Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.36 n.2 Texcoco May./Aug. 2018
https://doi.org/10.18781/r.mex.fit.1712-5
Phytopathological notes
Phytophthora capsici and P. drechsleri mating types A1 and A2 coexist in ornamental nursery plants
1Instituto de Investigaciones Agropecuarias y Forestales, Universidad Michoacana de San Nicolás de Hidalgo, km 9.5 Carretera Morelia-Zinapécuaro, CP. 58880, Tarímbaro, Michoacán, México
2Programa de Fruticultura, Colegio de Posgraduados, km 36.5 Carretera México-Texcoco, CP. 56230, Montecillo, Texcoco, Estado de México, México
3Facultad de Agrobiología, Universidad Michoacana de San Nicolás de Hidalgo, Paseo Lázaro Cárdenas esquina Berlín, Colonia Viveros, CP. 60090, Uruapan, Michoacán, México;
4Universidad Autónoma del Estado de Morelos, Avenida Universidad 1001, CP. 62209, Cuernavaca, Morelos, México.
The presence of both mating types of Phytophthora species in the same pot has not been reported in hosts of commercial nurseries in Mexico. Isolates of Phytophthora were collected in commercial nurseries of Mexico City and Morelos state during 2015. Mating types A1 and A2 of Phytophthora capsici and P. drechsleri were detected on wilted plants of Capsicum annuum (stems) and Petunia x hybrida (rhizosphere), respectively. Phytophthora isolates coinoculated in planta formed oospores under greenhouse conditions. Phytophthora was reisolated from inoculated plants, and identified with morphological and molecular tools. Mating types A1 and A2 of Phytophthora capsici and P. drechsleri occur simultaneously within nursery plants, this suggests that sexual recombination and genetic variation is occurring. Mating types A1 and A2 of Phytophthora capsici and Phytophtora dechsleri were detected for the first time in Capsicum annuum and Petunia x hybrid respectively, in Mexican nurseries.
Key words: Oomycetes; Oospores; Petunia; Capsicum annuum
No se ha reportado la presencia de ambos tipos de compatibilidad de especies de Phytophthora en plantas hospedantes sembradas en una misma maceta en viveros comerciales de México. En 2015, se colectaron aislados de Phytophthora en viveros comerciales en la Ciudad de México y en el estado de Morelos. Se detectaron los tipos de compatibilidad A1 y A2 de Phytophthora capsici y P. drechsleri en plantas marchitas de Capsicum annuum (tallos) y Petunia x hybrida (rizósfera), respectivamente. Los aislados de Phytophthora co-inoculados in planta formaron oosporas en condiciones de invernadero. Phytophthora fue re-aislada de plantas inoculadas e identificada mediante herramientas morfológicas y moleculares. Los tipos de compatibilidad A1 y A2 de Phytophthora capsici y P. drechsleri se presentan simultáneamente en plantas de invernadero, lo cual sugiere que están ocurriendo recombinación sexual y variación genética. Los tipos de compatibilidad A1 y A2 de Phytophthora capsici y Phytophtora dechsleri fueron detectados por primera vez en Capsicum annuum y Petunia x hybrida respectivamente, en viveros mexicanos.
Palabras clave: Oomicetes; oosporas; Petunia; Capsicum annuum
The ornamental plant Petunia x hybrid and chili pepper plants for home gardens are produced in Morelos state and Mexico City, two of the most important locations that grow ornamental nursery flower pot plants in Mexico (Mundo-Ocampo, 2006). Nevertheless, production is limited by root diseases caused by various Phytophthora species. P. capsici is a highly destructive pathogen with a host range of over 50 plant species including Solanaceous, Cucurbitaceous, Fabaceous and ornamental plants around the world (Lamour et al., 2012; Fernández-Pavía et al., 2013), on the other hand P. drechsleri, has been reported affecting over 25 horticultural and ornamental plant species (Lamour et al., 2012; Fernández-Pavía et al., 2013; Olson et al., 2011; Truong et al., 2012). In Mexico, one of the most important root pathogens is Phytophthora capsici, which is a highly destructive pathogen of chili pepper plants. These Phytophthora species are heterothallic, requiring the presence of opposite mating types (A1 and A2) for oospore formation. The occurrence of mating types of P. capsici and P. drechsleri in the same nursery on different hosts has been detected in previous studies conducted in our laboratory in Mexico (data not published). However, the presence of both mating types of these species in the same host in ornamental nurseries has not been documented. The objective of this study was to determine if both, A1 and A2, mating types of Phytophthora species can be present in the same host, and if oospores are formed in planta under greenhouse controlled conditions.
Phytophthora isolates were obtained from commercial nursery plants of Capsicum annuum and Petunia x hybrid with wilting symptoms in Morelos and Mexico City, Mexico, during 2015. Plant tissue of Capsicum annuum and Petunia x hybrid soil of the rhizosphere from diseased plants with wilt and root rot symptoms were analyzed for pathogen isolation. Plant tissues were rinsed with running water, small diseased tissue fragments were cut, plated out onto NARPH-V8 medium (Delvocid Instant (0.02 g L-1) [(50% natamycin, 50% lactose)], Ampicillin (0.27 g L-1), Rifampicin (0.01 g L-1), PCNB (0.10 g L-1), and Hymexazol (0.075 g L-1) and incubated at 25 °C in the dark. Soil samples of the rhizosphere were subjected to Rhododendron sp. leaves baiting. Briefly, six Rhododendron leaves were floated over flooded soil (10 g of soil:20 mL of sterile water) and incubated for 48 h at 25 °C, the petiole was removed, disinfested with 10% commercial bleach (0.6% sodium hypochlorite) for 30 sec, rinsed with sterile distilled water twice, blotted dry with sterile paper towels, transferred to NARPH-V8 plates, and incubated as above. Phytophthora isolates were identified by examination under a microscope and transferred to corn meal agar media (CMA, 17 g L-1) to obtain pure cultures by the hyphal tip method. Sporangia were produced and morphologically characterized by incubating V8 agar blocks plus mycelia, in sterile distilled water at 25 °C under continuous fluorescent light for three to five days. Mating type of the Phytophthora isolates was determined by pairing them with known A1 and A2 strains of P. capsici and P. cinnamomi on V8 agar at 25 °C in the dark and examined after 20 days. Additionally, opposite mating types of the Phytophthora capsici (A1 and A2) and P. drechsleri (A1 and A2) isolates were paired out. Pairing of opposite mating types in planta was carried out by inoculating Phytophthora isolates on healthy plants of Capsicum annuum and Petunia x hybrid. Inoculum consisted of six mm disks of V8 agar with mycelia and sporangia. One disk of a Phytophthora isolate A2 was placed on the base of the stem, while a second disk of a Phytophthora isolate A1 was placed three cm apart from the first disk. Control plants were inoculated with six mm disks of V8 agar without the pathogen. Plants were flooded for 24 h to favor disease development. Oospore formation in planta was confirmed by observing under a bright field microscope at 40x samples of necrotic stem tissue. Tissues were gently washed in soapy water, rinsed with distilled sterile water, clarified in boiling 96% ethanol for 40 sec, cooled down for 30 min, and repeating the ethanol clarification step for 40 sec, afterwards, the tissue was mounted on a glass slide.
Genomic DNA extraction was obtained by culturing the isolates on 2% clarified liquid V8 at 21 °C for 15 to 20 days, and the protocol by Möller et al., (1992) was followed with modifications as previously described by Robideau et al., (2011). At the final step, DNA pellet was resuspended in 0.1X TE buffer containing 50 μg/mL of RNase A and incubated at 65 °C for 10 min.
The ribosomal DNA ITS (internal transcribed spacer) region was amplified using primers UN-up18S42 (Bakkeren et al., 2000), UN-lo28S1220 (Bala et al., 2010), Oom-up18S67, UN-lo28S22 and ITS4 (Lévesque and De Cock 2004). Partial sequences of the mitochondrial gene COI (cytochrome c oxidase subunit 1) were amplified with primers OomCoxILevup and Fm85mod (Martin and Tooley, 2003), and β-tubulin with primers Oom-Btub-up415 and Oom-Btub-lo1401 (Bilodeau et al., 2007).
PCR amplifications were performed using the following conditions: all reactions had an initial denaturation step at 95 °C for 3 min and a final extension step at 72 °C for 8-10 min. The ITS region amplified with primers Oom-up18S67 and UN-lo28S1220 was performed with 35 cycles of 95 °C for 30 sec, 58 °C for 45 sec, and 72 °C for 2 min. The same region amplified with primers UN-up18S42 and ITS4 utilized 40 cycles of 95 °C for 30 sec, 68 °C for 45 sec, and 72 °C for 1.5 min.
Amplification conditions for COI were the same as those described by Robideau et al., (2011). Amplification of the β-tubulin gene for P. capsici isolates were 40 cycles of 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min. For the P. drechsleri isolates a touch-down PCR was performed under the conditions described by Blair et al., (2008), decreasing the temperature from 68 to 58 °C.
An Applied Biosciences Prism® 3130xl genetic analyzer was used to generate the DNA sequences from the amplification sequencing reactions. The sequences were assembled, edited to obtain consensus sequences, aligned with published sequences (NCBI, National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/), and analyzed using the Blast program (Geneious 8.1, http://www.geneious.com) to determine identity percentages. Sequences were submitted to GenBank.
A multi-locus phylogenetic analysis by maximum likelihood and Bayesian inference method, to confirm isolates identity with ex-type sequences was constructed in the science gateway CIPRES v. 3.3 (Miller et al., 2010).
Two P. drechsleri isolates with opposite mating types were obtained from the rhizosphere soil of a Petunia x hybrid plant. Two P. capsici isolates with opposite mating types were obtained from stem tissues of a C. annuum plant. Morphological and molecular characterization and phylogenetic analysis (Figure 1) confirmed the identity of the isolates. The accession numbers of the isolates are shown in Table 1. All isolates had 99% similarity with sequences available in the database Phytophthora - Q-bank (http://www.q-bank.eu/bioloMICSSequences.aspx?file=Bacteria&file=FUNGI&file=Arthropods&file=Nematodes&file=Phytopl&file=Plants&file=Virus&file=Sequence&wsize=20). Phytophthora capsici LEV6716 (A1) and LEV6717 (A2) and Phytophthora drechsleri LEV6731 (A1) and LEV6731 (A2) isolates were deposited in the Agriculture and Agri-Food Canada culture Collection, Ottawa, Canada.
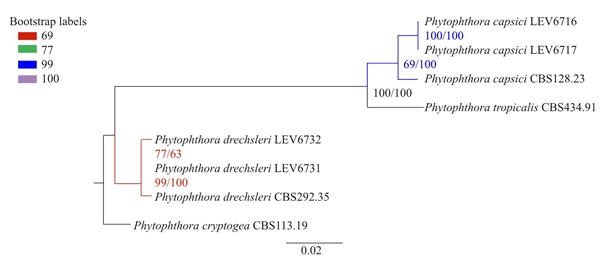
Figure 1 Phylogeny analysis derived from Maximum likelihood and Bayesian inference of concatenated ITS, β-tubulin and COI genes from Phytophthora capsici and P. drechsleri with Phytophthora cryptogea as an outgroup. Maximum likelihood bootstrap values and Bayesian inference posterior probabilities in percentages are indicated in the branch points, respectively. Scale bar indicates 0.02 substitutions per site per branch.
Table 1 Accesion numbers of the genes ITS, b-tubulin and COI, of the isolates.
|
Código aislado |
Especies |
Hospedante o sustrato |
GenBank número de acceso |
||
|
ITS |
β -tubulina |
CO1 |
|||
|
LEV6716 |
P. capsici |
Capsicum annuum |
MH025883 |
MH013476 |
MH013474 |
|
LEV6717 |
P. capsici |
Capsicum annuum |
MH025884 |
MH013477 |
MH013475 |
|
LEV6731 |
P. drechsleri |
Petunia x hybrida |
MH025881 |
MH013478 |
MH013472 |
|
LEV6732 |
P. drechsleri |
Petunia x hybrida |
MH025882 |
MH013479 |
MH013473 |
Capsicum annuum plants inoculated with both mating type isolates of P. capsici showed chlorosis, defoliation, wilting, and necrosis on the stem five days after inoculation (dai). Petunia x hybrid plants inoculated with A1 and A2 mating types of P. drechsleri showed wilt symptoms 10 dai. The isolates were reisolated from the lesions of the plants and identified by morphology confirming Koch’s postulates. Control plants remained healthy. Abundant oospores were observed in the epidermis of stems of C. annuum and Petunia x hybrid inoculated plants (Figures 2 and 3), seven and 10 dai, respectively.
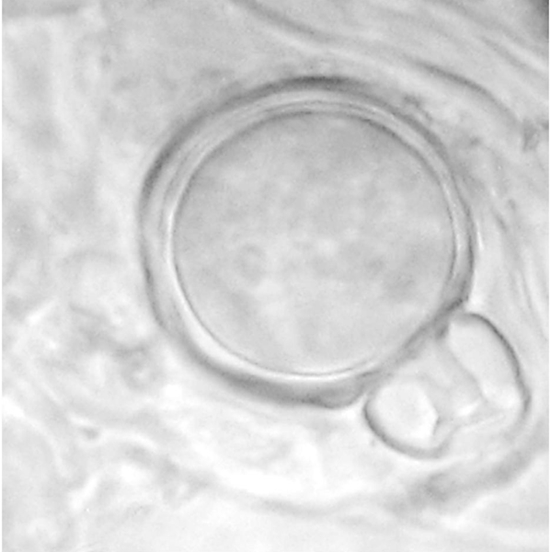
Figure 2 Oospore of Phytophthora capsici formed in planta in Capsicum annuum root tissues coinoculated with mating type A1 and A2 isolates.
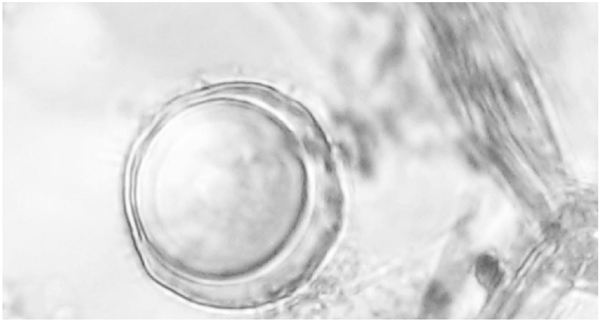
Figure 3 Oospore of Phytophthora drechsleri formed in planta in Petunia x hybrid root tissues coinoculated with mating type A1 and A2 isolates.
The presence of both mating types in isolates of P. capsici and P. drechsleri, in the same plant and rhizosphere, respectively indicates that sexual reproduction and genetic variation is occurring in nursery plants. Oospore formation could serve as primary inoculum (Lehtinen and Hannukkala, 2004), since reutilization of infested soil and pots occurs in nurseries throughout Mexico, contributing to the yearly presence of Phytophthora in nursery plants. Oospores can be long-term resistant structures in nurseries, allowing new host colonization and may introduce new recombinant genotypes of Phytophthora to new areas. Since the commercial nurseries sampled distribute plants to many states in Mexico, the dissemination of these pathogens into new agricultural, forestry and urban areas is highly probable. An additional risk factor is the broad host range of P. capsici and P. drechsleri (Erwin and Ribeiro, 1996; Lamour et al., 2012). Moreover, isolates with resistance to fungicides may develop and the disease will be more difficult to manage. This work showed that both mating types of P. capsici and P. drechsleri can occupy the same niche in nursery environments. Growers should improve management practices as recommended by Parke and Grünwald (2012), according to their economic resources, to reduce losses. This is the first report of mating types A1 and A2 of P. capsici and P. drechsleri detected in commercial nursery pot plants in Mexico.
Acknowledgment
We express our sincere gratitude to C. Andre Lévesque and Tara Rintoul from Agriculture and Agri-Food Canada department for their support. First author thanks to Consejo Nacional de Ciencia y Tecnología (CONACyT) for the doctoral scholarship (no. 254151).
REFERENCES
Bala K, Robideau GP, De´saulniers N, De Cock AWAM and Lévesque CA. 2010. Taxonomy, DNA barcoding and phylogeny of three new species of Pythium from Canada. Persoonia: Molecular Phylogeny and Evolution of Fungi 25:22-31. http://doi.org/10.3767/003158510X524754 [ Links ]
Bakkeren G, Kronstad JW and Lévesque CA. 2000. Comparison of AFLP fingerprints and ITS sequences as phylogenetic markers in Ustilaginomycetes. Mycologia 92:510-521. Disponible en línea: http://www.jstor.org/stable/3761510 [ Links ]
Bilodeau GJ, Lévesque CA, De Cock AWAM, Duchaine C, Brière S, Uribe P, Martin FN and Hamelin RC. 2007. Molecular detection of Phytophthora ramorum by real-time polymerase chain reaction using TaqMan, SYBR Green, and molecular beacons. Phytopathology 97:632-642. https://doi.org/10.1094/PHYTO-97-5-0632 [ Links ]
Blair JE, Coffey MD, Park SY, Geiser DM and Kang S. 2008. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology 45:266-277. https://doi.org/10.1016/j.fgb.2007.10.010 [ Links ]
Erwin DC and Ribeiro OK. 1996. Phytophthora Diseases Worldwide. American Phytopathological Society Press. St. Paul, Minnesota, USA. 562 p. [ Links ]
Fernández-Pavía SP, Díaz-Celaya M and Rodríguez-Alvarado G. 2013. Phytophthora in Mexico. Pp:215-221. In: Lamour K (eds.). Phytophthora: A global perspective. CABI Press. Boston, MA., USA. 244p. [ Links ]
Lamour KH, Stam R, Jupe J and Huitema E. 2012. The oomycete broadhost-range pathogen Phytophthora capsici. Molecular Plant Pathology 13:329-337. http://dx.doi.org/10.1111/j.1364-3703.2011.00754.x [ Links ]
Lehtinen A and Hannukkala A. 2004. Oospores of Phytophthora infestans in soil provide an important new source of primary inoculum in Finland. Agricultural and Food Science 13:399-410. Disponible en línea: http://www.mtt.fi/afs/pdf/mtt-afs-v13n4p399.pdf [ Links ]
Martin FN and Tooley PW. 2003. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia 95:269-284. Disponible en línea: https://www.ncbi.nlm.nih.gov/pubmed/21156613 [ Links ]
Miller MA, Pfeiffer W and Schwartz T. 2010. “Creating the CIPRES Science Gateway for inference of large phylogenetic trees” in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA pp 1-8. DOI: 10.1109/GCE.2010.5676129 [ Links ]
Möller EM, Bahnweg G, Sandermann H and Geiger HH. 1992. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies and infected plant tissues. Nucleic Acids Research 20:6115-6116. Disponible en línea: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC334490/ [ Links ]
Mundo-Ocampo J. 2006. El vivero ornamental. First Edition. Universidad Autónoma del Estado de Morelos. México. 461 p. [ Links ]
Olson HA, Carbone I and Benson DM. 2011. Phylogenetic history of Phytophthora cryptogea and P. drechsleri isolates from floriculture crops in North Carolina greenhouses. Phytopathology 101:1373-1384. https://doi.org/10.1094/PHYTO-11-10-0302 [ Links ]
Parke JL and Grünwald NJ. 2012. A systems approach for management of pests and pathogens of nursery crops. Plant Disease 96:1236-1244. https://doi.org/10.1094/PDIS-11-11-0986-FE [ Links ]
Robideau GP, De Cock AW, Coffey MD, Voglmayr H, Brouwer H, Bala K, Chitty DW, Désaulniers N, Eggertson QA, Gachon CMM, Hu CH, Küpper FC, Rintoul TL, Sarhan E, Verstappen ECP, Zhang Y, Bonants PJ, Ristaino JB and Lévesque CA. 2011. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Molecular Ecology Resources 11:1002-1011. http://dx.doi.org/10.1111/j.1755-0998.2011.03041.x [ Links ]
Truong NV, Burgess L and Liew E. 2012. Cross-infectivity and genetic variation of Phytophthora isolates from chilli and black pepper in Vietnam. Australasian Plant Pathology 41:439-447. Disponible en línea: https://link.springer.com/article/10.1007/s13313-012-0136-4 [ Links ]
Received: December 19, 2017; Accepted: March 02, 2018











 text in
text in 


