Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.36 n.2 Texcoco May./Aug. 2018
https://doi.org/10.18781/r.mex.fit.1801-1
Phytopathological notes
Organisms associated with damage to post-harvest potato tubers
1Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Unidad Integral de Diagnóstico, Servicios y Constatación, Carretera Federal México-Pachuca Km 37.5, CP. 55740, Tecámac, Estado de México
2Universidad Autónoma de Chihuahua, Facultad de Zootecnia y Ecología, CP. 31000, Chihuahua, México.
The potato (Solanum tuberosum L.) is one of the crops with the highest human consumption worldwide. The crop is threatened from its beginnings by different organisms; some of them cause superficial damage to the tubers, which reduce the quality and appearance and causes rejection by the consumer. Out of a total of 34 potato tuber samples destined for human consumption, obtained through directed visual sampling, at the Central de Abastos de Ecatepec, microorganisms associated with some physical damage or superficial alteration were identified; in addition, of atypical spots on the surface of tubers. Of the total samples, in the 50% were identified bacterial damages caused by Enterobacter aerogenes, Pseudomonas fluorescens and Streptomyces sp. In the 41% were identified fungi and protozoa like Fusarium oxysporum, F. solani, F. verticillioides, Rhizoctonia solani, Alternaria sp., Colletotrichum sp., Clonostachys sp., Geotrichum sp., and Spongospora subterranea f. sp. subterranea. 9% of the remaining samples, no organism related to the damage was identified.
Key words: Solanum tuberosum; microorganisms; fungi; bacteria
La papa (Solanum tuberosum L.) es uno de los cultivos de mayor consumo humano a nivel mundial. El cultivo es amenazado desde sus inicios por distintos organismos; algunos de ellos ocasionan daños superficiales en los tubérculos, que reducen la calidad y apariencia lo que provoca el rechazo por el consumidor. De un total de 34 muestras de tubérculos de papa destinados para consumo humano, obtenidas mediante muestreo visual dirigido, en la Central de Abastos de Ecatepec, se identificaron a los microorganismos asociados a algún daño físico o alteración superficial; además, de manchas atípicas en la superficie de tubérculos. Del total de las muestras en el 50% se identificaron a las bacterias Enterobacter aerogenes, Pseudomonas fluorescens y Streptomyces sp. En el 41% se identificaron a hongos y protozoarios como Fusarium oxysporum, F. solani, F. verticillioides, Rhizoctonia solani, Alternaria sp., Colletotrichum sp., Clonostachys sp., Geotrichum sp., y Spongospora subterranea f. sp. subterranea. Del 9% de las muestras restantes no se identificó ningún organismo relacionado al daño.
Palabras clave: Solanum tuberosum; microorganismos; hongos; bacterias
The potato (Solanum tuberosum L.) is one of the crops with the highest human consumption worldwide. Every year, 381 million tons of this crop are produced worldwide (FAO, 2014). In Mexico, it is number five in terms of food importance, after maize, wheat, beans and rice. Out of the total of the country’s production, 56% is used for consuming fresh, 29% for industrial use, and 15%, as a seed (Mora-Aguilar, 2014). Worldwide, roughly 70 diseases and physiological disorders have been reported to affect this crop and cause severe damages, particularly in tubers (Herrera and Scott, 1993; Stevenson, 2001). Some of the main symptoms of the diseases that affect tubers are root nodules, spots, and rotting (Fiers et al., 2012), which may be caused by fungi, bacteria, nematodes and viruses. There are also other factors that, aside from the physical appearance, can downgrade the quality of the tubers, leading to rejection from consumers; however, Fiers et al. (2010) mentioned that surface spots only affect the epidermis of the tubers, and do not alter their taste or nutritional properties (Jemison et al., 2008; Vázquez-Carrillo et al., 2013). Some of these surface alterations that affect the peridermis of the tubers are a result of the presence of pathogens such as Colletotrichum coccodes, Helminthosporium solani, Rhizoctonia solani, Spongospora f. sp subterranea and Streptomyces spp., as well as, in many cases, a means of entry for opportunist microorganisms that lead to the rotting of tubers. Fungi species such as Fusarium sp. frequently damage relevant damages to tubers on fields and in storage worldwide; in the latter, it may affect up to 60% of the production (Boyd, 1972). Other conditions may also appear that lead to poor quality and appearance. They may be due to mechanical damages, insects, abiotic factors such as humidity and temperature, the use of chemical products, nutritional deficiencies, and other damages of unknown causes, known as atypical spots (Friedmans, 1960; Stevenson et al., 2001; Fiers, 2010; Naerstad et al., 2012). On the other hand, the bacteria and fungi that cause rotting or more severe damage to the peridermis of tubers produce a wide range of enzymes such as pectinases, cellulases, xylanases and proteases, responsible for the maceration of tissues and cell death (Olivieri et al., 2004). Symptoms include dry or soft rotting, discoloring of the tuber and annular rotting, and they are caused by fungi such as Fusarium spp., Verticillium spp.; bacteria such as Pectobacterium carotovorum subsp. carotovorum, Pectobacterium carotovorum subsp. atrosepticum, Ralstonia solanacearum, and others (de Haan et al., 2008; Czajkowski et al., 2011; Fiers et al., 2012; Gashgari and Gherbawy, 2013). Due to the above, the aim of this study was to identify the microorganisms related to the symptoms and damages potato tubers in postharvest, produced for human consumption which can be found in commercial places, such as the Central de Abastos of Ecatepec.
Sampling. Potato tubers destined for human consumption were collected in August, 2017, from the Central de Abastos de Ecatepec, State of Mexico. For this, samples were taken, aimed at those that displayed some physical damage or superficial alteration, as well as atypical spots. Each tuber was wrapped in a paper towel and placed inside polyethylene bags. They were then transported to the Mycology and Bacteriology Lab of the National Plant Health Reference Center. They were classified according to the symptoms and/or typical damages caused by the presence of fungi, bacteria or insects, such as necrosis, dry rotting, spots, corklike lesions, black scabs, obtaining a total of 34 samples, which were processed on the same day for their diagnosis.
Isolation of fungi. Photographs were taken of symptoms or damages from each sample suspected of having fungi and bacteria. Later, 1 cm2 sections were taken from the stains in tubers and the transition from healthy to diseased tissue. They were disinfected with 4% sodium hypochlorite for 1 minute, washed three times with distilled water for 3 minutes each, and dried using sterilized paper towels. They were then placed in a PDA (potato-dextrose-agar) medium in 90 x 15 mm Petri-dishes. On the other hand, in order to promote the growth of microorganisms on the tuber, humidity chambers were performed for each tuber and symptom planted in PDA, two samples were taken from each tuber, and they were disinfested following the method described above. In both cases, incubation was performed at a temperature of 22±2 °C and a photoperiod of 12 hours. After five days, from the dishes that presented fungal culture, we performed monospore culture growth in new dishes with PDA. The identification was carried out using taxonomical codes and morphometric characteristics (Sneh et al., 1991; Barnett and Hunter, 2006; Leslie and Summerell, 2006; Seifert and Gams, 2011).
In the case of tubers with symptoms of pustules and root nodules suspected to have been caused by Spongospora subterranea, the present structures were observed under the microscope, histological cuts were made, and permanent preparations were made for the identification of S. subterranea using taxonomic codes. To confirm the presence of Spongospora subterranea f sp. subterranea a PCR test was carried out using specific Sos1 (5’-CCTGGGTGC-GATTGTCTGTT-3’) and Sps2 (5’-CACGCCAATGGTTAGA-GACG-3’) primers reported by Bell et al. (1999).
Isolation of bacteria. In order to identify bacteria, tubers were used with signs of either rotting or necrosis. Using the same methodology described by the isolation of fungus, we obtained the bacterial strains in King’s B agar; biochemical tests were run on each one, using serological and enzyme techniques (ELISA) (Schaad et al., 2001), as well as pathogenicity tests in potato tubers (Goszczynska et al., 2000).
Out of the total samples analyzed, 50% tested positive for bacteria, 41% for fungi, and 9% tested negative for any organism related to the damage. In regard to fungi, we identified: Fusarium sp. (F. oxysporum, F. solani, F. verticillioides), Rhizoctonia solani, Alternaria sp., Colletotrichum sp., Clonostachys sp., Geotrichum sp., and the protozoa Spongospora subterranea f sp. subterranea (Figures 1 and 2). The species Alternaria, Colletotrichum, Clonostachys, Fusarium, Rhizoctonia, have been related previously to the colonization of the surfaces of tubers (Fiers et al., 210; Naerstad et al., 2012; Gherbawy and Gashgari, 2013; Zimudzi et al., 2017).
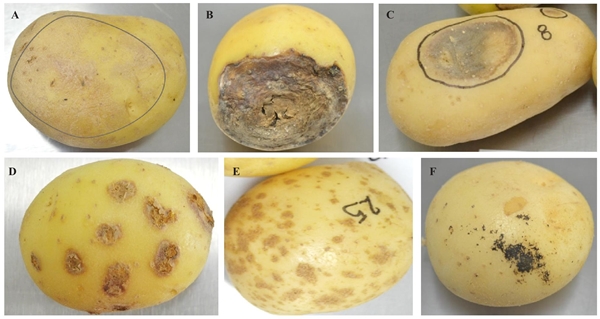
Figure 1 A) Potato tubers with dark surface spots related to Clonostachys sp. and Colletotrichum sp. B) Dry rotting, where the fungi Fusarium sp., Rhizoctonia solani, Alternaria sp., and Geotrichum sp. were identified. C) Sinking of the heart of the tuber. D) Pustules or circular scabs on the surface of the tuber, characteristics caused by Spongospora subterranea. E) Spots or corklike lesions in polyhedral shapes, damages characteristically caused by Streptomyces sp. F) Hard, black scabs, similar to soil, that are sclerotia formed by Rhizoctonia solani on the periderm of the tuber.
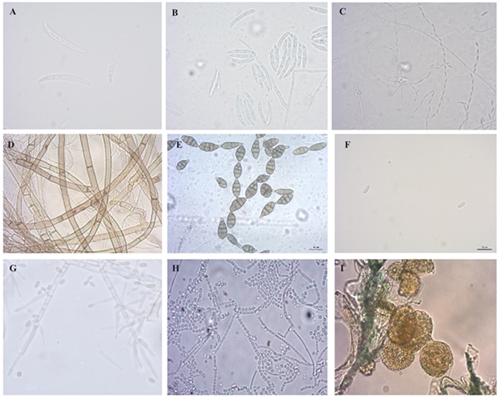
Figure 2 Microorganisms (fungi and protozoa) isolated from potato tubers. A) Fusarium oxysporum macroconidia. B) Fusarium solani macroconidia. C) Fusarium verticillioides chain conidia. D) Rhizoctonia solani hyphae. E) Alternaria sp., conidia. F) Colletotrichum sp., conidia. G) Clonostachys sp., phialides and conidia. H) Geotrichum sp., arthroconida I) Spongospora subterranea f. sp. subterranea sporosori.
On the other hand, in the tubers that displayed symptoms of necrosis or rotting, we identified Enterobacter aerogenes and Pseudomonas fluorescens (Figure 3). Likewise, the bacteria Streptomyces sp., was found in 11.7% of the tubers and only defined at the genus level. This bacteria is very commonly reported in potato tubers, and produces severe symptoms such as irregular, brown corklike lesions, or lesions in polyhedral shapes that can connect and give the tuber a poor appearance and quality (Fiers, 2010; Fiers et al., 2010).
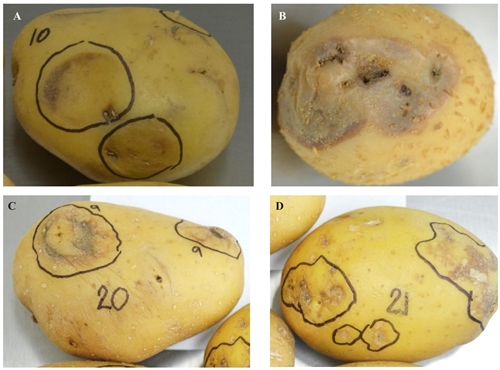
Figure 3 Potato tubers with symptoms of necrosis in the peridermis of the tuber; in all cases, the bacteria Enterobacter aerogenes and Pseudomonas fluorescens were identified.
Potato tubers can display different surface damages that downgrade their commercial value. Some are caused by pathogens or insects, or by abiotic factors or another series of alterations of unknown causes, that is, physiological disorders. Fiers et al. (2010), reported Fusarium spp., Rhizoctonia spp., Penicillium spp., Alternaria spp., Clonostachys spp. as the most common genera that colonize the surface of potato tubers in storage conditions. Likewise, Gherbawy and Gashgari (2013), identified Fusarium, Penicillium, Ilyonectria, Alternaria, and Rhizoctonia, in a study, as the most common genera isolated in different types of symptoms in potato tuber spots. On the other hand, Naerstad et al. (2012) pointed out that the pathogens that most commonly produce spots in tubers, and reduce yield and quality, are Rhizoctonia solani, Spongospora subterranea f. sp. subterranea, Helminthosporium solani, Colletotrichum coccodes, Fusarium sp. and Streptomyces sp. Regarding Fusarium sp., the results obtained in this investigation coincide with reports from earlier studies, since it was identified as being one of the genera most frequently isolated from the peridermis of potato tubers (Chelkowski, 1989; Fiers et al., 2010; Gherbawy and Gashgari, 2013; Zimundzi et al., 2017) and the species F. oxysporum has been isolated from most of the cultivars studied (Manici and Cerato, 1994; Zimundzi et al., 2017). Apart from Fusarium spp., other fungi were found to colonize the same spots of the tubers, such as Alternaria sp., Colletotrichum sp., Clonostachys sp., Rhizoctonia solani, and Geotrichum sp. The latter has not been reported to colonize or cause damages to the crop or potato tubers, but is found throughtout the soil, although only one specie is important as a pathogenic agent: Geotrichum candidum, a specie that has been reported as the causaling agent of bitter rotting in post-harvest citrus fruits (Brown 1988; López-García et al., 2003; Talibi et al., 2012), as well as soft rottings in post-harvest strawberry (Fraire-Cordero et al., 2003) and other crops, and therefore, further studies are recommended in order to determine their pathogenic condition. In the case of potato tubers, it has not been related as a pathogenic agent, and could be considered as a pollutant on the surface of the potato tubers in post-harvest or as a suppressor of the pathogenic microorganisms in this study.
The different genera and species of fungi that have been identified as colonizing the surface of the periderm of potato tubers, not only have a pathogenic behavior on the crop, but some genera may have an antagonistic behavior with pathogenic organisms, which have been evaluated to determine their potential as biocontrol agents. The fungus Clonostachys spp., known for its antifungal capability and mycoparasitic action against pathogens, produces a wide range of volatile organic compounds. Studies show that Clonostachys sp. presents antibiosis and an effective colonization of the lesions caused by mechanical damages, avoiding the entry of pathogenic agents (Gan et al., 2007; Assefa, 2013), and limiting the growth of other organisms in the potato tuber (Gan et al., 2007). On the other hand, the species Gliocladium roseum (anamorph: Clonostachys rosea) has been reported as a pathogen in potato and a causing agent of dry rotting (Theron, 1991).
Danyluk et al. (2013), pointed out that the microbiota that dominates in freshly-harvested orchards is composed of bacteria Enterobacter, Bacillus spp., Pantoea spp., Cyanobacterium, Erwinia spp., Pectobacterium and Pseudomonas, resulting from contact with the soil, air and water. In the areas damaged by necrosis or rotting in some of the tubers collected in this investigation, bacteria were identified and we found a marked delimitation that stopped the rot from advancing, which may suggest that the antagonistic bacteria identified as Enterobacter aerogenes and Pseudomonas fluorescens stopped the necrotrophic growth of a pathogenic agent that may have been colonizing the tuber. El-Ghaouth et al. (1998) mentioned that these organisms cause no damage when in contact with plant tissues. It is worth pointing out that different species of Enterobacter spp. and Pseudomonas fluorescens have been reported as biocontrol agents, since they can cure diseases such as dry rotting in potato tubers, caused by Fusarium spp., by producing different antifungal metabolites (Schisler, 1994), and can also reduce the severity of the disease by up to 25% (Chelkowski, 1989; Schisler et al., 1995; Schisler et al., 2000). Pseudomonas fluorescens was also reported as a biocontrol agent for bacteria such as Erwinia carotovora subsp. atroseptica, since it produces the antimicrobial component 2,4-diacetylphloroglucinol (DAPG), which inhibits the growth of this bacteria in in vitro conditions (Cronin et al., 1997).
In 9% of the samples analyzed, no clear relation was established between a microorganism and the damages or symptoms in the tuber; symptoms were identified as brown cavings in the center of the tuber. The main causes of the physiological disorders are a response of the plant to stress, including inadequate cultural practices during planting, including the choice of susceptible cultivars, handling and storage, extreme temperatures, soil pH, humidity levels, and inadequate levels of nutrients (Fiers, 2010; Mikitzel, 2014). Zotarelli et al. (2013), mention that the leaching of nutrients such as nitrate lead to nutritional stress in the plant, leading to physiological alterations, such as the brown center, hollow heart, necrosis due to internal heat, cracking, and others.
The aim of this study was to identify the organisms related to damage in potato tubers. In conclusion, we found a diversity of pathogenic microorganisms colonizing one same tuber, as well as different bacteria known for being antagonistic of pathogenic microorganisms. In this study, the fungus Alternaria sp. was isolated more frequently in the tubers collected. Symptoms of either necrosis or rotting, or both, were found to be consistently associated to the antagonistic bacteria Enterobacter aerogenes and Pseudomonas fluorescens, limiting the advancement of the necrosis in the potato tuber tissue. However, further research is required to understand the interaction of these organisms on the surface of potato tubers.
LITERATURA CITADA
Assefa JT. 2013. Postharvest Biological control of Fusarium dry-rot diseases in potato tubers using Clonostachys rosea strain IK726. Disponible en línea: https://stud.epsilon.slu.se/5248/12/assefa-jima_t_130130.pdf (consulta, octubre 2017). [ Links ]
Barnett HL and Hunter BB. 2006. Illustrated Genera of Imperfect Fungi, 4th. (Ed.), APS Press. The American Phytopathological Society, St. Paul, Minnesota, USA. 218p. [ Links ]
Bell KS, Roberts J, Verrall S, Cullen DW, Williams NA, Harrison JG and Claxton JR. 1999. Detection and quantification of Spongospora subterranea f. sp. subterranea in soils and on tubers using specific PCR primers. European Journal of Plant Pathology 105:905-915. https://doi.org/10.1023/A:1008782309333 [ Links ]
Boyd AEW. 1972. Potato storage diseases. Review of Plant Pathology 51:297-321. Disponible en línea: https://www.cabi.org/isc/FullTextPDF/2006/20063049509.pdf [ Links ]
Brown GE. 1988. Efficacy of guazatine and iminoctadine for control of postharvest decays of oranges. Plant Disease 72:906-908. Disponible en línea: https://www.apsnet.org/publications/PlantDisease/BackIssues/Documents/1988Articles/PlantDisease72n10_906.PDF [ Links ]
Chelkowski J. 1989. Toxinogenic of Fusarium species causing dry rot of potato tubers. Pp 435-440. In: Chelkowski J. (ed.). Fusarium: Mycotoxins, Taxonomy and Pathogenicity. Elsevier Publishing Company, New York, USA. 492 p. https://doi.org/10.1002/food.19900340624 [ Links ]
Cronin D, Moënne-Loccoz Y, Fenton A, Dunne C, Dowling DN and O’gara F. 1997. Ecological interaction of a biocontrol Pseudomonas fluorescens strain producing 2, 4-diacetylphloroglucinol with the soft rot potato pathogen Erwinia carotovora subsp. atroseptica. FEMS Microbiology Ecology 23:95-106. https://doi.org/10.1111/j.1574-6941.1997.tb00394.x [ Links ]
Czajkowski R, Perombelon MC, van Veen JA and van der Wolf JM. 2011. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant pathology 60:999-1013. https://doi.org/10.1111/j.1365-3059.2011.02470.x [ Links ]
Danyluk MD, Fatica MK, Brar PK, McEgan R, Valadez AM, Schneider KR and Trinetta V. 2013. Fruits and Vegetables. Chapter 50. In: Salfinger Y and Tortorello ML. (Eds.). Compendium of methods for the microbiological examination of foods. 5th edition. American Public Health Association (APHA Press). Washington, D.C. USA. 515,533,561pp. https://doi.org/10.2105/MBEF.0222.055 [ Links ]
El-Ghaouth A, Wilson CL and Wisniewski M. 1998. Ultrastructural and cytochemical aspects of the biological control of Botrytis cinerea by Candida saitoana in apple fruit. Phytopathology 88:282-291. https://doi.org/10.1094/PHYTO.1998.88.4.282 [ Links ]
FAO (Organización de las Naciones Unidas para la Alimentación y la Agricultura). 2014. FAOSTAT. Food and agriculture data. Disponible en línea: http://www.fao.org/faostat/es/#data/QC (consulta, octubre 2017). [ Links ]
Fiers M. 2010. Origins of the blemishes of potato tubers: from the soil microbiology to the pedoclimatic environment. Food and Nutrition. Université de Bourgogne, France. 261p. Disponible en línea: https://tel.archives-ouvertes.fr/tel-00572491/document (consulta, octubre 2017). [ Links ]
Fiers M, Chatot C, Edel-Hermann V, Le Hingrat Y, Konate AY, Gautheron N and Steinberg C. 2010. Diversity of microorganisms associated with atypical superficial blemishes of potato tubers and pathogenicity assessment. European Journal of Plant Pathology 128:353-371. https://doi.org/10.1007/s10658-010-9657-2 [ Links ]
Fiers M, Edel-Hermann V, Chatot C, Le Hingrat Y, Alabouvette C and Steinberg C. 2012. Potato soil-borne diseases. A review. Agronomy for Sustainable Development 32:93-132. https://doi.org/10.1007/s13593-011-0035-z [ Links ]
Friedmans BA. 1960. Market diseases of fresh fruits and vegetables. Economic Botany 14:145-156. https://doi.org/10.1007/BF02860016 [ Links ]
Fraire-Cordero MDL, Yáñez-Morales MDJ, Nieto-Angel D y Vázquez-Gálvez G. 2003. Hongos patógenos en fruto de fresa (Fragaria x ananassa Duch.) en postcosecha. Revista Mexicana de Fitopatología 21:285-291. Disponible en línea: http://www.redalyc.org/html/612/61221307 [ Links ]
Gan Z, Yang J, Tao N, Liang L, Mi Q, Li J and Zhang KQ. 2007. Cloning of the gene Lecanicillium psalliotae chitinase Lpchi1 and identification of its potential role in the biocontrol of root-knot nematode Meloidogyne incognita. Applied Microbiology and Biotechnology 76:1309-1317. https://doi.org/10.1007/s00253-007-1111-9 [ Links ]
Gashgari RM and Gherbawy YA. 2013. Pathogenicity of some Fusarium species associated with superficial blemishes of potato tubers. Polish Journal of Microbiology 62:59-66. [ Links ]
Gherbawy YA and Gashgari RM . 2013. Mycobiota associated with superficial blemishes of potato tubers. Food Biotechnology 27:137-151. https://doi.org/10.1080/08905436.2013.781947 [ Links ]
Goszczynska T, Serfontein JJ and Serfontein S. 2000. Introduction to practical phytobacteriology; a manual for phytobacteriology by SAFRINET, SDC Switzerland. 83p. Disponible en línea: https://www.researchgate.net/publication/237021880_Introduction_to_Practical _Phytobacteriology_A_manual_for_phytobacteriology [ Links ]
de Haan EG, Dekker-Nooren TC, van den Bovenkamp GW, Speksnijder AG, van der Zouwen PS and van der Wolf JM. 2008. Pectobacterium carotovorum subsp. carotovorum can cause potato blackleg in temperate climates. European Journal of Plant Pathology 122:561. https://doi.org/10.1007/s10658-008-9325-y [ Links ]
Herrera JE and Scott GJ. 1993. Factores limitantes a la producción y usos de la papa: resultados de la encuesta a los programas nacionales de América Latina. Revista Latinoamericana de la papa 5:122-134. Disponible en línea: http://www.papaslatinas.org/ojs/index.php/rev-alap/article/viewFile/63/65 [ Links ]
Jemison Jr JM, Sexton P and Camire ME. 2008. Factors influencing consumer preference of fresh potato varieties in Maine. American Journal of Potato Research 85:140-149. https://doi.org/10.1007/s12230-008-9017-3 [ Links ]
Leslie JF and Summerell BA. 2006. The Fusarium laboratory manual. Blackwell Publishing Professional, Iowa, USA. 388p. Disponible en línea: http://onlinelibrary.wiley.com/doi/10.1002/9780470278376.fmatter/pdf (consulta, octubre 2017). [ Links ]
López-Garcı́a B, Veyrat A, Pérez-Payá E, González-Candelas L and Marcos JF. 2003. Comparison of the activity of antifungal hexapeptides and the fungicides thiabendazole and imazalil against postharvest fungal pathogens. International Journal of Food Microbiology 89:163-170. https://doi.org/10.1016/S0168-1605(03)00118-1 [ Links ]
Manici LM and Cerato C. 1994. Pathogenecity of Fusarium oxysporum f. sp. tuberosi isolates from tubers and potato plants. Potato Research 37:129-134. https://doi.org/10.1007/BF02358713 [ Links ]
Mikitzel L. 2014. Tuber physiological disorders. Chapter 14. Pp: 237. In: Navarre R, Pavek MJ. (eds.). The Potato: Botany, Production and uses. CABI, USA. 382p. http://doi.org/10.1079/9781780642802.0000 [ Links ]
Mora-Aguilar R. 2014. Consumo y mercado de la papa en México. XXVI Congreso bienal de la Asociación Latinoamericana de la Papa (ALAP). Disponible en línea: https://consumoymercadodepapa.wordpress.com/2014/11/28/consumo-y-mercadeo-de-la-papa-en-mxico/ (consulta, abril 2018). [ Links ]
Naerstad R, Dees MW, Le VH, Holgado R and Hermansen A. 2012. Occurrence of skin blemish diseases (scab and scurf) in Norwegian potato production. Potato Research 55:225-239. http://doi.org/10.1007/s11540-012-9221-x [ Links ]
Olivieri FP, Maldonado S, Tonon CV and Casalongue CA. 2004. Hydrolytic activities of Fusarium solani and Fusarium solani f. sp eumartii Associated with the Infection Process of Potato Tubers. Journal of Phytopathology 152:337-344. http://doi.org/10.1111/j.1439-0434.2004.00851.x [ Links ]
Schisler DA. 1994. Selection and performance of bacterial strains for biologically controlling Fusarium dry rot of potatoes incited by Gibberella pulicaris. Plant Disease 78:251-255. https://doi.org/10.1094/PD-78-0251 [ Links ]
Schisler DA, Kurtzman CP, Bothast RJ and Slininger PJ. 1995. Evaluation of yeasts for biological control of Fusarium dry rot of potatoes. American Journal of Potato Research, 72:339-353. https://doi.org/10.1007/BF02849331 [ Links ]
Schisler DA, Slininger PJ, Kleinkopf G, Bothast RJ and Ostrowski RC. 2000. Biological control of Fusarium dry rot of potato tubers under commercial storage conditions. American Journal of Potato Research 77:29-40. https://doi.org/10.1007/BF02853659 [ Links ]
Schaad NW, Jones JB and Chum W. 2001. Laboratory Guide for Identification of Plant Pathogenic Bacteria. 3rd Ed. APS Press, St. Paul, MN, USA 398p. [ Links ]
Seifert KA and Gams W. 2011. The genera of Hyphomycetes-2011 update. Persoonia 27:119-129. http://dx.doi.org/10.3767/003158511X617435 [ Links ]
Sneh B, Burpee L and Ogoshi A. 1991. Identification of Rhizoctonia species. 3rd print. APS press. St Paul, Minnesota, USA. 133p. [ Links ]
Stevenson WR, Loria R, Franc GD and Weingartner DP. 2001. Compendium of potato diseases. Second Edition. The American Phytopathological Society Press. St. Paul, MN, USA. 144p. http://doi.org/10.1046/j.1365-3059.2002.06934.x [ Links ]
Theron DJ. 1991. Dry rot of potatoes caused by Gliocladium roseum. Plant Pathology 40:302-305. http://doi.org/10.1111/j.1365-3059.1991.tb02380.x [ Links ]
Talibi I, Askarne L, Boubaker H, Boudyac, EH, Msanda F, Saadi B, Ait Ben and Aoumar A. 2012. Antifungal activity of Moroccan medicinal plants against citrus sour rot agent Geotrichum candidum. Letters in Applied Microbiology 55:155-161. http://doi.org/10.1111/j.1472-765X.2012.03273.x [ Links ]
Vázquez-Carrillo MG, Rubio-Cobarruvias OA, Salinas-Moreno y Santiago-Ramos D. 2013. Usos alternativos de la papa en el Estado de México Disponible en línea: https://www.researchgate.net/profile/David_Santiago-Ramos/publication/260437185_Usos_alternativos_de_la_papa_en_el_Estado_de_Mexico/links/004635315013a361e4000000/Usos-alternativos-de-la-papa-en-el-Estado-de-Mexico.pdf (consulta, octubre 2017). [ Links ]
Zimudzi J, Coutinho TA and Van der Waals JE. 2017. Pathogenicity of Fungi Isolated from Atypical Skin Blemishes on Potatoes in South Africa and Zimbabwe. Potato Research 60:119-144. https://doi.org/10.1007/s11540-017-9345-0 [ Links ]
Zotarelli L, Reyes-Cabrera JE, Worthington CM, Hutchinson C, Byrd S, Gergela D y Rowland DL. 2013. Trastornos fisiológicos de la papa-Necrosis por calor interno. Departamento del Ciencias para la Horticultura, Servicio de Extensión Cooperativa de la Florida, Instituto de Alimentos y Ciencias Agrícolas, Universidad de la Florida. (UF/IFAS). 4p. Disponible en línea: https://edis.ifas.ufl.edu/pdffiles/HS/HS122100.pdf (consulta, octubre 2017). [ Links ]
Received: January 09, 2018; Accepted: April 16, 2018











 text in
text in 


