Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.36 no.3 Texcoco oct./dic. 2018
https://doi.org/10.18781/r.mex.fit.1806-2
Scientific articles
Induction of defense response in tomato plants against Forl by garlic extract
1 Centro de Investigación en Alimentación y Desarrollo, A.C. Carretera A la Victoria Km. 0.6, CP. 83304, Hermosillo, Sonora.
2 Instituto Tecnológico de Sonora, 5 de febrero 818 sur, Colonia Centro, CP. 85000 Ciudad Obregón Sonora.
A garlic extract (EA) was evaluated to induce the defense of tomato plants against Fusarium oxysporum radicis-lycopersici (Forl), by increasing the endogenous concentration of salicylic acid (AS) and jasmonic acid (AJ). Spraying 1 and 2% of EA on plants intentionally infected with 1x107 spores.mL-1 of Forl reduced in 73.53% the severity of the disease produced by Forl with respect to the infected control. The height was similar (p≤0.05) in plants treated with EA and untreated. The accumulation of AS in plants spayed with 1 and 2% of EA presented two significant increases (p≤0.05), in the second and fifth weeks after spraying, with higher concentrations than the infected and non-infected controls in 11 and 6 times for the first and 2.7 times and 3.8 times for the second, respectively. The AJ was induced from the first week after the treatments, increasing 2.5 and 1.8 times more than the infected and non-infected controls, maintaining those values throughout the evaluation. The results show that the EA reduced the development of the disease caused by Forl to tomato plants mediated by an increase in the endogenous concentration of AS and AJ.
Key words: SAR; severity; salicylic acid; jasmonic acid
Se evaluó un extracto de ajo (EA) para inducir la defensa de plantas de tomate contra Fusarium oxysporum radicis-lycopersici (Forl), mediante el incremento de la concentración endógena de ácido salicílico (AS) y ácido jasmónico (AJ). La aspersión de 1 y 2 % del EA sobre plantas intencionalmente infectadas con 1x107 esporas mL-1 de Forl, redujeron en 73.53% la severidad de la enfermedad producida por Forl respecto al control infectado. La altura fue similar (p≤0.05) en plantas tratadas con EA y no-tratadas. La acumulación de AS en plantas asperjadas con 1 y 2% de EA, presentaron dos incrementos significativos (p≤0.05), en la segunda y quinta semana después de las aspersiones, con concentraciones mayores que los controles infectado y no-infectado en 11 y 6 veces para el primero y en 2.7 y 3.8 veces para el segundo, respectivamente. El AJ se indujo desde la primera semana después de los tratamientos, incrementando 2.5 y 1.8 veces más que los controles infectado y no-infectado, manteniendo esos valores durante toda la evaluación. Los resultados muestran que el EA redujo el desarrollo de la enfermedad provocada por Forl a plantas de tomate mediado por un incremento en la concentración endógena de AS y AJ.
Palabras clave: SAR; severidad; ácido salicílico; ácido jasmónico
Phytopathogen attack is one of the most disturbing aspects of tomato (Solanum lycopersicum) production, either when grown in an open field, a greenhouse or a mesh-protected system. Among the most important tomato diseases is crown rot (TCR) caused by the saprophyte fungus Fusarium oxysporum f. sp. radicis-lycopersici (Forl), which reduces harvested yields up to 50% (Apodaca et al., 2004). The pathogen can survive in the soil for long periods of time and infects plants through cuts in the roots or when new roots are forming (Szczechura et al., 2013). A good strategy for controlling the disease is to use resistant varieties and grafting (Carrillo-Fasio et al., 2003). However, this disease is controlled mainly by chemical fungicides (McGovern, 2015), but it has been reported that once the fungus penetrates the plant tissue, chemical control becomes ineffective (Apodaca et al., 2002). A method that could potentially reduce Forl disease severity is to induce plant resistance to the pathogen (Ojha and Chatterjee, 2012). It has been reported that plants that are susceptible to pathogens, and that, after being treated with biotic and abiotic agents that affect the host’s physiology, quickly and coordinatedly activate their defense system (El-Kallal, 2007; Arzoo et al., 2012). Plant extracts or their components have been reported to be defense inducers in different crops (Baysal et al., 2002; Zaker and Mosallanejad, 2010; Arzoo et al., 2012). Of particular interest are extracts that can induce salicylic acid (SA) and jasmonic acid (JA), which are signal molecules that have an important role in regulating the signaling network involved in inducing plant defense responses against pathogens (Ryan and Moura 2002; Van Loon et al., 2006). A plant’s defense is more effective when the acquired resistance is systemic, because it is effective against a wide range of pathogens (Gozzo and Faoro, 2013). Systemic acquired resistance implies an increase in mobile signals, such as SA and JA, two compounds that are involved in the expression of genes that activate defense mechanisms in plant tissues far away from where the pathogen attack began and that extend the defense to non-infected plant parts as well (Ojha and Chatterjee, 2012). Therefore, induced defense helps plants to effectively resist attacks from other pathogens (Kachroo and Robin, 2013). In this case, resistance induction is a non-conventional pathogen control technique where the resistance-inducing agent may not have a direct effect on the pathogen (Arzoo et al., 2012). In this research, garlic extract was applied to tomato plants in order to induce resistance against Forl by quantifying SA and JA accumulation as biochemical parameters, and disease severity, plant height and chlorophyll content as resistance parameters.
MATERIALS AND METHODS
Plant material
We used 21-day old seedlings of tomato (Solanum lycopersicum) var. Tiny tim cherry, which were propagated from certified seed in a commercial greenhouse. The seedlings were taken to a growth chamber and kept at a temperature of 25 °C, under a 16 h light/8 h darkness (100 µmol m-2 s-1 of luminous intensity) photoperiod and 80% relative humidity. When the seedlings reached 28 days after germination, a 2% rooting stimulator (Magic root) was applied; 45 days after germination, the seedlings were inoculated with Forl and immediately transplanted to 3-kg bags containing prepared substrate (Nutrigarden). After the seedlings were transplanted, treatments were assigned and randomly applied to each group of plants.
Garlic extract (EA).
The extract was prepared using 10 g of fresh garlic cloves (var. Río Sonora) that were blended in 14 mL of distilled water for 1 min at room temperature. The mixture was centrifuged at 15000 x g at 4 °C for 20 min, then 6 mL of ethanol were added to the supernatant and the mixture was centrifuged again at 8000 x g at 4 °C for 20 min (Jansen et al., 1987). The supernatant represented the garlic extract.
Obtaining Forl spores
The Fusarium oxysporum f. sp. radicis-lycopersici strain was molecularly identified using the ITS1-5.8S ADNr-ITS2 sequencing technique and/or D1/D2 domains of the 28S ADNr gene at the Genomic Biotechnology Center of the Instituto Politécnico Nacional (Access number: 90436915:DO452455.1 in the NCBI database). The Forl strain was donated by the Plant Biotechnology Laboratory of ITSON Centro’s CIBAA. It was grown in an agar-potato-dextrose (PDA) culture medium and incubated at 28 °C for 7 days. It was re-cultivated by taking a small piece of mycelium and inoculating it in fresh PDA at 28 °C for 7 days, or until the fungus produced enough spores to prepare a solution of 1x107 spores/mL. The spore suspension was prepared by adding 5 mL of distilled water containing 0.01% of 0.02% Tween 80 on the surface of the dish containing the fungus; then the surface was scraped using a glass rod, and the liquid containing fungus spores was collected and filtered through gauze to remove the mycelium; the filtrate was centrifuged at 5000 x g. The collected spores were re-suspended in sterilized water until reaching a concentration of 1x107 spores/mL, quantified with a Neubauer chamber.
Tomato seedlings inoculated with Forl.
Forty-five days after germination, the tomato seedlings were taken from the seedbed and their roots were gently washed to remove the substrate. Using a previously sterilized scalpel, small cuts were made on the adventitious roots, which were immediately submerged in a Forl inoculating solution (1X107 spores/mL) for 30 min. The seedlings were then transplanted as described in the previous paragraph.
Treatments to induce resistance in tomato plants
Groups of 10 seedlings per treatment with three replications were formed. Six treatments were evaluated, as shown in Table 1. The first three treatments were used as controls. Seedlings were inoculated with Forl (1x107 spores mL-1) before transplanting. In treatments with EA, the extract was sprayed on the leaves 7 and 14 days after inoculation with Forl and transplanting.
Table 1 Treatments used to induce resistance in tomatoes plants infected with Forl.
| Tratamiento | Plantas |
| 1 | Testigo absoluto |
| 2 | Raíces heridas |
| 3 | Raíces heridas e inoculadas con Forl |
| 4 | Raíces heridas e inoculadas con Forl + EA 0.5% |
| 5 | Raíces heridas e inoculadas con Forl + EA 1% |
| 6 | Raíces heridas e inoculadas con Forl + EA 2% |
Evaluation of disease severity
To evaluate the response of the tomato plants to the pathogen, we used a hedonic scale proposed by Apodaca et al. (2004) and Clavijo-Castro (2014). Plants were classified after the fifth true leaf emerged, and each plant was evaluated using a scale of 1-5 (Table 2), according to disease intensity. To quantify disease severity, the parameters chlorophyll index (UC) and height (cm) were evaluated every 7 days after the treatment was applied for 49 days. Chlorophyll content was measured with a SPAD 502 meter (Minolta), a device that measures absorption in 650 and 940 nm wave lengths to estimate chlorophyll levels. Measurements were made in triplicate on the third leaf to obtain the chlorophyll index. Plant height was measured from the base of the stem to the tip using a flexometer.
Table 2 Hedonic scale used to evaluate disease severity caused by Forl in tomato plants.
| Clase | Intensidad de la enfermedad |
| 0 | Sin síntomas visibles a la enfermedad |
| 1 | Puntos necróticos en hipocotílo |
| 2 | Hojas marchitas, oscurecimiento en la base del hipocotílo o disminución en el crecimiento de la planta |
| 3 | Marchitez, lesión necróticas 1-5 cm y disminución en el crecimiento de la planta |
| 4 | Lesión necrótica 6-10 cm, defoliación y disminución en el crecimiento. |
| 5 | Muerte de la planta |
The severity was calculated by applying the formula proposed by Towsend and Heuberguer (1943) (1).
Severity= (Σnv/ 5N) x 100 (1)
Where:
S= Severity
n= Plants per category
v= Value of the category
N= Plants per experimental unit
Quantification of salicylic acid and jasmonic acid
The content of SA and JA was determined on leaves before and after transplanting, both on treated and non-treated plants, every 7 days for 49 days. The leaves were cut from the plants, wrapped in aluminum foil, immediately frozen in liquid nitrogen and stored at -80 °C until they were analyzed. To quantify metabolites, we used the methodology proposed by Guzmán-Téllez et al. (2014) and Arbona and Gómez-Cárdenas (2008). We started by cutting 1 g of leaves and placing them in tubes (30 mL) to which 10 mL of deionized water were added. This was homogenized with an Ultra-Turrax dispersing machine. The tubes were centrifuged (Galaxy 7D Centrifuge, VWR, USA) at 4000 g for 45 min at 4 °C. The supernatant was collected, and its pH adjusted to 3.0 with 15% acetic acid. Two 3-ml samples of the pH 3 supernatant were prepared, and 2 mL of ethyl ether were added to each tube. The tubes were shaken to recover the organic phase, which was then dried under a stream of gaseous nitrogen. The dry residue obtained was re-suspended in 500 µL of the A/B (50/50) mobile phase that was prepared for determination in high performance liquid chromatography (HPLC). It was then filtered using a 0.45 µm PTFE filter (Pall Gelman Acrodisc filters). The filtrate was used to determine SA and JA content with a liquid chromatography system (Agilent Technologies Model 1260 Infinity) controlled by the OpenLab ChemStation software (Agilent Technologies). The equipment consists of a quaternary pump, a diode-array detector and a 7725i Rehodyne manual injector; we used a 250 x 4.6 mm C18 column (Phenomenex Luna) of 5 µm particle. The mobile phase consisted of an A phase containing 94.9% H2O, 0.1% HCOOH (formic acid) and 5% CH3CN (acetonitrile) and a B phase containing 5% H2O, 94.9% CH3CN and 0.1% HCOOH, at a 60 (A):40 (B) ratio, for which we used an isocratic method at 1 mL per min during 20 min at room temperature. The detection wave length used for both compounds was 303 nm. Pure salicylic acid and jasmonic acid compounds were used to determine the calibration curves (Sigma Aldrich), the retention time of each compound and calculate the concentration. The calibration curves ranged as follows: 0.02-0.1 µg g-1 and 0.1-1.0 µg g-1 for AS, and 0.05-0.1 µg g-1 and 0.1-1.0 µg g-1 for AJ.
Experimental design and data analysis
We used a completely randomized design with 6 treatments and three replications. The experiment was conducted twice for validation. Data were analyzed using ANOVA (one-way) and NCSS statistical software version 2007 (Number Cruncher Statistical System, Kaysville, Utah). Seven-week data on the chlorophyll index, height, severity, and salicylic and jasmonic acid concentration were variable responses. The means were compared using the Tukey-Kramer method at a p≤0.05 probability level. The chlorophyll index and height were analyzed using descriptive statistics.
RESULTS AND DISCUSSION
Chlorophyll content in tomato plants
The chlorophyll content in all tomato plants a week after transplanting was between 41 and 44 UC (Figure 1). These values are within the range (41.3-56.11 UC) reported for tomato plants that were measured under conditions similar to ours (Mendoza et al., 1998; Peñuelas-Rubio et al., 2017). The chlorophyll content in plants used as absolute controls and controls with roots with cuts on them decreased starting on the third week after transplanting, a fact that is associated with the plants’ demand for nitrogen during their rapid vegetative growth (Nurzyeki, 2013). However, the control plants that had roots with cuts and that had been inoculated with Forl showed a significant decrease in UC beginning the second week after transplanting, with significantly lower chlorophyll values (p≤0.05) than the other two control groups (Figure 1). The decrease in UC could be due to the presence of Forl, as reported by Mendoza et al. (1998) and Hiderman et al. (1992).
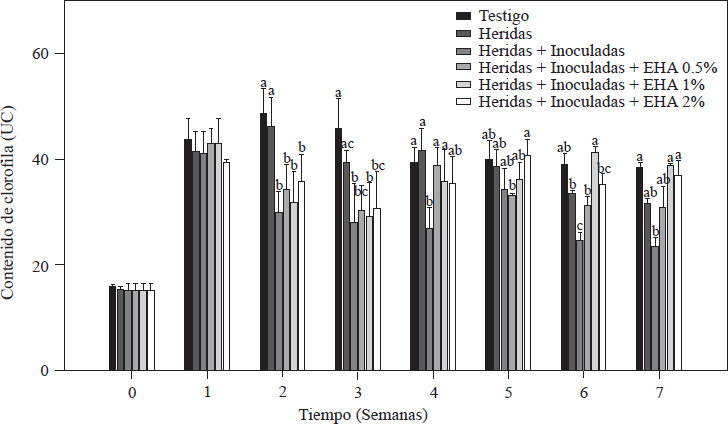
Figure 1 Chlorophyll content in tomato plants non-inoculated and inoculated with Forl and treated with different concentrations of garlic extract. The values are the means of three repetitions of 10 plants each. The vertical bars represent the standard deviation (n=3). Different letters indicate a significant difference between treatments, according to the Tukey-Kramer method at a p≤0.05 probability level. The experiment was conducted twice.
Plants treated with EA were able to increase the UC. Specifically, plants treated with 1 and 2% of EA had significantly increased values of UC (p≤0.05), so during the last two weeks of evaluation they were able to reach the values of the absolute control, whose chlorophyll value was of 41.3 UC, and plants treated with 1 and 2% of EA with values of 41.5 and 40.5 UC, respectively. Massacci et al. (2008) reported that in plants infected with F. oxysporum, photosynthetic processes do not take place, and this decreases the photosynthetic assimilation rate. These results are in agreement with data obtained in this evaluation, where the lowest chlorophyll values (24.9 UC) were observed in the group of control plants that had roots with cuts and that had been inoculated but not treated with EA so that they were not able to recover the chlorophyll values as the plants treated with EA did. UC is an indirect measure of the amount of N in a plant (Vázquez et al., 2012), so it is assumed that plants treated with EA developed well even when they were infected with Forl.
Height of the tomato plants
The results of measuring the height of the tomato plants are shown in Figure 2. During the first three weeks after inoculation and transplanting, all the plants grew and there were no significant differences between the plants non-treated and treated with EA (p≤0.05). After the fourth week, there were marked differences in growth within the groups of plants; for example, plants treated with 1 and 2% EA grew the most, and plants treated with 2% EA reached 67.8 cm, while plants treated with 1% EA reached 62.2 cm; these values were similar (p≤0.05) to the value of the absolute control: 61.9 cm. Plants treated with 0.5% EA reached an average height of 53.5 cm, which is higher than the 34.9 cm reached by the control with cut + inoculated roots. Both groups showed values that are statistically lower (p≤0.05) than the values of treatments with 1 and 2% of EA (Figure 2). Growth decrease is a characteristic symptom of plants infected by Forl (Apodaca et al., 2004; Peñuelas-Rubio et al., 2017), due to the proliferation of the pathogen in the plant’s vascular bundles, which means the spores are disseminated towards the apex in the xylem flow (Hadian et al., 2011). This is in agreement with the results observed in control plants with cuts in the roots + Forl inoculation that were not treated with EA; the disease established itself and was propagated intercellularly. The treatment with 0.5% EA induced the lowest level of resistance in tomato plants because the group of plants sprayed with EA were the lowest in height of the three EA treatments. The effect of garlic as a promoter of vegetative and reproductive growth in pathogen-infected plants was reported by Fatema and Ahmad (2005) in peanut plants infected by nematodes, and by Chohan and Perveen (2015) in tomato plants infected with Fol. In both cases, aqueous garlic solutions improved the plant’s growth response and were able to control the pathogen. In this study, 1 and 2% concentrations of EA improved plant development and induced resistance to Forl.
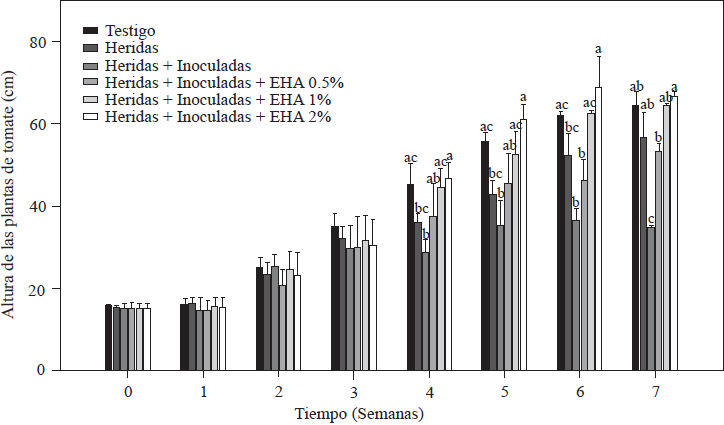
Figure 2 Evaluation of the height of tomato plants non-inoculated and inoculated with Forl and treated with different concentrations of garlic extract. The values are the means of three repetitions with 10 plants each. The vertical bars represent the standard deviation (n=3). Different letters indicate a significant difference between treatments, according to the Tukey-Kramer method at a p≤0.05 probability level. The experiment was conducted twice.
Disease severity of tomato plants infected with Forl
Results of the evaluation of disease severity caused by Forl in tomato plants are shown in Figure 3. All plants inoculated with Forl showed characteristic symptoms of the disease at different levels, including leaf wilt and defoliation, slow growth and stem damage in the form of spots and necrotic lesions on vascular bundles. As expected, the absolute control and the plants with cut roots did not develop disease symptoms because they were not inoculated with the fungus (Figure 3). The minimum value of disease severity was 26.4% in the group of plants treated with 1 and 2% EA, followed by 33.4% in plants treated with 0.5% EA. The most affected plants were those in the control group with cut roots + inoculation, which showed visible symptoms starting the second week after inoculation and 100% severity in the seventh week. These results show the positive effect of applying EA, because disease severity was reduced by 66 to 73% compared to the severity level in the control plants (cut + inoculated), which means that the plants treated developed fewer symptoms. The effect on disease severity caused by Forl in tomato plants treated with EA could be due to the accumulation of metabolites such as SA and JA, which induce defense mechanisms, as demonstrated by Shah and Zeier (2013) and El Oirdi et al. (2011), where these metabolites induced the expression of genes involved in plant defense under stress conditions. Based on this result, we can conclude that the exogenous application of EA acted as an activator of systemic acquired resistance in tomato plants.
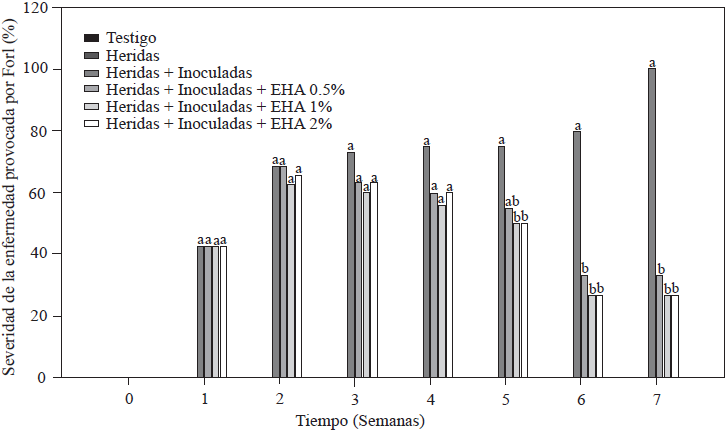
Figure 3 Percentage of disease severity caused Forl in non-inoculated and inoculated tomato plants that were treated with different concentrations of garlic extract. The values are the means of three replications of 10 plants each. Different letters indicate a significant difference between treatments, according to the Tukey-Kramer method at a p≤0.05 probability level. The experiment was conducted twice.
Quantification of salicylic acid (SA)
The results of SA quantification in tomato plants are shown in Figure 4. There were variations in the SA content beginning in the first week of inoculation and basal levels of 0.10 µg g-1 in the absolute control plants, a value within the range (0.10-10 µg g-1) reported for Solanaceae (Rivas-San Vicente and Plasencia, 2011). In cut and inoculated plants, an initial concentration of 0.24 µg g-1 was quantified, which was twice the concentration in the absolute control. In plants inoculated with Forl and treated with 1 and 2% of EA, there was a significant increase (p≤ 0.05) in the SA content in the third week after inoculation. In plants treated with 2% EA, we found values that were 11 and 6 times higher than the values of the control treatments within the same week; for the treatment with 1% EA, we found values 8 and 4.5 times higher than the values of their respective controls. After that increase, SA levels decreased in the following weeks but increased again in the sixth week (Figure 4). As of the fifth week, SA accumulation in the control plants (absolute control and cut + inoculated plants) increased and reached a maximum concentration of 0.78 y 0.82 µg g-1, respectively. These increases in the control treatments occurred two weeks after the increase in the group of plants treated with 1 and 2% EA. Increases in SA content were between 2 and 5 times higher than the basal values reported in plants at the onset of flowering, and in plants infected with necrotic pathogens such as Fusarium oxysporum (Swarupa et al., 2014). This fact may explain the increase in SA content in the control plants from week five to seven. However, increases five times higher than SA concentration that occurred in the third week in plants treated with 1 and 2% EA may be associated with the treatment applied rather than with the presence of the pathogen, given that the maximum SA values in treatments with 1 and 2% of EA were consistent with the reduced symptoms observed when disease severity was evaluated (Figure 3). In support of this deduction, Ojha and Chatterjee (2012) applied SA to tomato plants infected with F. oxysporum and observed a significant increase in plant resistance to pathogen attack when the activity of enzymes related to the defense system increased. The greatest increases in enzyme activity took place 21 days after SA application. This result is in agreement with the results of the present study, which show that the highest SA values were quantified the third week after treatment application. The protective effect against Forl in plants treated with EA, which improved their growth and development, was also reported by Hayat et al., (2012), who by increasing endogenous SA concentrations were able to significantly improve plant growth characteristics, including height, leaf number and color, shoot diameter, dry and fresh weight, and photosynthetic rate.
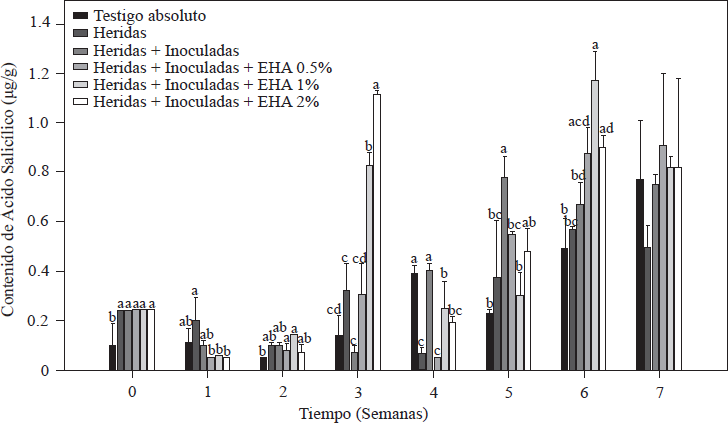
Figure 4 Concentration of salicylic acid in tomato plants inoculated and non-inoculated with Forl and treated with different concentrations of garlic extract. The values are the means of three repetitions with 10 plants each. The vertical bars represent the standard deviation (n=3). Different letters indicate a significant difference among treatments, according to the Tukey-Kramer method at a p≤0.05 probability level. The experiment was conducted twice.
Based on the results of our study, increased SA concentration coincides with an improvement in plant development, as well as resistance to the pathogen, which agrees with the important protective role of SA against pathogens, probably because of its ability to induce the transcription of defense proteins (PR) which codify enzymes such as chitinases, glucanases, endohydrolases and β-1, 3-glucanase that contribute to systemic acquired resistance (SAR) against different pathogens because of its antifungal activity (Heil and Bostock, 2002; Hayat et al., 2010).
Quantification of jasmonic acid (JA)
The results of JA quantification in tomato plants are shown in Figure 5. Plants with cut roots + plants inoculated with Forl, as well as those to which EA was applied increased their JA content, with initial values of 0.89 µg g-1 in plants cut and/or inoculated with Forl, which was significantly higher (p≤0.05) than the value of 0.74 µg g-1 of the absolute control plants. As of the second week, application of 1 and 2% EA produced an increase 2.5 times the value of the absolute control, and 1.8 times the value of the cut + Forl control. These JA values remained significantly higher (p≤0.05) in the third week after inoculation and after transplanting plants treated with 1 and 2% EA. In all treatments, except for the absolute control, JA content was gradually reduced as of the fourth week until the end of the evaluation but maintained high levels, which indicates that the plant was responding to a stress condition (Ryan and Moura, 2002). From this result we can infer that 1 and 2% EA concentrations are enough to increase endogenous JA concentrations in tomato plants, which in turn can induce plant defense responses (Kravchuk et al., 2011). JA is known to be a key regulator that stimulates defense responses in synergy with SA and reduces symptoms of diseases caused by pathogens (Pieterse et al., 2012) by activating the expression of genes involved in plant defense (Ryan, 2000). It has been reported that JA induces protease and polyphenol oxidase inhibitors, and shikimate pathway compounds specifically in tomato plants, which reduces pathogens (El Oirdi et al., 2011). It is well accepted that plants need to activate induced systemic resistance that depends on JA to fight necrotrophic pathogens (Glazebrook, 2005). Therefore, protecting tomato plants from Forl as was done in this study could be related to rapid JA induction and quantified endogenous concentrations (Rahman et al., 2012). However, simultaneous activation of the defense systems that depend on SA and JA results in increased resistance to pathogens (Mur et al., 2006). This is in agreement with the observed increases in endogenous SA and JA and the reduction of disease symptoms caused by Forl in tomato plants.
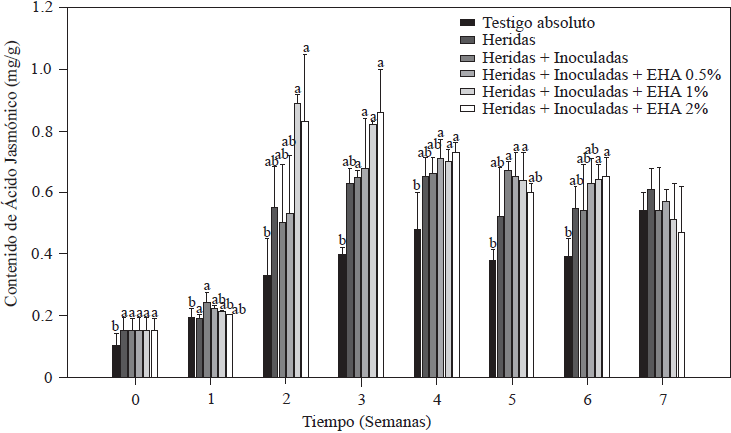
Figure 5 Concentration of jasmonic acid in tomato plants non-inoculated and inoculated with Forl and treated with different concentrations of garlic extract. The values are the means of three repetitions with 10 plants each. The vertical bars represent the standard deviation (n=3). Different letters indicate a significant difference between treatments, according to the Tukey-Kramer method at a p≤0.05 probability level. The experiment was conducted twice.
Acknowledgment
The authors wish to thank CONACYT for the scholarship granted to Ileem Aguilar Gastélum to her Master’s degree.
REFERENCES
Apodaca M, Zavaleta E, García R, Osada S y Valenzuela J. 2002. Frecuencia de campos infestados con Fusarium oxysporum f. sp. radicis-lycopersici en Sinaloa México y su control. Revista Mexicana de Fitopatología 20:1-7. Disponible en línea: http://www.redalyc.org/articulo.oa?id=61220101 [ Links ]
Apodaca M , Zavaleta E , Osada S , García R yValenzuela J . 2004. Hospedantes asintomáticos de Fusarium oxysporum Schlechtend. f. sp. radicis lycopersici W.R. Jarvis y Shoemaker en Sinaloa, México. Revista Mexicana de Fitopatología 22:7-13. Disponible en línea: http://www.redalyc.org/articulo. oa?id=61222102 [ Links ]
Arbona V and Gómez-Cadenas A. 2008. Hormonal modulation of citrus responses to flooding. Journal of Plant Growth Regulation 27:241-250. DOI: 10.1007/s00344-008-9051-x [ Links ]
Arzoo K, Biswas S and Rajik M. 2012. Biochemical evidences of defence response in tomato against fusarium wilt induced by plant extracts. Plant Pathology Journal 11:42-50. DOI: 10.3923/ppj.2012.42.50 [ Links ]
Baysal O, Laux P and Zeller W. 2002. Further studies on the induced resistance effect of plant extract from Redera helix against fire blight (Erwinia amylovora). Acta Horticulturae 590:273-277. DOI: 10.17660/ActaHortic.2002.590.40 [ Links ]
Carrillo FJA, Montoya RTJ, García ERS, Cruz OJE, Márquez ZI y Sanduño BJ. 2003. Razas de Fusarium oxysporum f. sp. lycopersici Snyder y hansen, en tomate (Lycopersicon escuelentum Mill.) en El Valle de Culiacán, Sinaloa, México. Revista Mexicana de Fitopatología 21:123-127. Disponible en línea: http://www.redalyc.org/articulo.oa?id=61221205 [ Links ]
Clavijo CSD. 2014. Búsqueda de resistencia a la pudrición causada por Fusarium spp. en Capsicum. Tesis de Maestría en Ciencias Agrarias. Universidad Nacional de Colombia. Palmira, Colombia, 75 pp. Disponible en línea: http://www.bdigital.unal.edu.co/47659/1/29673462_Sharon.pdf [ Links ]
Chohan S and Perveen R. 2015. Phytochemical analysis and antifungal efficacy of rhizome extracts of various plants against fusarium wilt and root rot of tomato. International Journal of Agriculture and Biology 17:1193-1199. DOI: 10.17857/IJAB/15.0055 [ Links ]
El Oirdi M, El Rahma TA, Rigan L, El Hadram A, Rodrigue MC, Daay F, Vojno A and Bouarab K. 2011. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. The Plant Cell 23:2405-2421. https://doi.org/10.1105/tpc.111.083394 [ Links ]
El-KallaL SM. 2007. Induction and modulation of resistance in tomato plant against fusarium wilt diseases by bioagent fungi (Arbuscular mycorrhiza) and/or hormonal elicitors (Jasmonic acid and Salicylic acid): 1-Changes in growth, some metabolic activities and endogenous hormones related to defense mechanism. Australian Journal of Basic Applied Science 1:691-705. Disponible en línea: https://pdfs.semanticscholar.org/42bb/00a3ee771688eba8485814214110d11cb703.pdf [ Links ]
Fatema S and Ahmad MU. 2005. Comparative efficacy of some organic amendments and a nematicide (Furadan-3G) against root-knot on two local varieties of groundnut. Plant Pathology Journal 4:54-57. DOI: 10.3923/ppj.2005.54.57 [ Links ]
Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review Phytopatholy 43:205-227. DOI: 10.1146/annurev.phyto.43.040204.135923 [ Links ]
Gozzo F and Faoro F. 2013. Systemic acquired resistance (50 years after discovery): moving from the lab to the field. Journal of Agricultural and Food Chemistry 61:12473-12491. DOI: 10.1021/jf404156x. [ Links ]
Guzmán TE, Montenegro DD and Benavides MA. 2014. Concentration of salicylic acid in tomato leaves after foliar aspersions of this compound. American Journal of Plant Sciences 5:2048-2056. DOI: 10.4236/ajps.2014.513220 [ Links ]
Hadian S, Rahnama K, Jamali S and Eskandari A. 2011. Comparing Neem extract with chemical control on Fusarium oxysporum and Meloidogyne incognita complex of tomato. Advances in Environmental Biology 5:2052-2057. Disponible en línea: http://www.aensiweb.com/old/aeb/2011/2052-2057.pdf [ Links ]
Hayat Q, Hayat S, Alyemeni MN and Ahmad A. 2012. Salicylic acid mediated changes in growth, photosynthesis, nitrogen metabolism and antioxidant defense system in Cicer arietinum L. Plant Soil Environmental 58:417-423. DOI: 10.17221/232/2012-PSE [ Links ]
Hayat Q , Hayat S , Irfan M, Ahmad A . 2010. Effect of exogenous salicylic acid under changing environment: A review. Environmental and Experimental Botany 68:14-25. https://doi.org/10.1016/j.envexpbot.2009.08.005 [ Links ]
Heil M and Bostock RM. 2002. Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Annals of botany 89:503-512. DOI: 10.1093/aob/mcf076 [ Links ]
Hiderman J, Makino A, Kurita Y, Mae T and Ojima K. 1992. Changes in the levels of chlorophyll and light-harvesting chlorophyll a/b protein of PS II in rice leaves aged under different irradiances from full expansion through senescence. Plant and cell physiology 33:1209-1214. https://doi.org/10.1093/oxfordjournals.pcp.a078375 [ Links ]
Jansen H, Müller B and Knobloch K. 1987. Allicin characterization and its determination by HPLC. Planta Médica 53:559-562. DOI: 10.1055/s-2006-962811 [ Links ]
Kachroo A and Robin GP . 2013. Systemic signaling during plant defense. Current Opinion in Plant Biology 16:527-533. DOI: 10.1016/j.pbi.2013.06.019 [ Links ]
Kravchuk Z, Vicedo B, Flors V, Camanes G, González BC, García AP. 2011. Priming for JA-dependent defenses using hexanoic acid is an effective mechanism to protect Arabidopsis against B. cinerea. Journal of Plant Physiology 168:359-366. DOI: 10.1016/j.jplph.2010.07.028 [ Links ]
Massacci A, Nabiev SM, Pietrosanti L, Nematov SK, Chernikova TN, Thor K, Leipner J. 2008. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied bygas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiology and Biochemistry 46:189-195. https://doi.org/10.1016/j.plaphy.2007.10.006 [ Links ]
McGovern RJ. 2015. Management of tomato disease caused by Fusarium oxysporum. Crop Protection 73:78-92. http://doi.org/10.1016/j.cropro.2015.02.021 [ Links ]
Mendoza M, González GA, Santelises AA, Etcheveres JD y Rincón JA. 1998. Estimación de la concentración de nitrógeno y clorofila en tomate mediante un medidor portátil de clorofila. Terra 16:135-141. Disponible en línea: https://www.chapingo.mx/terra/contenido/16/2/art135-141.pdf [ Links ]
Mur L, Kenton P, Atzorn R, Miersch O and Wasternack C. 2006. The Outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiology 140:249-262. https://doi.org/10.1104/pp.105.072348 [ Links ]
Nurzyeki J. 2013. Effect of substrates on nutrient content in root zone and leaves of greenhouse tomato. Acta Scientiarum Polonorum. Hortorum Cultus 12:169-178. Disponible en línea: http://www.acta.media.pl/pl/full/7/2013/000070201300012000050016900178.pdf [ Links ]
Ojha S and Chatterjee NCh. 2012. Induction of resistance in Tomato plants against Fusarium oxysporum f. sp. licopersici mediated through salicylic acid and Trichoderma harzianum. Journal of Plant Protection Research 52:220-225. https://doi.org/10.2478/v10045-012-0034-3 [ Links ]
Peñuelas RO, Arellano GM, Verdugo FA, Chaparro ELA, Hernández RSE, Martínez CJL y Vargas AIC. 2017. Larrea tridentata extracts as an ecological strategy against Fusarium oxysporum radicis-lycopersici in tomato plants under greenhouse conditions. Revista Mexicana de Fitopatología 35:360-376. DOI: 10.18781/R.MEX.FIT.1703-3 [ Links ]
Pieterse CM, Van der Does D, Zamioudis C, Leon RA, Van Wees SCM. 2012. Hormonal modulation of plant immunity. Annual Review of Cell and Development Biology 28:489-521. DOI: 10.1146/annurev-cellbio-092910-154055 [ Links ]
Rahman TA, Oirdi ME, Gonzalez LR, Bouarab K . 2012. Necrotrophic pathogens use the salicylic acid signaling pathway to promote disease development in tomato. Molecular Plant Microbe Interaction 25:1584-93. DOI: 10.1094/MPMI-07-12-0187-R [ Links ]
Rivas SVM and Plasencia J. 2011. Salicylic acid beyond defence: its role in plant growth and development. Journal of experimental Botany 62:3321-3338. DOI: 10.1093/jxb/err031 [ Links ]
Ryan CA. 2000. The systemin signaling pathway: differential activation of plant defensive genes. Biochimica et Biophysica Acta 1477:112-121. https://doi.org/10.1016/S0167-4838(99)00269-1 [ Links ]
Ryan CA and Moura DS. 2002. Systemic wound signaling in plants: A new perception. Proceeding of the National Academy of Science. 99:6519-6520. https://doi.org/10.1073/pnas.112196499 [ Links ]
Shah J and Zeier J. 2013. Long-distance communication and signal amplification in systemic acquired resistance. Frontiers in Plant Science 4:30. DOI: 10.3389/fpls.2013.00030 [ Links ]
Swarupa V, Ravishankar K and Rekha A. 2014. Plant defense response against Fusarium oxysporum and strategies to develop tolerant genotypes in banana. Planta 239:735-751. DOI: 10.1007/s00425-013-2024-8 [ Links ]
Szczechura W, Staniaszek M and Habdas H. 2013. Fusarium oxysporum f. sp. radicis-lycopersici-the cause of Fusarium crown and root rot in tomato cultivation. Journal of Plant Protection Research 53:172-176. DOI: 10.2478/jppr- 2013-0026 [ Links ]
Towsend GR and Heuberger JW. 1943. Methods for estimating losses caused by diseases in fungicide experiments. Plant Disease Reporter 27:340-343. Disponible en línea: https://eurekamag.com/research/025/008/025008582.php [ Links ]
Van Loon L, Rep M and Pieterse CM. 2006. Significance of inducible defense-related proteins in infected plants. Annual Review of Phytopathology 44:135-62. DOI: 10.1146/annurev.phyto.44.070505.143425 [ Links ]
Vázquez M, Jiménez S, Torres I, Anaya I, Mendoza H y Guevara R. 2012. Comportamiento de plantas de tomate (Solanum lycopersicum) asperjadas con ácido salicílico cultivadas bajo diferentes condiciones climáticas en invernadero. Ciencia@UAQ, 5:1-9. Disponible en línea: http://www.uaq.mx/investigacion/revista_ciencia@uaq/ArchivosPDF/v5-n1/articulo6.pdf. [ Links ]
Zaker M and Mosallanejad H. 2010. Antifungal activity of some plant extracts on Alternaria alternata, the causal agent of alternaria leaf spot of potato. Pakistan Journal of. Biological Science 13:1023-1029. DOI: 10.3923/pjbs.2010.1023.1029 [ Links ]
Received: June 13, 2018; Accepted: July 20, 2018











 texto en
texto en 


