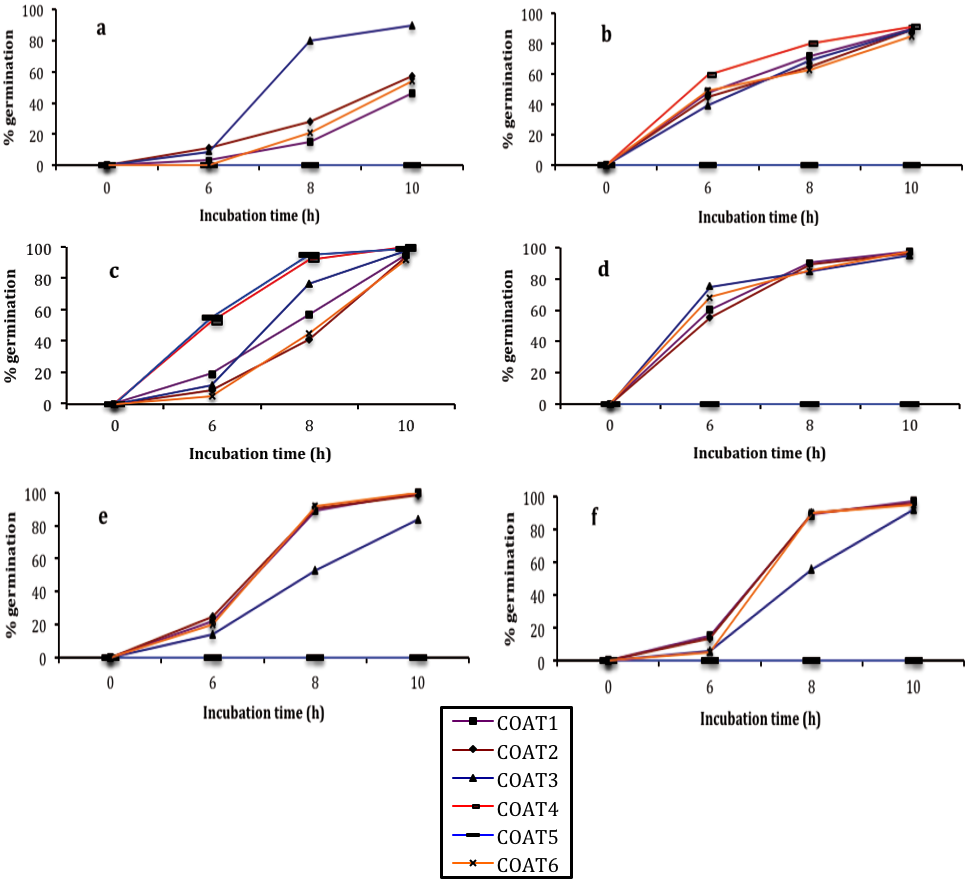
Figure 2 Germination process of conidial fungi during a 10 h incubation period of a) A. alternata (fig), b) C. gloeosporioides (papaya), c) C. gloeosporioides (soursop), d) C. fragariae (custard apple), e) R. stolonifer (papaya) and f) R. stolonifer (strawberry) and subjected to different coating nanoformulations. Vertical bars indicate mean standard deviation
In general, the results of our research coincide with those previously published by Zahid et al. (2013) and Correa-Pacheco et al. (2017) in which the effective control of C. gloeosporioides isolated from dragon fruit (Hylocereus undatus) and avocado (Persea americana) and treated with chitosan nanoemulsions resulted from the low molecular weight chitosan 1%, and nanostructured formulations chitosan-thyme essential oil 1-5 %, respectively, in terms of inhibition of mycelia and conidial germination of the fungus compared to the untreated control. In other research carried out by Khalili et al. (2015) it was demonstrated a better efficacy of thyme EO when encapsulated in chitosan and benzoic acid - made nanogels, in comparison with free thyme EO, against A. flavus. In those studies, the best fungicidal concentration of 300 mg L-1 under sealed conditions was enough to inhibit significantly the number of fungal colonies, while at 700 mg L-1, the shelf life of the treated tomatoes was extended up to one month. In this research, the major fungicidal effect seems to be mostly from the thyme essential oil and its concentration, results that agree with various other researchers. For example, Barrera-Necha et al. (2009) and Sellamuthu et al. (2013) stated the antifungal activity of thyme EO in vitro and on avocado fruit against C. gloeosporioides. In other reports, a noticeable fungicidal effect was also reported on A. flavus, A. alternata and F. oxyspsorum with low concentrations (1.0-8-0 µg mL-1) from two different species of the genus Thymus: T. kotschyanus and T. daenensis (Mohammadi et al., 2014) while the minimal concentration of 62.5 µg mL-1 was exhibited on A. flavus to avoid spore germination with this same EO species (Pekmezovik et al., 2015). The above finding results clearly state the traditional results of the thyme EO either alone or incorporated with other substances; however, a problem for most EO and their respective major compounds is their high volatility when applied alone. As stated by Correa-Pacheco et al. (2017) ‘to overcome the volatility aspect of EO, the incorporation of nanomaterials and antimicrobials, including EO into edible coatings will give new properties to the nanostructured coating, in addition, their synergistic effects can improve’.
The development of new fungi-control treatments with environmentally friendly compounds by employing nanotechnology can offer effective and more reliable means for controlling microorganism. In this research chitosan-thyme EO nanoparticles at the highest concentrations (5%), tested either independently or integrated in a formulation (50, 53 and 55 %) gave a notable in vitro growth control on the evaluated fungi, but it is necessary to study their effects of in situ control assessments.
LITERATURA CITADA
Barrera-Necha LL, Garduño-Pizaña G and García-Barrera J. 2009. In vitro antifungal activity of essential oils and their compounds on mycelial growth of Fusarium oxisporum f. sp. gladioli (Massey) Snyder and Hensen. Plant Pathology Journal 8:17-21. Disponible en línea: http://www.docsdrive.com/pdfs/ansinet/ppj/2009/17-21.pdf [ Links ]
Beyki M, Zhaveh S, Khalili TS, Rahmani-Cherati T, Abollahi A, Mansour B, Bayat Tabatabaei M and Mohsenifarc A. 2014. Encapsulation of Mentha piperita essential oils in chitosan-cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Industrial Crops and Products 54:310-319. https://doi.org/10.1016/j.indcrop.2014.01.033 [ Links ]
Correa-Pacheco ZN, Bautista-Baños S, Marquina-Valle MA and Hernández-López M. 2017. The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides growth in vitro and on cv Hass and fruit quality. Journal of Phytopathology 165:297-305. DOI: 10.1111/jph.12562 [ Links ]
Foladori G. 2009. La gobernanza de las nanotecnologías. Sociológica 24:125-153. Disponible en línea: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0187-01732009000300006 [ Links ]
Khalili ST, Mohsenifar A, Beyki M , Zhaveh S . Rahmani-Cherati T , Abdollahi A, Bayat M and Tabatabaei M. (2015) Encapsulation of Thyme essential oils in chitosan-benzoic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. LWT-Food Science and Technology 60:502-508. https://doi.org/10.1016/j.lwt.2014.07.054 [ Links ]
Mohammadi A, Nazari H, Imani S and Amrollahi H. 2014. Antifungal activities and chemical composition of some medicinal plants Activite´s antifongiques et composition chimique de quelques plantes médicinales. Journal of Mycologie Médicale 24:e1-e8. http://dx.doi.org/10.1016/j.mycmed.2014.02.006 [ Links ]
Pekmezovic M, Rajkovic K, Barac A, Senerovic´ L and Arsic Arsenijevic V. 2015. Development of kinetic model for testing antifungal effect of Thymus vulgaris L. and Cinnamomum cassia L. essential oils on Aspergillus flavus spores and application for optimization of synergistic effect. Biochemical Engineering Journal 99:131-137. https://doi.org/10.1016/j.bej.2015.03.024 [ Links ]
Saharan V1, Mehrotra A, Khatik R, Rawal P, Sharma SS and Pal A. 2013. Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. International Journal of Biological Macromolecules 62:677-683. https://doi.org/10.1016/j.ijbiomac.2013.10.012 [ Links ]
Sellamuthu P, Sivakumar D and Soundy P. 2013. Antifungal activity and chemical composition of thyme, peppermint and citronella oils in vapor phase against avocado and peach postharvest pathogens. Journal of Food Safety 33:86-93. DOI: 10.1111/jfs.12026 [ Links ]
Sivakumar D and Bautista-Baños S . 2014. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Protection 64:27-37. http://dx.doi.org/10.1016/j.cropro.2014.05.012 [ Links ]
Sotelo BME, Bautista BS, Aldana LL, Solorza FJ, Jiménez AA, Barrera NLL, Valverde AG y Plascencia JM. 2015. La nanotecnología en el control de microorganismos patógenos e insectos de importancia económica. In: C. Laréz VC, Koteich KS y López GF eds. Nanotecnología: fundamentos y aplicaciones. Comisión de Publicaciones del Departamento de Química de la Facultad de Ciencias, Universidad de los Andes, Caracas Venezuela 307-321. [ Links ]
Sotelo-Boyás M, Correa-Pacheco Z, Bautista-Baños S , Gómez and Gómez Y. 2017. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. International Journal of Biological Macromolecules 103:409-414. http://dx.doi.org/10.1016/j.ijbiomac.2017.05.063 [ Links ]
Yien L, Mohammad N, Sarwar A and Katas H. 2012. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. International Journal of Biomaterials 2012:1-9. http://dx.doi.org/10.1155/2012/632698 [ Links ]
Zahid N, Alderson P, Ali A, Maqbool M and Manickam S. 2013. In vitro control of Colletotrichum gloeosporioides by using chitosan loaded nanoemulsions. Acta Horticulturae 1012:769-774. DOI: 10.17660/ActaHortic.2013.1012.104 [ Links ]
Received: March 13, 2018; Accepted: August 19, 2018











 text in
text in 


