Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.36 no.3 Texcoco Out./Dez. 2018
https://doi.org/10.18781/r.mex.fit.1805-3
Phytopathological notes
Antifungal property of honey on in vitro development of Colletotrichum gloeosporioides
1 Instituto de Biociencias, Universidad Autónoma de Chiapas. Boulevard Príncipe Akishino s/n. Colonia Solidaridad 2000. Tapachula Chiapas, CP. 30798, México.
In addition to the high toxicity of chemicals for phytosanitary control of fungal diseases in field conditions, the microorganism causing disease is not completely eliminated. Therefore, products of natural origin, both plant and animal, have generated interest for the control of pests and diseases in plants. Thus this study aimed to evaluate the antifungal activity of honey in the in vitro growth of Colletotrichum gloeosporioides. Nine samples of honeys were evaluated, corresponding to three bees species: Melipona solani, M. beecheii and Scaptotrigona mexicana. We registered a decreased colony diameter of C. gloeosporioides at higher concentration of honey, with lower 40% growth of the colony compared to the control treatment within 12 days of incubation. In general, growth rate of the fungus colony in the three types of bee honey was in average, 40% lower than the control treatment. Inhibition rate value observed in bee honeys is 70% higher than that obtained with the fungicide Chlorothalonil®.
Key words: mycelial growth; Melipona solani; Melipona beecheii; Scaptotrigona mexicana; Clorotalonil®
Aunado a la alta toxicidad de los productos químicos para el control fitosanitario de las enfermedades fúngicas a nivel de campo, el microorgansimso causante de enfermedad no es completamente eliminado, por lo que, productos de origen natural tanto vegetal como animal han generado interés para el control de plagas y enfermedades en las plantas. El presente trabajo tuvo como objetivo principal evaluar la actividad antifúngica de mieles de abeja en el crecimiento in vitro de Colletotrichum gloeosporioides. Se evaluaron nueve muestras de mieles, correspondientes a tres especies de abejas: Melipona solani, M. beecheii y Scaptotrigona mexicana. Se encontró que el diámetro de la colonia de C. gloeosporioides fue menor a mayor concentración de miel, con un 40% menos crecimiento de la colonia respecto al tratamiento testigo a los 12 días de incubación. En general la tasa de crecimiento de la colonia del hongo en las mieles correspondientes a los tres tipos de abeja fue en promedio de 40% menos que el testigo. El valor de porcentaje de inhibición observada en las mieles de abeja fue 70% mayor a lo obtenido con el fungicida Clorotalonil.
Palabras clave: crecimiento micelial; Melipona solani; Melipona beecheii; Scaptotrigona mexicana; Clorotalonil®
In this century, products used in the control of plant health do not counteract disease development, since they do not eradicate the problem due to the low efficiency of penetration into the cuticle, the activity of the active principle and the accumulation in plant tissue (Campa et al., 2017; Pérez et al., 2017). Therefore, the development of alternative products with antifungal activity and of a microbial, plant or animal nature are currently an option and a reality (Correa et al., 2015; Ramírez et al., 2016). Recent studies have identified honey as an alternative in the control of fungi in vitro, since it has a natural origin, derived from the process of transformation of nectar by bees (Apis mellifera).
Honey presents a variety of neutracetical properties (Ramalivhana et al., 2014) and has also been used as a medicinal alternative for centuries (Vallianou et al., 2014). Recent studies have found a relation between the anti-microbial properties and the concentration and composition of honey (Fangio et al., 2007; Pimentel et al., 2013). Its anti-fungal action has been observed against the fungal genera of Trichophyton, Microsphorum, Aspergillus, Penicillium and Candida (Moussa et al., 2012; Londoño-Orozco et al, 2008; Montenegro et al., 2009; Olaitan et al., 2007). Thus the aim of the present study was to evaluate the anti-fungal activity of honey in the in vitro growth of Colletotrichum gloeosporioides.
Honey samples
Honey samples were collected during February and March 2016, in meliponaries from the “Asociación de Meliponicultores del Soconusco S. C. del R. L.” in the municipal areas of the Soconusco, Chiapas: Tapachula, Tuxtla-Chico and Cacahoatán.
Nine honey samples were used from three bee species: Melipona solani (n=3, from Trinidad (MsTa), San Jerónimo (MsSjb) and Izapa (Mslc)), M. beecheii (n=2, from Tapachula (MbTa) and (MbTb) and Scaptotrigona mexicana (n=4, from Cacahoatán (Smca), Francisco y Madero (SmFMb), Izapa I (Smic), Izapa II (Smid)). The honey was collected from three different boxes in the meliponaries in order to obtain a mixed honey sample per site, which was taken using sterile 5 ml syringed, and it was stored in properly labelled jars. They were stored at -4 °C until their analysis.
Anti-fungal activity of honey
The anti-fungal activity of honey was evaluated by antagonizing it in the laboratory with a C. gloeosporioides strain from the collection of the Bioscience Institute of the Autonomous University of Chiapas. The strain was reactivated in a potato-dextrose-agar (PDA) medium without honey and a pH of 6.0. The biotrial was carried out with a PDA medium mixed with a honey solution, and for this, we prepared dishes with a volume of solution (in concentrations of 25, 50 and 100 % honey) of 100 µl for every 20 mL of medium. After the strain inoculation (0.5 cm culture discs), the Petri dishes were incubated at 32 °C for 12 days.
Mycelial growth was measured every third day using a Vernier (Stainless Hardebed®) with a capacity of 0-150 mm (minus the size of the inoculant disc).
The growth rate of every culture was determined with the following equation:
where µ was growth rate of the culture, Db was the diameter of the culture (mm) in time “b”, Da was the diameter of the culture in time “a”; and “tb” and “ta” were the time in which the evaluation was carried out.
Determination of anti-fungal activity was queivalents comparable to Clorotalonil®
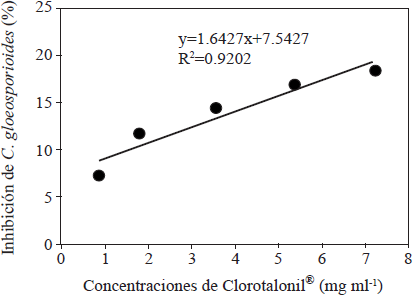
The anti-fungal activity of the honey was converted to equivalents of Chlorothalonil® (2, 4, 5, 6-tetrachloroisophthalonitrile), which is a chemical fungicide, commonly used in the control of C. gloeosporioides. For this, we determined the growth inhibition (%) of this fungus mediated by the Chlorothalonil® by prepating solutions with the concentrations 0, 0.9, 1.8, 3.6, 5.4 y 7.2 mg ml-1. Using the data obtained, we created a callibration curve (Figure 1).
Analysis of results
To analyze the diameter of the culture, its growth rate and inhibition, and equivalents of Chlorothalonil, an analysis of variance was carried out, followed by a mean comparison using Tukey’s test (p<0.05). The statistical program used was Infostat 2015. All treatments had nine repetitions.
In the treatments with honey, a relation was found between the honey concentration and the size reached by the fungal culture (r=-0.87, p<0.05)i.e., the diameter of the culture decreased as the concentration of honey increased. Starting on day 9 (Figure 2), in the concentrations of 25 and 50% of honey, significant differences were observed (p<0.05) in the diameter of the culture amongst the different types and concentration of honey applied. On average, the maximum diameters reached by the fungal cultures, regardless of the honey type, were 31.1, 20.4 and 3.2 mm for concentrations 25, 50 and 100%, respectively, unlike the control, which was 50 mm (Figure 2).

Figure 2 Growth kinetics of C. gloesporioides in different concentrations of honey (honey concentrations: 25%, 50% and 100%)
Typical growth rate (µ) was in the range of 0.020 to 0.023 h-1, with honey concentrations of 25 and 50%, unlike in the control, where it was 0.025 h-1. At a concentration of 100% of honey, the range was between 0.004 h-1 and 0.008 h-1. In general, the growth rate of the culture of C. gloeosporioides in honey was 40% less than in the control (Cuadro 1).
Table 1 Effect of the use of honey on the speed of growth and mycelial inhibition of C. gloeosporioides and its comparison with Clorotalonil® equivalents.
| Tratamiento | Concentración (%) |
Velocidad de crecimiento (h-1) |
Inhibición micelial (%) |
Equivalentes de Clorotalonil® (mg ml-1) |
| Testigo | 0 | 0.025 a* | 0 | 0 |
| SMCA | 25 | 0.023 bc | 40.4 i* | 29.2 f* |
| 50 | 0.020 e | 66.1 c | 44.8 b | |
| 100 | 0.006 h | 95.2 a** | 62.5 a** | |
| SMIC | 25 | 0.023 bc | 39.0 jkl | 28.3 f |
| 50 | 0.020 e | 63.6 d | 43.3 c | |
| 100 | 0.004 i | 95.2 a** | 62.5 a | |
| SMFMB | 25 | 0.023 bc | 38.5 kl | 28.0 f |
| 50 | 0.021 de** | 59.1 e | 40.5 d | |
| 100 | 0.007 g | 94.6 ab | 62.1 a** | |
| SMID | 25 | 0.023 bc | 39.0 ijkl | 28.3 f |
| 50 | 0.021 de** | 58.5 ef | 40.2 d | |
| 100 | 0.008 f | 94.4 ab | 62.1 a | |
| MSTA | 25 | 0.024 b | 39.8 ijk | 28.8 f |
| 50 | 0.021 de | 57.7 fg | 39.5 d | |
| 100 | 0.007 g | 94.0 ab | 61.8 a | |
| MSSJB | 25 | 0.023 bc | 40.0 ij | 28.9 f |
| 50 | 0.020 e | 63.4 d | 43.2 c | |
| 100 | 0.007 g | 94.6 ab | 62.2 a | |
| MBTB | 25 | 0.024 b | 38.1 l | 27.8 f |
| 50 | 0.021 de | 58.3 ef | 40.1 d | |
| 100 | 0.008 f | 94.7 ab | 62.2 a | |
| MSLC | 25 | 0.024 b | 34.8 ll | 25.8 g |
| 50 | 0.022 cd | 56.5 g | 38.9 d | |
| 100 | 0.007 g | 93.8 b | 61.7 a | |
| MBTA | 25 | 0.024 b | 33.1 m | 24.7 g |
| 50 | 0.022 cd | 54.3 h | 37.6 e | |
| 100 | 0.007 g | 93.5 b | 61.5 a |
*p≤ 0.05.
** Same letters indicate no difference between averages
For inhibition activity of C. gloeosporioides, highly significant differences were found (p<0.0001) between the honey from different bees, and it was also found that regardless of the concentration of honey, those coming from S. mexicana (Smca and Smic) inhibited the development of the C. gloeosporioides by 5.2%, while in honey from M. solani (Mssjb and Msta), M. becheii (Mbtb) and from S. mexicana (Smid and Smfmb) inhibition action was between 94% and 95%. The honey with the lowest inhibition values were M. solani (Mslc) and M. becheii (Mbta), with valued of 93.87 and 93.53%, respectively. However, inhibition was still high.
Finally the anti-fungal activity of the related honey in equivalents of Chlorothalonil® (eC) was also related to the concentration of honey (r=0.98, p<0.05). The highest concentration related to eC with inhibition action on C. gloeosporioides was displayed by honey from S. mexicana (Smca and Smic) with values of 62.5 mg ml-1, 72.7 mg ml-1 y 62.5 mg ml-1, 72.6 mg ml-1, respectively.
The time taken for the growth of the C. gloeosporioides culture without honey to reach the inner diameter of the Petri dish was 12 days (Figure 2). Unlike the treatments with honey, the growth of the control displayed a sigmoidal shape.
The reduction in mycelial growth of the fungus could be related to the bioactive components of honey, since similar results were reported in the development of Aspergillus spp and Penicillium spp fungal cultures when incubated in the presence of Apis honey (Olaitan et al., 2007). Lira (2003) and Jasso et al. (2007, 2011) coincided in that the inhibiting effect on the growth of the mycelium of pathogenic fungi for plants lies in the bioactive compounds of the biological products used, as in the case of plants.
Scaptotrigona or Melipona honey showed potential for use as a product with antifungal action when using a concentration of 500 µL of honey for every 100 mL of solution, reaching a level of inhibition of 95.0%, in comparison with commercial products that can be applied across a wide range of concentrations that range from 0.5 mg kg-1 to 500 mg kg-1 (Rodríguez et al., 2008; Warnke et al., 2009).
The anti-fungal action observed on C. gloeosporioides growth in the presence of honey at different concentrations could be due to the concentration of phenols, flavonoids and pH value, which have been reported by Marín et al. (2017). The structural similarity of these compounds found in the honey with that shown by the commercial chemical molecules used for the same purpose (Pérez-Cárdenas et al., 2013; Gregorí, 2005) suggests that the antifungal mechanism observed on the honey studied in this investigation could be associated with the alteration of cell division, the permeability of cell membranes and the intracellular transportation in the fungal mycelium.
Scaptotrigona and Melipona honey showed a potential for the control of the pathogenic fungus C. gloeosporioides and also reduces the mycelial growth rate by 95%, which makes it 40% more efficient than the commercial fungicide Clorotalonil®. The use of honey in the control of plant pathogenic fungi could be a solution alternative within a comprehensive management.
LITERATURA CITADA
Campa SP, Vallejo CS, Corrales MC, Martínez TMA y Vargas AI. 2017. Reducción en la incidencia de la pudrición gris en uva de mesa por el efecto de volátiles de un extracto de ajo. Revista Mexicana de Fitopatología 35:493-508. http://dx.doi.org/10.18781/r.mex.fit.1707-1 [ Links ]
Correa NYM, Palomino GLR y Marino MO. 2015. Actividad antioxidante y antifúngica de piperaceaes de la flora colombiana. Revista Cubana de Plantas Medicinales 20(2). Disponible en línea: http://www.revplantasmedicinales.sld.cu/index.php/pla/article/view/125/122 [ Links ]
Fangio M, Jurlina M and Fritz R. 2007. Antimicrobial activity of honey of south east province of Buenos Aires in front of Escherichia coli. Revista Argentina de Microbiología 39:120-123. [ Links ]
Gregori VBS y Gregori V. 2005. Estructura y actividad de los antifúngicos. Revista Cubana de Farmacia 39:15. Disponible en línea: https://www.researchgate.net/publication/237767583_Estructura_y_actividad_de_los_antifungicos [ Links ]
Jasso RD, Hernández CD, Angulo SJL, Rodríguez GR, Villareal QJA and Lira SRH. 2007. Antifungal activity in vitro of Fluorensia spp. extracts on Alternaria sp., Rhizoctonia solani and Fusarium oxysporum. Industrial Crops and Products 25:111-116. https://doi.org/10.1016/j.indcrop.2006.08.007 [ Links ]
Jasso RD , Rodríguez GR , Hernández CD , Aguilar GCN, Saénz GA, Villareal QJA and Moreno ZLE. 2011. in vitro antifungal activity of extracts of Mexican Chihuahua desert plants against postharvest fruit fungi. Industrial Crops and Products 34:960-966. https://doi.org/10.1016/j.indcrop.2011.03.001 [ Links ]
Lira SRH . 2003. Estado actual del conocimiento sobre las propiedades biocidas de la gobernadora (Larrea tridentata (D. C.) Coville). Revista Mexicana de Fitopatología 21:214-222. Disponible en línea: http://www.redalyc.org/articulo.oa?id=61221217 [ Links ]
Londoño OA, Penieres CJG, García TCG, Carrillo ML, Quintero MML, García VSE, Mendoza SMA y Cruz STA. 2008. Estudio de la actividad antifúngica de un extracto de propóleo de la abeja Apis mellifera proveniente del Estado de México. Tecnología en Marcha 21:49-55. Disponible en línea: https://www.researchgate.net/publication/267271152_Estudio_de_la_actividad_antifungica_de_un_extracto_de_propoleo_de_la_abeja_Apis_mellifera_proveniente_del_estado_de_Mexico [ Links ]
Marín SI, Torres de SR, Grajales CJ, Adriano AML y Albores FV. 2016. Actividad antimicrobiana de mieles de abeja sin aguijón en la region Soconusco, Chiapas, Mexico. Pp:741-743. In: Reyes de LBO, Pérez AEJ, Arévalo DMA (eds.). Congreso Mesoamericano de Investigación UNACH. Chiapas, México. 653p. [ Links ]
Montenegro G, Salas F, Peña RC y Pizarro R. 2009. Actividad antibacteriana y antifúngica de mieles monoflorales de Quillaja saponaria, especie endemica de Chile. Phyton. 78:141-146. Disponible en línea: http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1851-56572009000200010 [ Links ]
Moussa A, Noureddine D, Saad A, Abdelmelek A and Abdelkader B. 2012. Antifungal activity of four honeys of different types from Algerian honeys against pathogenic yeasts: Candida albicans and Rhodotorula sp. Asian Pacific Journal of Tropical Biomedicine 2:554-557. DOI: 10.1016/S2221-1691(12)60096-3 [ Links ]
Olaitan P, Adeleke O and Ola I. 2007. Honey: a reservoir for microorganisms and an inhibitory agent for microbes. African Health Science 3:159-165. Disponible en línea: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2269714/ [ Links ]
Pérez VL, Santana Y, García O, Lovaina Y, Pérez MM, Rodríguez JA y Avila R. 2017. Eficacia de fungicidas antioomycetes en la desinfección de hijos de piña MD2 para el control de Phytophthora nicotianae var. parasitica Dastur. Revista de Protección Vegetal 32:1-13. Disponible en línea: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1010-27522017000200007&lng=es&nrm=iso [ Links ]
Pérez CJE, Hoyos ZAM y Cárdenas HC. 2013. Sensibilidad antimicótica de diferentes especies de hongos aislados de pacientes con micosis ungueal en la ciudad de Manizales (Caldas, Colombia). Biosalud 11:26-39. Disponible en línea: http://www.scielo.org.co/pdf/biosa/v11n2/v11n2a04.pdf [ Links ]
Pimentel R, Da Costa C, Albuquerque M and Duvoisin J. 2013. Antimicrobial activity and rutin identification of honey produced by the stingless bee Melipona compressipes manaosensis and commercial honey. BMC Complementary and Alternative Medicine 13:1-13. DOI: 10.1186/1472-6882-13-151 [ Links ]
Ramalivhana JN, Obi CL, Samie A, Iweriebor BC, Uaboi EP, Idiaghe JE and Momba MNB. 2014. Antibactereial activity of honey and medicinal plant extracts against gram negative microorganisms. African Journal Biotechnology 13:616- 625. DOI: 10.5897/AJB11.892 [ Links ]
Ramírez GSI, López BO, Espinoza ZS y Wong VA. 2016. Actividad antifúngica de hidrodestilados y aceites sobre Alternaria solani, Fusarium oxyspurum y Colletotrichum gloeosporioides. Revista Mexicana de Ciencias Agrícolas 7:1879-1891. Disponible en línea: http://cienciasagricolas.inifap.gob.mx/editorial/index.php/agricolas/article/view/99/92 [ Links ]
Rodríguez TJL, Alcázar FL, Cuesta I, Alastruey IA, Monzón A and Mellado E. 2008. Clinical relevance of resistance of antifungal. International Journal of Antimicrobial Agents. 32:111-113. DOI: 10.1016/S0924-8579(08)70010-4 [ Links ]
Vallianou G, Gounari P, Skourtis A, Panagos J and Kazazis C. 2014. Honey and its anti-inflammatory, anti-bacterial and anti-oxidant properties. General Medicine 2:132. DOI: 10.4172/2327-5146.1000132 [ Links ]
Warnker HK, Becker TS, Podschun R, Sivananthan H, Springer N, Russo P, Wiltfang A, Fickenscher H and Sherry E. 2009. The battle against multi-resistant strains: renaissance of antimicrobial essential oils as promissing force to figth hospital-acquired infections. Journal of Cranio-Maxillofacial Surgery 37:392-397. DOI: 10.1016/j.jcms.2009.03.017 [ Links ]
Received: May 16, 2018; Accepted: July 30, 2018











 texto em
texto em