Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.36 no.2 Texcoco Mai./Ago. 2018
https://doi.org/10.18781/r.mex.fit.1711-1
Phytopathological notes
First Report of Colletotrichum spp. in fruits of allspice (Pimenta dioica) in Veracruz, Mexico
1Departamento de Ingeniería Forestal, División de Ciencias Forestales, Universidad Autónoma Chapingo, Km 38.5 Carretera México-Texcoco, Estado de México, CP. 56230, México
2Instituto de Fitosanidad, Colegio de Posgraduados, Km. 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Estado de México, CP. 56230, México.
Allspice is being affected by anthracnose, a disease that attacks fruits, causing dark brown-dark spots, until necrosis invades the fruits and causes them to fall. Between 2015 and 2016 in the north of Veracruz, allspice fruits were collected with and without symptoms of anthracnose. Isolates were obtained, for a total of 13 monosporic isolates, which were submitted to morphological, pathogenic and molecular characterization. For the morphological characterization, the isolates were seeded in potato dextrose agar media and described by 11 variables. In the pathogenic characterization, the isolates were inoculated on fruits of three locations, with and without injury prior to inoculation; the controls were inoculated with sterile distilled water. For the molecular characterization, the deoxyribonucleic acid was extracted by the CTAB extraction and purification method. The ITS region was amplified with primers ITS4, ITS5. All isolates were pathogenic in fruits after 11 days of inoculation. Morphological characterization identified two species: C. acutatum and C. gloeosporioides. Molecularly, the results of the morphological identification and of two additional species were confirmed: C. fragariae and C. boninense.
Key words: spice; anthracnose; morphological; pathogenic and molecular characterization
La pimienta gorda se ha visto afectada por antracnosis, enfermedad que ataca frutos provocando manchas marrón-oscuras hundidas, hasta necrosar los frutos. Entre 2015-2016 en el norte de Veracruz se colectaron frutos de pimienta gorda con y sin síntomas de antracnosis, de estos se obtuvieron 13 cultivos monospóricos; posteriormente fueron caracterizados morfológica, patogénica y molecularmente. Para la caracterización morfológica los aislados se sembraron en medio de papa dextrosa agar y descritos mediante 11 variables. Para la caracterización patogénica fueron inoculados en frutos de pimienta gorda de tres localidades, con y sin herida previa a la inoculación, los testigos fueron tratados con agua destilada estéril. Se evaluó la incidencia y severidad. Para la caracterización molecular se extrajo el ácido desoxirribonucleico por el método de extracción y purificación CTAB. La región del ITS fue amplificada con los primers ITS4, ITS5. La caracterización morfológica identificó dos especies: C. acutatum y C. gloeosporioides. Todos los aislados resultaron patogénicos en los frutos después de 11 días de la inoculación. Molecularmente se confirmaron los resultados de la identificación morfológica y de dos especies adicionales: C. fragariae y C. boninense.
Palabras clave: especia; antracnosis; caracterización morfológica; patogénica y molecular
Allspice is a plant used in the culinary arts and it is produced in tropical areas of the coastal areas of the Gulf of Mexico. More than 15 thousand tons of allspice are produced in municipal areas of the Sierra del Totonacapan and distributed nationwide, as well as to countries in the European Union. Its production is currently being affected by fungi that attack leaves, stem, flowers, and mainly the flowers of this plant, which has contributed to the loss in quality of the product, and has lead to investigations on the cause of such reduction (Carballo PI. 2016, abril 10).
Anthracnose is a disease that affects plant species of different botanical families from hot and humid areas, caused by different species of the genus Colletotrichum. The symptoms commonly caused by this pathogen are dark brown concave spots on leaves, stems, flowers or fruits, leading to the death of tissues (Rivera, 2007).
In August of 2014, M. C. Silvia E. García Díaz (personal communication) performed a diagnosis on allspice fruits, stems and leaves with material from the field in Northern Veracruz, and found symptoms of anthracnose; the disease caused losses of 20 to 50% of the production in pre and post harvest. Due to a lack of information on this fungus in the host, this investigation was carried out between 2015 and 2016, in order to detect the presence of the pathogen with certainty. Because of this, the aim of our investigation was to characterize, pathogenically and molecularly, different isolations obtained from allspice fruits (Pimenta dioica L. Merrill) with symptoms of anthracnose and determine the causal agent.
The sampling and collection of plant material (leaves, stems and fruits) with symptoms of anthracnose was carried out in three orchards alternated with allspice and basic crops, located in Northern Veracruz (Location 1: La Fábrica, Coxquihui (20.177222°/-97.560278°); Location 2: Chapultepec, Espinal (20.204444°/-97.545000°) and Location 3: Sta. Isabel, Espinal (20.191111°/-97.529722°). Three samples were taken from each orchard, one for each tree chosen, based on the presence of symptoms, and the phenological state of the trees was the stage of fructification. The first experiment was carried out in July of 2015, and the second one, in August of 2016 (production season), in the Postharvest Diseases Laboratory of the Instituto de Fitosanidad-Fitopatología of the Colegio de Postgraduados, Campus Montecillos. From each collection sitee, fruits were collected with symptoms of the disease, disinfected with sodium hypochlorite at 1% for 1 min, washed using sterile distilled water, and dried with sterile paper towels. Later, they were placed in humidity chambers at 25 ± 1 °C for five days. After finding signs of the pathogen on the surface of the fruits, preparations were made and the fungus of interest was confirmed. From these, we obtained monosopre cultures in a PDA culture medium (39 gr in 1L water), following the dilution and dispersion method (Echandi, 1971). Each monospore culture developed in the culture medium for seven days. For the morphology, discs were taken from those cultures, each 0.5 cm in diamater, four repetitions were planted per isolation in Petri dishes with a PDA medium (Bioxon), they were incubated at 28 °C and their growth was evaluated every 48 h until the pathogen filled the dish. The following macroscopic variables were recorded after 10 days: 1) Growth in diameter; 2) Margin of the colony; 3) Type of mycelia; 4) Color of colony; 5) Concentric growth rings; 6) Sporulated colony. After each isolation, preparations were made to characterize the microvariables. The microscopic The microscopic variables evaluated were: 7) Type of conidia; 8) Sexual stage; 9) Color and dimension of appressoria, using a compound miscrospoe (Micro Star, American Optical) with a 40x lens to observe these characteristics; 10) Dimensions of 50 conidia pero isolation; amd 11) Dimensions of 50 appressoria. The appressoria were obtained after 5 days of preparing conidia in humidity chambers to induce their formation. A total random experimental design was used and the data were analyzed in two ways: 1) applying a multivariate analysis by clusters for the variables used in the identification, thus obtaining a clustering dendrogram of the morphological characteristics to know which species were involved in the development of symptoms of anthracnose in allspice; 2) a normal analysis of variance was carried out, in which the diameters of six day old colonies were compared. Mathematical analyses were carried out using the statistical analysis package SAS® System for Windows V9, 2002. The identification of the Colletotrichum isolations in allspice fruits was based on the morphological and morphometric, as well as the clustering obtained from the multivariate analysis compared with the descriptions and keys by (Bailey et al., 1996; Barnett y Hunter, 1998).
To verify that the symptoms observed in the fruits gathered in the field were caused by the isolations obtained, Koch’s postulates (Agrios, 2002). Healthy fruits were gathered (approximately 300 allspice fruits) and 13 Colletotrichum spp. monospore isolations obtained from allspice. In order to increase the inoculant, the isolations were planted in corn meal agar (CMA). Using the developed spores, a mother solution was prepared, and the concentration was determined using a hemocytometer, adjusted to 1x106 spores/mL. The fruits were disinfected using sodium hypochlorite (NaClO) at 1% for 1 min and rinsed twice with sterile distilled water and left to dry for 15 min. They were then placed in Petri dishes that were, in turn, placed in humidity chambers. Fruit inoculation was carried out with the spore suspension; for two isolation that displayed sporulation, we used discs, 0.5 cm in diamater, which contained mycelium. We placed 5 µL of the conidia suspension on fruits without lesions (w/o/l) and with lesions (w/l), made with a dissection needle, to a depth of 1 mm. In the controls, 5 µL of distilled water were applied in fruits w/o/l and w/l, per sampling site. The humidity chambers were sealed and incubated at 23±1 °C, with natural light for 11 days. To evaluate the pathogenicity, we determined the presence or absence of circular or deformed necrotic spots, the percentage of incidence, and the severity of the fungus on the fruit. Incidence was evaluated as a parecentage of fruits affected by the pathogen. For the analysis, the data of w/o/l and w/l were groupd by isolation and location, using equation (1), suggested by Anculle and Álvarez (1999). Severity was evaluated as the percentage of tissue affected by the pathogen, using the visual scale by Anculle and Alvarez (2006), modified and adapted to the experiment; considering the degree of the condition graded visually in each fruit, it was valued using severity equation 2 according to Anculle and Alvarez (1999).
The treatments were a combination of isolations, inoculation and host method, established under a total random design with five repetitions inoculated with a lesion (w/w) and five without a lesion (w/o/l), comapring the degree of damage on the fruit, the isolation and the sampling site for the variable of severity; for the variable of incidence, we used a multivariate analysis comparing the number of damaged fruits w/l and w/o/l, the isolations and the incidence on the three sampling sites. Mathematical analyses were performed using the statistical analysis package SAS® System for Windows V9, 2002. To confirm Koch’s postulates, we reisolated the pathogen, extracting fragments of tissue and/or sporulation, which were deposited in PDA, observed and we compared the macro and microscopic variables of the reisolations with which the original allspice fruits were presented. The experiment was carried out twice.
Monospore cultures from all 13 isolations were incubated for 10 days at a room temperature of 23 ±1 °C in a PDA culture medium. A sample of mycelium was taken from each isolation and deposited into an Eppendorf tube to be maceraed. DNA was extracted with the CTAB extraction and purification method (Wagner et al., 1987). Later, DNA concentration was quantified using a NanoDrop 2000 Spectrophotometer (Thermo Scientific). We completely amplified genes ITS-1, ITS-2 and 5.8S, and partially, gene 18S and 28S of the 13 Colletotrichum spp. isolations; primers ITS 5 and ITS 4 were used. The PCR amplification and sequencing (ITS) of the DNA extracted were carried out using the Sanger method in the company Macrogen (Korea). The sequences of the 13 isolations were compared with those deposited in the National Center for Biotechnology Information (NCBI), with the support of the Blast tool. The sequences with the greatest similarities were extracted from the data bank for phylogenetic analyses and then, along with those obtained from the fruits, they were aligned (Clustar W) and processed using the UPGMA method in the program Mega 6 (Molecular Evolutionary Genetic Analysis), to obtain a phylogenetic analysis.
Thirteen Colletotrichum spp. monospore cultures were obtained, out of which four isolated allspice fruit species were identified as presenting symptoms of anthracnosis (Figure 1). The growth in diameter was similar for most isolations, except for three with a slow growth, identified as C. acutatum. Out of all the cultures, 69% presented a circular growth and edge, while 31% presented a circular growth and a wavy edge, although most presented concentric rings. Andrades et al. (2009) and Chowdappa et al. (2012), described cultures with regular edges and a circular growth; Morales et al. (2009) and Saldarriaga et al. (2008), observed a formation of growth rings in some of their isolations. On the other hand, 15% of the isolations presented aerial mycelia and 54% was flat; 8% presented a dense mycelium and in 23%, it was scarce fue escaso. Similar results were presented by Saldarriaga et al. (2008), since 70% of their strains presented a thin, superficial growth, mycelial aggregates. The color of the culture was initially white-salmon, and later turned dark gray; 54% had a salmon-white-gray, 31% was white-gray, 8% was salmon and 7% were white cultures; similar percentages were reported by Dominguez et al. (2012). On the other hand, Freeman et al. (1998), report that C. acutatum developed salmon-colored conidial masses, and C. gloeosporioides, a gray sporulation. Out of the isolations, 77% produced unicellular hyaline conidia with rounded edges and only 23% presented conidia with one rounded edge, and another acute edge; Villanueva et al. (2008), reported the formation of hyaline, aceptados, cyllindrical and straight. Robles (2015) mentioned that 83% of his isolations developed unicellular, hyaline conidia with rounded edges, 11% presented conidia with one rounded edge and another acute edge, and 6% of conidia with both ends being acute. Villanueva et al. (2008), reported that the C. gloeosporioides conidia presented obtuse ends, but some are narrow, and C. Fragariae, and obtuse end and another narrow end. The length and width of the conidia ranged between 18.1 - 44.9 µm and 4.2 - 13.6 µm, respectively; the total average for the size of conidia was 28.4 x 8.4 µm. Statistically, no significant differences were found in the length and width (Pr>F: <0.0001; α: 0.05; LSD length and width of conidium: 1.2891 and 0.4377). Similar results were reported by Villanueva et al. (2008). Irregular appressoria were observed, with different shapes, and with a dark brown color. Length ranged between 9.2 and 90.2 µm, while the width ranged between 5.9 and 57.1 µm; the average was 25.9 x 15.5 µm; statistically, no significant differences were found in length (Pr>F: 0.0014, α: 0.05, LSD: 6.0527) and width (Pr>F: 0.0001, α: 0.05, LSD: 3.4877). Robles (2015) and Oliveira et al. (2005) reported similar results to those obtained in this experiment; Villanueva et al. (2008), observed that the appressoria formed by C. fragariae, C. gloeosporioides and C. orbiculare were dark brown, and with similar shapes and sizes; C. boninense promotes appressoria in short chains or solitary, brown-colored, with thick walls, whole or crenate edges, rarely lobulated, with irreguar shapes, but commonly in the shape of a projectile 4.5-18 x 4-11 µm according to Damm et al. (2012). Only one isolation presented asci with hyaline ascospores, slightly curves and with rounded vertices, and the rest had no presence. Robles (2015), reports a scarce formation of perithecia in a PDA culture medium. A multivariate analysis was carried out using data obtained from the 11 macroscopic and microscopic characteristics, and an eigenvalue of 2.07 was found for the four clusters or groups. This information was confirmed using the value of the Pseudo F that peaked at 21.9 for the four clusters or groups. These values affirm that when analyzing the groups alongside the evaluated, the 13 isolations can be classified into four groups.
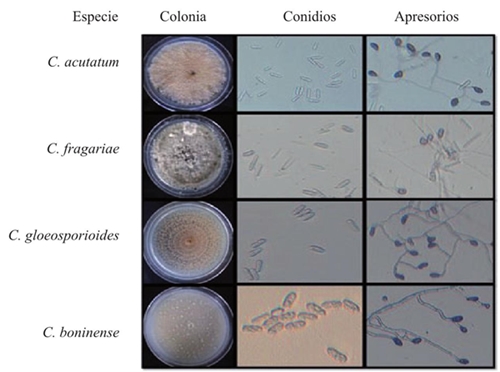
Figure 1 Macroscopic and some microscopic characteristics of the Colletotrichum spp. species obtained from allspice fruits in Northern Veracruz.
The aggressiveness of the isolations was variable in the form of inoculation (w/l and w/o /l), type of inoculation (suspension and section of growth media) and between isolations; these differences helped prove that some isolations are capable of inducing more aggressiveness than others, regardless of the condition of the inoculation. Some allspice fruits presented brown, circular necrotic spots on the epidermis of the fruit, concave, moist and soft lesions. As the infection progressed, the spots turned brownish-maroon to dark; salmon-colored, pink to orange sporulation appeared on the lesions, and in some cases, white mycelia formed from the inicial lesion, until it covered the fruits. These results coincide with those reported by Somashekhara et al. (2013).
Incidence percentages obtained ranged from 20 to 100% from Colletotrichum spp. on the total amount of fruits. A higher incidence was observed in fruits from location 2, which was lower than in location 1. Bogantes and Mora (2013) reported a high incidence (70%) but low severity in papaya fruits. The multivariate analysis found an eigenvalue of 6.32 in two clusters or groups. This information was confirmed when reviewing the value of Pseudo F that reached a maximum point of 20.9 for the two highest clusters or groups. On the other hand, the results of the severity evaluation showed that it was higher in allspice fruits from location2, and to a lesser extente, in locations 1 and 3 (Pr>F: <.0001, α:0.05, LSD: 6.9888; difference in averages of 35.644 A in location 2 against 5.604 B in location 1 ) (Figure 2). In this way, high percentages for severity were also obtaine in inoculations, and a null percentage was observed in inoculations with water (Pr>F: <.0001, α: 0.05; LSD: 13.778; difference in averages of 16.773 A for suspension against 0.000 B in inoculation with water). The severity of each isolation in fruits from location 2 was of 100% (Pr>F: 0.0094, α: 0.05 y LSD: 20.636), invading the fruits completely, with abundant sporulation, and in some, white to gray mycelia developed. Similar results were obtained by Bogantes and Mora (2013), obtaining a percentage of 100% for papaya fruits affected by anthracnose, with an average of 36% for severity in fruits.

Figure 2 Colletotrichum spp. isolations that cause the highest degree of severity in allspice ftuirs.
The reisolations obtained from the inoculated fruits displayed symptoms and signs with similar macro and micro morphological characteristics to those of the original isolations, complying with Koch’s 4° postulate in a satisfactory manner.
The ITS region of the thirteen isolations obtained from allspice fruits was amplified, and this helped compare and determine the fungus species. The results obtained from the GenBank with the Blast tool of the amplified sequences revealed that the 13 existing isolations were divided into four species of the genus Colletotrichum: C. acutatum, C. fragariae, C. gloeosporioides and C. boninense. Using the sequences, we created a phylogenetic tree (Figure 3), which shows the clustering of associates species. According to the similar characteristics of the isolations, three groups can be distinguished: the group with the highest number of isolations is composed of the species C. gloeosporioides, and within this set with analogous characteristcs is C. fragariae; the second group differs by a lower proportion of the largest set and includes the species C. boninense; and the last group that desynchronizes molecularly and morphologically from the other isolations includes the species C. acutatum.
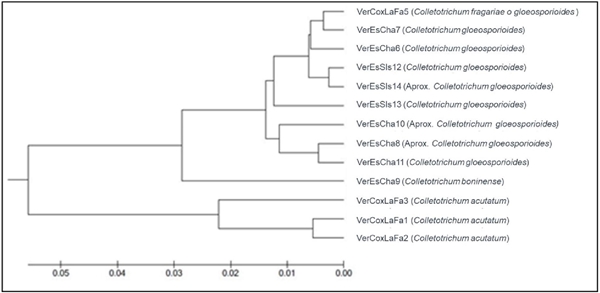
Figure 3 Phylogenetic tree for the 13 Colletotrichum spp. isolations obtained from allspice fruits in Northern Veracruz.
The use of molecular techniques for this investigation was of great importance, since the species belonging to this genus show a great genetic variability, which is expressed in morphological variability, virulence, and aggressiveness (Freeman et al., 2000).
Thirteen monospore isolations were obtained from Colletotrichum spp., from allspice fruits taken from three locations. Uisng morphological characterizations, we identified C. acutatum (23%), a species of slow growth, with rounded and sharp edges, and C. gloeosporioides (77%), a pathogen of fast growth, with conidia with rounded edges; the variable of sexual state of phase was found in an isolation. In pathogenic characterization, we found a variation in the aggressiveness between Colletotrichum isolations on allspice fruits, finding a higher number of aggressive isolations in fruits in the location of Chapultepec, Espinal, Ver., which helped affirm that, at least for this investigation, pathogenicity does depend in the location from which the isolation is taken. Out of the two methods of inoculation (w/l and w/o/l), the best results were obtained using the method w/l, which showed a higher incidence and severity of Colletotrichum in the fruits; controls displayed no symptoms. Molecular identification confirmed the results of morphological characterization and showed the presence of two additional species, therefore, out of the 13 isolations, three belonged to C. acutatum, one belonged to the species C. fragariae, eight belong to the species C. gloeosporioides, and one to C. boninense. The molecular results coincided with a high percentage of similarity (97 to 99%) with the species reported in the GenBank for other hosts.
LITERATURA CITADA
Agrios GN. 2002. Fitopatología. Second Edition. Limusa. México. 837p. [ Links ]
Anculle A y Álvarez R. 1999. Evaluación de enfermedades de plantas. Estudio Arequipa, Perú. Disponible en línea: https://es.scribd.com/document/151857253/Evaluacion-de-Enfermedades-de-Las-Plantas [ Links ]
Anculle A y Álvarez R. 2006. Evaluación de enfermedades de plantas. Arequipa, Perú: Senasa. Disponible en línea: http://www.bioline.org.br/pdf?cg09021 [ Links ]
Andrades I, Yender F and Labarc J. 2009. Evaluation of anthracnose (Colletotrichum sp) in Annona muricata L. Giant type in the sector Moralito, Zulia State, Venezuela. Revista UDO Agricola 9:148-157. Disponible en línea: http://udoagricola.orgfree.com/V9N1UDOAg/V9N1Andrades148.htm [ Links ]
Bailey JA, Nash C, Morgan LW, O´Connell RJ and TeBeest DO. 1996. Molecular Taxonomy of Colletotrichum species causing Anthracnose on the Malvaceae. Phytopathology 86:1076-1083. http://dx.doi.org/10.1094/Phyto-86-1076 [ Links ]
Barnett H. and Hunter B. 1998. Illustrated Genera of Imperfect Fungi. Fourth Edition. APS Press. Minnesota, USA. 197p. [ Links ]
Bogantes A y Mora N. 2013. Incidencia y Severidad de la Antracnosis en líneas e híbridos de papaya (Carica papaya). Agronomía Mesoamericana 24:411-417. Disponible en línea: http://www.scielo.sa.cr/scielo.php?pid=S165913212013000200017&script=sci_arttext&tlng=en#Corres1 [ Links ]
Carballo PI. 2016. Apoya IPN a productores de pimienta de Papantla. El Diario Martinense. Disponible en línea: http://diarioelmartinense.com.mx/estado/regional/36165-apoya-ipn-a-productores-de-pimienta-de-papantla.html [ Links ]
Chowdappa P, Somashekar C, Bharghavi R, Sandhya H and Prasad P. 2012. Morphological and molecular characterization of Colletotrichum gloeosporioides (Penz) Sac. Isolates causing anthracnose of orchids in India. Biotechnology Bioinformation Bioengeening 2:567-572. Disponible en línea: http://bioscipub.com/journals/bbb/pdf/567-572.pdf [ Links ]
Damm U, Cannon PF, Woudenberg JHC, Johnston PR, Weir BS, Tan YP and Crous PW. 2012b. The Colletotrichum boninense species complex. Studies in Mycology 73:1-36. http://dx.doi.org/10.3114/sim0002 [ Links ]
Dominguez GI, Mohali CSR, Marin MMA y Pino MHB. 2012. Caracterización y variabilidad genética de Colletotrichum gloeosporioides sensu lato en plantaciones de palma aceitera (Elaeis guineensis Jacq.) en Venezuela. Tropical Plant Pathology 37:108-122. Disponible en línea: http://www.scielo.br/scielo.php?pid=S1982-56762012000200003&script=sci_abstract&tlng=es [ Links ]
Echandi E. 1967. Obtención de cultivos monospóricos por los métodos de dilución y rayado. Pág. 16. In: Echandi E (eds.). Manual de laboratorio para Fitopatología General. Turrialva. 51p. Disponible en línea: https://books.google.com.mx/books?id=9I0gAQAAIAAJ&pg=PA16&lpg=PA16&dq=Obtenci%C3%B3n+de+cultivos+monosp%C3%B3ricos+por+los+m%C3%A9todos+de+diluci%C3%B3n+y+rayado&source=bl&ots=Q0FKvqrC7w&sig=5BjxvuFLWKYUuvmpl6aOXSnSTb4&hl=es&sa=X&ved=0ahUKEwi6opKeoMPTAhWC [ Links ]
Freeman S, Katan T and Shabi E. 1998. Characterization of Colletotrichum species resposible for Anthracnose diseases of various fruits. Plant Disease 82:596-604. http://dx.doi.org/10.1094/PDIS.1998.82.6.596 [ Links ]
Freeman S, Minz D, Jurkevitch E, Maymos M and Shabi E. 2000. Molecular analyses of Colletotrichum species from almond and other fruits. Phytopathology 90:608-614. http://doi.org/10.1094/PHYTO.2000.90.6.608 [ Links ]
Morales GJL, Azpìroz RHS y Pedraza SME. 2009. Caracterización cultural, morfológica, patogénica e isoenzimatica de aislados de Colletotrichum gloeosporioides Penz. Causante de la antracnosis en aguacate (Persea americana Mill) en Michoacán, México. Revista UDO Agrícola 9:848-856. Disponible en línea: https://dialnet.unirioja.es/servlet/articulo?codigo=3394147 [ Links ]
Oliveira R, Moral J, Bouhmidi K. y Trapero A. 2005. Caracterización morfológica y cultural de aislados de Colletotrichum spp. causantes de la antracnosis del olivo. Boletín de sanidad vegetal 31:531-548. Disponible en línea: http://helvia.uco.es/xmlui/handle/10396/2420 [ Links ]
Rivera G. 2007. Conceptos Introductorios a la Fitopatología (1 ed.). EUNED, ed.) Costa Rica. Disponible en línea: https://books.google.com/books?id=xpTHXEWG_t8C&pg=PA6&lpg=PA6&dq=Conceptos+Introductorios+a+la+Fitopatolog%C3%ADa&source=bl&ots=OQNO_7ozWd&sig=SjX_YMlwt5cJucIh8oKsQBUrA34&hl=es-419&sa=X&ved=0ahUKEwjlgfiAh7rLAhXq74MKHb8mBkQQ6AEIITAB#v=onepage&q&f=false [ Links ]
Robles L. 2015. Caracterización morfológica, molecular, patogenicidad cruzada y Resistencia a productos químicos en aislados de Colletotrichum spp. obtenidos de frutos de aguacate a nivel nacional. Colegio de Postgraduados, Campus Montecillos. Fitosanidad-Fitopatología, Texcoco de Mora, Edo. México. Tesis Doctoral. Pp. 167. [ Links ]
Saldarriaga CA, Castaño Z y Arango I. 2008. Caracterización del agente causante de la antracnosis en tomate de árbol manzano y mora. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 32:145-156. Disponible en línea: https://www.researchgate.net/publication/257652278_Caracterizacion_del_Agente_Causal_de_la_Antracnosis_en_Tomate_de_Arbol_Manzano_y_Mora [ Links ]
Somashekhara A, Vasanthakumari M, Mahishi P, Mallikarjunaswamy G and Shivanna M. 2013. Prevalence and Severity of Anthracnose of Yam (Dioscorea alata and D. bulbifera) caused by Colletotrichum gloeosporioides in Bhadra Wildlife Sanctuary in Karnataka. Journal Mycology Plant Pathology 43:282-290. Disponible en línea: https://www.researchgate.net/publication/256838587 [ Links ]
Villanueva A, Yañez MJ y Hernandez AM, 2008. Especies de Colletotrichum en Chirimolla (Annona cherimola Mill.). Agrociencia 42:689-701. Disponible en línea: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952008000600009 [ Links ]
Wagner D, Furnier G, Saghay-Maroof M, Williams S, Dancik B and Allard R. 1987. Chloroplast DNA polymorphisms in lodgepole and jack pines and their hybrids. Proceedings of the National Academy of Science USA (pp. 2097-2100). Disponible en línea: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC304592/ [ Links ]
Received: November 03, 2017; Accepted: April 29, 2018











 texto em
texto em 


