Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.36 no.2 Texcoco may./ago. 2018
https://doi.org/10.18781/r.mex.fit.1712-1
Phytopathological report
First report of Cladosporium cladosporioides, a fungus that causes rot in zapote mante fruits in Mexico
1Laboratorio de Fisiología y Biología Molecular de la Interacción Planta-Patógeno-Vector. Postgrado en Fitosanidad-Fitopatología, Colegio de Postgraduados, Km. 36.5, Carretera México-Texcoco. Montecillo, Texcoco, México, C.P. 56230, México
2Campo Experimental Valle de México. Carretera Texcoco-los Reyes-km. 13.5 Texcoco, Coatlinchan, C.P. 56250, Texcoco, México.
The consumption of zapote mante (Pouteria campechiana) in Mexico has increased, being the municipality of El Mante, Tamaulipas, one of the main producers. In a non-commercial garden, sunken spots of brown color coalesced in fruits. The objective of this study was to identify the causal agent of the spot of fruit de zapote mante. With the fungi isolated, the Koch postulates were amplified, and the ITS region of the rDNA was amplified by PCR using the universal primers ITS1-ITS4. Morphological and molecular analysis indicated that the causal agent of the fruit blotch is the fungus Cladosporium cladosporioides. To our knowledge, this is the first report of this pathogen affecting this zapote mante in Mexico.
Key words: sunken spots; fungi; Koch postulate; PCR
El consumo del zapote mante (Pouteria campechiana) en México se ha incrementado, siendo el municipio de El Mante, Tamaulipas, uno de los principales productores. En un huerto no comercial se observaron en frutos manchas hundidas de color marrón que coalescen. El objetivo de este estudio fue identificar el agente causal de la mancha del fruto de zapote mante. Con los hongos aislados se realizaron los postulados de Koch, se amplificó y secuencio la región ITS del ADNr mediante PCR utilizando los iniciadores universales ITS1-ITS4. El análisis morfológico y molecular indicó que Cladosporium cladosporioides es el agente causal de la mancha del fruto. Hasta donde se sabe, este es el primer reporte de este patógeno afectando a zapote mante en México.
Palabras clave: mancha hundida; hongo; postulados de Koch; PCR
The genus Pouteria includes species for fruit production potential, among which is the zapote mante, also called yellow or canistel zapote (P. campechiana). This species is native to southern Mexico and Central America. In Mexico, its production and marketing are increasing, as well as its regional consumption as fresh food or to manufacture processed foods, oils for the cosmetics industry, or as medication. This sapotacea is produced in the municipality of El Mante, Tamaulipas, Mexico, where fruits with sunken spots that cause rot have been observed. No one knows when the disease appeared nor the pathogen that causes it. The objective of this research was to identity the causal agent by performing pathogenicity, morphological and molecular tests.
In a non-commercial orchard in Ciudad Mante, municipality of El Mante, Tamaulipas, Mexico, 30 zapote mante fruits were collected that showed brown to black sunken spots (1-3 cm in diameter) on most of the epicarp which, in time, coalesced. The fruits were disinfected with a 1% sodium hypochlorite solution for 3 min and rinsed three times with sterile distilled water. Then they were placed in a humid chamber at 27 °C, and when sporulation began, the fungal structures were placed on a petri dish containing potato-dextrose-agar (PDA 39 g L-1 of water). This procedure was used for each collected fruit and a total of 180 isolates were obtained and kept on petri dishes with PDA at 27 °C in total darkness.
The morphology of the isolates was described with temporary and permanent preparations. The fungal structures were observed under a microscope and measured at least 50 times each. Later, the keys of Barnett and Hunter (1998) and Bensch et al., (2012) were used to determine the genus. Based on the keys, the obtained isolates were termed MTCc (144 isolates), MTCO (22 isolates) and MT1 (14 isolates).
In the pathogenicity tests, only the MTCc and MTCO isolates (treatments) were used, and in each case, the morphotype was used most frequently. Forty symptomless ripe zapote mante fruits were disinfected with 1% sodium hypochlorite and rinsed with sterile distilled water. Two levels of inoculum (1 x 106 and 1 x 104 conidia per mL) were evaluated and used to inoculate two groups of 20 fruits. A conidial suspension was obtained from monosporic cultures of the MTCc and MTCO isolates. In the fruits of each treatment small cuts were made using a sterile needle, and then sprayed with 3 mL of the conidial suspension. Small cuts with a sterile needle were also made on the 20 control fruits, and sprayed with sterile distilled water. All 60 fruits in each treatment were placed in a moisture chamber at 27 °C for 12 days, during which the fruit developed symptoms; when the symptoms became evident, isolates of the mycelium growing on the symptoms were made. This experiment was repeated one more time. It should be noted that in both assays, neither the isolate called MTCO nor the control treatment induced symptoms in the inoculated fruits. In contrast, the MTCc isolate at each level of inoculum induced symptoms very similar to those observed in the field. The re-isolates obtained were named MTCc-pp and kept on petri dishes containing PDA at 27 °C in total darkness.
For the molecular characterization of the MTCc isolate and of the isolates obtained from the pathogenicity test (MTCc-pp), total genomic DNA was extracted. We used 200 mg of mycelium grown for five days in PD (potato dextrose) liquid medium and kept in a thermo-shaker at 112 rpm. The 200 mg of mycelium were incubated for 1 h at 60 °C in 1,000 µL of extraction buffer (NaCl 0,7 M, Tris 0,1 M pH 7,5, 0,01 M EDTA pH 8,0, 1% ß-ME and 1% CTAB) and moderately shaken every 5-10 min. Subsequently, 200 µL of NaAc 3 M were added, and placed at -20 °C for 20 min, followed by centrifugation at 10,000 rpm for 10 min. For DNA precipitation, 750 µL of the supernatant were taken and placed in a new 1.5 mL tube; then, 750 µL of cold isopropanol were added and kept at -20 °C for 20 min. After this period, they were centrifuged for 10 min at 12,000 rpm, the supernatant was discarded, the pellet was washed three times with 100 µL of cold 70% ethanol, dried at room temperature and re-suspended in 30 µL of water free of DNase. Finally, the DNA was stored at -20 °C.
For the polymerase chain reaction, the universal primers ITS1F (5´- CTTGGTCATTTAGAGGAAGTAA-3´) and ITS4R (5´- TCCTCCGCTTATTGA TATGC-3´) were used. The mixture for the PCR with a final volume of 25 µL consisted of DNase-free water, 1X reaction buffer, MgCl2 2.5 mM, dNTP´s 2 mM, 1U of Taq DNA polymerase (Promega®), 1 pmol primers and 100 ng/µL of DNA, with the following amplification conditions: one cycle at 94 °C for 5 min, 30 cycles at 94 °C for 30 s, 55 °C for 30 s and 72 °C for one min. The amplification products were visualized in 1% agarose gel + ethidium bromide in a Gel-Doc 2,000 photo-documentation system. Later, the products of the MTCc and MTCc-pp isolates obtained through PCR were purified using the Wizard® SV gel kit and PCR Clean-up System (Promega) and sequenced using an automatic 16-capillary DNA sequencer (Applied Biosystems, model 3130xl).
The obtained sequences were assembled using the Mega 7 program, where they were cleansed to correct possible sequencing errors; then they were aligned to obtain a consensus sequence of 572 base pairs, which was compared to the sequences reported in the National Center for Biotechnology Information (NCBI) database using the Blast tool. Then a phylogenetic analysis was performed following the Neighbor-Joining method with 5,000 bootstrap replications.
From the obtained isolates, two fungi were identified: Colletotrichum sp. (MTCO) and Cladosporium sp. (MTCc); a third one produced a sclerosed stroma (MT1), but was not identified. Cladosporium sp. was the most frequently present fungus and showed an 80.5% association with the disease lesions. The Cladosporium sp. isolates showed similar culture traits consistent with those reported for this genus (Bensch et al., 2012). The presence of nodulous macronematous cylindrical conidiophores far from each other, with a single conidiogenic cell was observed under a compound microscope; conidia had one or no septum and were 3 to 5 μm wide, which are characteristics reported for C. cladosporioides (Bensch et al., 2012).
In the pathogenicity tests using the two evaluated inoculum levels, sunken areas were observed in the epicarp 48 h after inoculation. At 72 h, brown spots were observed on the sunken areas which later became necrotic and coalesced on the fruit. White mycelium grew on the spots and had morphological structures very similar to those of the MTCc isolate. The re-isolates obtained coincided with the culture and morphological traits of the isolate, which proved that this isolate produced the sunken spots in zapote mante (Figure 1).
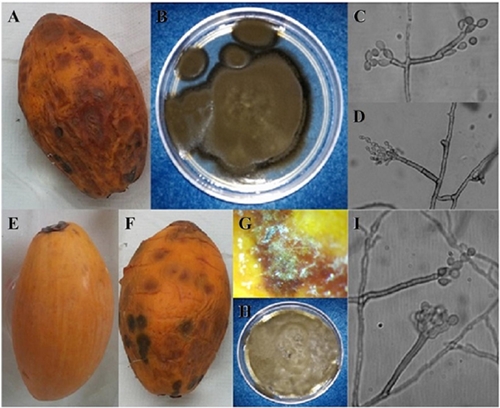
Figure 1 Morphological characterization and pathogenicity tests were used to identify Cladosporium cladosporioides in zapote mante fruits (Pouteria campechiana) in Ciudad Mante, Tamaulipas, Mexico. A) Fruit with brown sunken spots; B) MTCc isolate; C and D) macronematous and elipsoidal conidiophores of Cladosporium cladosporioides. E-I) Pathogenicity tests; E) Fruit used as a control; F) Inoculated fruit showing brown sunken areas; G) Presence of morphological structures on the MTCc isolate on inoculated fruit; H) Re-isolate obtained from inoculated fruits in pathogenicity tests; I) Conidiophores and conidia of Cladosporium cladosporioides.
The obtained sequences were analyzed using the NCBI BLAST tool and the results showed that they were 99% similar to the sequences reported for Cladosporium cladosporioides. The sequence of the MTCc isolate was deposited in the GenBank (access number KP788715). In the phylogenetic analysis, the identity of the MTCc isolate was established based on its close relationship with isolates reported as Cladosporium cladosporioides.
The symptoms observed coincide with those described for Cladosporium cladosporioides, which affects tangerine (Citrus reticulata), papaya (Papaya carica), passion fruit (Passiflora edulis) and mango (Mangifera indica L.) (Guillen-Sánchez et al., 2007; Vásquez et al., 2012; Tashiro et al., 2013), and demonstrates the impact of this pathogen on different fruit crops; it also reduces their commercial value due to the unpleasant appearance of the epicarp. As far as we know, this is the first time in Mexico that Cladosporium cladosporioides has been reported to produce necrotic spots on zapote mante fruits.
Literatura citada
Barnett HL and Hunter BB. 1998. Illustrated genera of imperfect fungi. Fourth Edition. APS Press. St. Paul, Minn. 218p. [ Links ]
Bensch K, Braun U, Groenewald JZ and Crous PW. 2012. The genus Cladosporium. Studies in Mycology 72:1-401. DOI: 10.3114/sim0003 [ Links ]
Guillén-Sánchez D, Yañez-Morales M.J, Téliz-Ortíz D, Siebe-Grabach C and Bautista-Baños S. 2007. Morphological and molecular characterization of Cladosporium tenuissimum Cooke (Deuteromycotina: Hyphomycetes) on mango tree panicles: symptoms, pathogenicity and severity of the fungus. Fruits 62:361-368. DOI: 10.1051/fruits:2007032 [ Links ]
Tashiro N, Noguchi M, Ide Y and Kuchiki F. 2013. Sooty spot caused by Cladosporium cladosporioides in Postharvest Satsuma mandarin grow in heated greenhouses. Journal of General Plant Pathology 79:158-161. DOI: 10.1007/s10327-013-0430-1 [ Links ]
Vásquez LA, Hernández CE, Mora A JA, Nava DC y Sánchez GF. 2012. Etiología y epidemiología de la necrosis de flores y frutos juveniles del papayo (Carica papaya L.) en Guerrero, México. Agrociencia 46:757-767. Disponible en línea: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952012000800002 [ Links ]
Received: December 04, 2017; Accepted: March 23, 2018











 texto en
texto en 


