Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.36 no.2 Texcoco may./ago. 2018
https://doi.org/10.18781/r.mex.fit.1711-3
Scientific articles
Effect of pH and temperature on the growth and antagonistic activity of Bacillus subtilis on Rhizoctonia solani
1Unidad de Biotecnología e Ingeniería Genética de Plantas, Centro de Investigación y de Estudios Avanzados-IPN, Unidad Irapuato Km. 9.6 Libramiento Norte Carretera Irapuato-León, CP. 36821, Irapuato, Guanajuato, México
2Departamento de Alimentos, División de Ciencias de la Vida. Campus Irapuato-Salamanca. Universidad de Guanajuato, Km9 carretera Irapuato-Silao, CP. 36500, Irapuato, Guanajuato, México
3Facultad de Agricultura y Zootecnia, Universidad Juárez del Estado de Durango, Ej. Venecia, Gómez Palacio, CP. 35000, Durango, México.
In this study, the effects of environmental conditions (pH and temperature) on the growth rate and the inhibitory effect of different strains of Bacillus subtilis on Rhizoctonia solani were evaluated. Strains of B. subtilis were grown in potato infusion broth. The physiological behavior and the growth rate (dN / dt) of the strains were analyzed under different pH and temperature conditions. The strains behaved differently, which allowed establishing optimum and suboptimal growth conditions for each strain. Cell-free supernatants obtained under different growth conditions were used in a quantitative test of in vitro antagonist activity against R. solani. This study showed that the inhibitory effect of the strains occurs mainly in the stationary phase.
Key words : biocontrol; growth rate; antagonism; physiological state
En este estudio, se evaluaron los efectos de las condiciones ambientales (pH y temperatura) sobre la tasa de crecimiento y el efecto inhibitorio de diferentes cepas de Bacillus subtilis sobre Rhizoctonia solani. Las cepas de B. subtilis se cultivaron en caldo de infusión de papa. Se analizaron el comportamiento fisiológico y la tasa de crecimiento (dN / dt) de las cepas bajo diferentes condiciones de pH y temperatura. Las cepas mostraron un comportamiento diferente, lo que permitió establecer las condiciones óptimas y subóptimas de crecimiento para cada cepa. Los sobrenadantes exentos de células obtenidos en diferentes condiciones de crecimiento se usaron en una prueba cuantitativa de actividad antagonista in vitro frente a R. solani. Se demostró que el efecto inhibitorio de las cepas se presenta principalmente en la fase estacionaria.
Palabras clave: biocontrol; tasa de crecimiento; antagonismo; estado fisiológico
The genus Bacillus includes a variety of industrially important species that are commonly used in the fermentation industry (Veith et al., 2004; Rey et al., 2004; Fujinami y Fujisawa, 2010) Bacillus subtilis, as with many in the Bacillus genus, is an extremely common bacterium. It is found in soil, water, air, and decomposing plant matter (Ashlee, et al., 2008.). Bacteria in the Bacillus genus are spore-forming, which means that they create a thick wall which surrounds their DNA and other internal cell structures. In this way, they are hardy and impervious to extreme temperatures, chemicals, environmental factors, even some types of radiation. As a consequence, they can be use in industrial processes. Bacteria are highly responsive to genetic mutation, resulting in experimental uses in a laboratory setting (Ashlee et al., 2008; Schallmey et al.,2004). Like most of its closest relative B. subtilis is non-pathogenic, the relative ease of cultivation and genetic manipulation, and the efficient secretion of proteins and metabolites (Matarante et al., 2004). Furthermore, many gram-positive bacteria that inhabit complex ecological communities, such as those within soil and aquatic environments, produce a great variety of special compounds that are valuable for their pharmacological and antimetabolic properties. Bacillus subtilis is used to produce many antibiotics, such as difficidin, oxydifficidin, subtilosin A, bacillomyin B, and bacitracin, bacilysocin which is helpful in treating bacterial skin infections and preventing infection in minor cuts and burns (Shelburne et al., 2007; Stein 2005; Tamehiro et al., 2002) Bacillus subtilis is also used as a fungicide (Savluchinske et al., 2004). The bacteria colonize the root system, leaving no room for fungal diseases organisms; it is used on agricultural seeds of vegetables, its multicellular behavior and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol agent (Bais et al.,2004; Gong et al., 2006; Leclére et al., 2005; Nagórska et al., 2007). The production of these metabolites may be crucial to develop strategies to adapt themselves to the soil environment occupied by the plant, which contributes to the survival of the organism in complex ecological systems. The genetically diverse bacilli show promising results for the promotion of growth on several crops and the biological control of various plant pathogens (Bapat y Shah 2000; Bernal et al. 2002; Kim 2010). Unfortunately, the inherent variable performance of most biocontrol agents between field locations and cropping seasons has hampered the commercial development of biological agents for use in agriculture (Pal y Gardener, 2006). Most of this variability has been attributed to differences between properties of the natural habitats of biocontrol agents and the places where they are applied (O’Callagman et al., 2001; Zhang et al., 2005). Understanding the environmental factors that regulate the biosynthesis of antimicrobial compounds by strains of Bacillus is an essential step in improving their antagonistic activities, which have not been adequately described (Bloemberg y Lugtenberg, 2001). Physiological studies are necessary to develop and optimize the use of biocontrol agents as plant protectants. It is important to establish the relationship between proliferation and status of the bacteria, as well as the influence of environmental factors that modulate the biosynthesis of antifungal compounds. Other factors that must be considered are the type and concentration of carbon and nitrogen sources, oxygen tension, osmotic pressure, pH, temperature, and water availability (Kamney, 2008). These studies will provide essential information for the selection of new strains and products for the biological control of plant pathogens. In the current study, the effects of pH and temperature on antagonistic activity of different strains of Bacillus subtilis were evaluated, while keeping in mind the physiological stage of development.
MATERIALS AND METHODS
Microbial strains, culture media and conditions
Rhizoctonia solani, was obtained from the ecological biochemistry laboratory collection at Cinvestav-Irapuato. This strain was primarily grown on potato-infusion broth (Johnson y Curl, 1972). The strain BEB-8 and BEB-13 of Bacillus subtilis were isolated from the rhizosphere of a potato field in the state of Guanajuato, México. Putative identification was made using Biolog GP2 MicroPlates (Biolog, Hayward, CA, USA). The microplates were inoculated according to the manufacturer´s instructions. The Bacillus subtilis PY-79 strain (Molecular Bacteriology Laboratory; Cinvestav-Irapuato) was used as a negative control for antagonistic activity; while Bacillus subtilis Kodiak (GBO3) obtained as a dry spore formulation (Bayer Crop Science Kansas City, MO US) was used as a positive control for biological activity. For long-term storage, bacterial cultures were maintained at -80 °C in potato-infusion agar containing 20% glycerol.
Bacterial growth rate
Growth rate studies were carried out in flasks containing 100 mL of potato-infusion broth. Pre-inoculum densities were adjusted to an initial population density of 105 cfu /mL. An inoculum (1% v/v) of the different strains of Bacillus subtilis was added to the liquid medium and incubated at 28 °C, with continuous stirring at 120 rpm; the optimal density was monitored at 535 nm on a 50 Cary Spectronic spectrophotometer (Varian, Australia Pty Lid) every hour for up to 18 h. The bacterial concentration (cfu/ mL) was estimated in plates containing the potato infusion with 1.5% (w/v) agar (BD, Bioxon, Becton-Dickinson), using an Automated Spiral Plater 4000 (Spiral Biotech, Inc. USA). The kinetic parameters and the time of occurrence of the phases of growth were obtained by the Verhulst-Pearl (Slater, 1985) or the logistic model, according to the following equation:
This model employs a non-linear correlation factor statistically calculated by the SYSTAT software (Inc. program for Windows V11.0).
Effect of pH and temperature
To study the influence of pH and temperature on bacterial growth, the pH of potato-infusion broth was adjusted to 5, 6, 7 and 8, with HCl 1M. The cultures initiated with a 1% v/v inoculum were incubated at 15 °C, 28 °C and 37°C with continuous agitation at 120 rpm for 18 h. Bacterial growth was determined as described above. All analyses were carried out in triplicate. The optimal conditions for the maximum growth rate (dN/dtmax) were determined using the statistical program S-Plus 4.0 for Windows.
Effect of the physiological state on antagonistic activity
Under optimal growth conditions (dN/dtmax), potato-infusion broth inoculated with 3% (v/v) of a cellular suspension (No) was incubated at the pre-determined pH and temperature with continuous agitation at 120 rpm for the time required to reach the desired phase of growth. The cells were collected by centrifugation at 10,000 g for 20 min 0 °C (J2-MC Centrifuge Beckman Germany) at all stages of growth. The sterilized (at 115 °C for 15 min) cell-free supernatant (CFS) of each of the treatments was used for the quantitative test of the antagonistic activity. All analyses were carried out in triplicate.
Quantitative test of antagonistic activity
Antimicrobial activity was determined in vitro by measuring inhibition of mycelial growth in potato-infusion-agar medium supplemented with the obtained CFS. Melted Potato-infusion agar was cooled to 45 °C and homogenously supplemented with 3 mL of CFS of each strain culture. A 5 mm diameter disc from an agar culture of the Rhizotonia solani belonging to the AG3 anastomosis group (donated by Dr. Gil Virgen of the University of Guadalajara) was inoculated at the center of Petri dishes containing the different supplemented media. Plates were incubated at 28 °C and fungi growth was measured at 24, 48, 72 and 96 h after start incubation. Each CFS was done in triplicate. The fungal growth observed on non-supplemented medium for 96 h was used as a control. Growth inhibition (%) was calculated as the ratio of mean diameter of fungal colony in medium with CFS / mean diameter of fungal colony in medium without CFS multiplied by 100 and the result was subtracted to 100.
AFLP analysis
The AFLP protocol used was similar to that reported by Vos et al. (1995), with the exception that two, rather than three selective nucleotides were used in order to generate an adequate number of bands for analysis. Total bacterial genomic DNA was digested with the restriction enzymes EcoRI and MseI, or the Tru 91 isoschizomer. The oligonucleotide primers used for the pre-amplification step were 5´-AGCTGCGTACCAATTC/A-3´ and 5´GACGATGAGTCCTGAGTAA/A-3´. The pre-amplification step was followed by a second selective amplification using similar primers but with two selective nucleotides. The EcoRI+AG primer was combined with the MseI primer +AA, AC, AG and AT. The EcoRI primer used in the second amplification reaction was radioactively labeled by using T4 kinase. AFLP reactions with primers having none or a single selective nucleotide were performed for 20 cycles with the following cycle profile: a 30 s DNA denaturation step at 94°C, a 1 min annealing step at 56°C , and a 1 min extension step at 72°C. AFLP reactions with primers having two selective nucleotides were performed for 36 cycles with the following cycle profile: a 30 s DNA denaturation step at 94°C, a 30 s annealing step (see below), and a 1 min extension step at 72°C. The annealing temperature in the first cycle was 65 °C, was subsequently reduced each cycle by 0.7°C for the next 12 cycles, and was continued at 56°C for the remaining 23 cycles. The amplification products of the second reaction were analyzed by electrophoresis on a polyacrylamide sequencing gel and visualized via autoradiography. Electrophoresis was performed at constant power, 110 W, for -2 h. Finally, the gel was analyzed in a Li-Cor Automatas sequencer mod. 4200 (Li-Cor Lincoln, Nebraska), the bands were captured and scored by use of LI-COR software.
Statistical Analyses
The design of the treatments was completely random. Data were subjected to ANOVA by using the version 2.5 FAUANL statistical software, for Microsoft Windows (University of Nuevo Leon, Mexico). The significance of the treatments was determined by the magnitude of the F value (P < 0.05). For the differences among treatment means, the least significant difference (LSD) test was applied to obtain 95% simultaneous confidence intervals.
RESULTS
The AFP analyses based on clustering of dice-similarity coefficients (Figure 1) revealed three genetic groups of bacilli. Group I includes B subtilis Kodiak and LS213 B. amyloliquefaciens, group II includes BEB-13 and PY-79 B subtilis; and group III includes BEB-8 B subtilis. The physiological characterization of these bacteria confirmed that they are different strains. According the Biolog database (Biolog System equipment), the microplate for the BEB-8 isolated showed the highest similarity SIM values (0.786) with B. subtilis providing a typical metabolic profile response for this strain (data not shown). The pH and temperature effects on the rates of growth (dN/dt) for the different strains are shown in Figure 2. The surface areas represent the growth as a function of pH and temperature. ANOVA analysis indicated that temperature, pH, and the interaction between the two factors were highly significant for the growth rate (dN/dt) of the PY-79 strain (Figure 2a). Based on this, two optimal growth conditions were determined to be 28 °C/pH 5 and 28 °C/pH 8 (Table 1A-II), which produced the maximum rates of growth of 2.19x105 and 2.36 x 105 cfu/ mL/ h respectively (Table 1A-III). For the Kodiak strain, the temperature and its interaction with pH were significant to produce the maximum rate of growth (Figure 2b). This maximum was observed at 28 °C to 5 pH units (Table 2A-II), showing no statistically significant differences in the values of the dN/dt in this range (Table 2A-III). In contrast, the BEB-8bs growth rate was strongly influenced by pH, but temperature and its interaction with pH showed no differences significance (Figure 2c). Optimal conditions of growth were obtained at pH 5 and 8 independently of the incubation temperature, reaching a maximum dN/dt value of 3.5 to 4 x 108 cfu /mL/h, respectively. The observed growing patterns were quite different for the different strains of B. subtilis (Table 1A-II and 1A-III).

Figure 1 AFLP fingerprints of the Bacillus strains using the 32-P labeled EcoRI primer E+AG with three MseI primers MseI+AA (I), MseI+AC (II) and MseI+AT (III) (a). Dendogram of different Bacillus strains based on AFLP analysis of the samples with EcoRI+AG and MseI-two primers (b). AFLP fragments were analyzed and dendograms were generated as described in materials and methods. B. subtilis Kodiak (1), B. subtilis PY-79 (2), B. amyloliquefaciens LS213 (3), Pseudomonas sp. (4), B. subtilis BEB-8bs (5), B. subtilis BEB-DN (6)
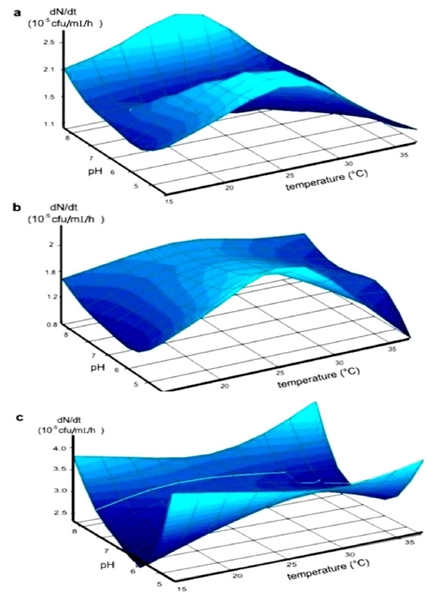
Figure 2 Effect of pH and temperature on the rate of growth (dN/dt) of the Bacillus subtilis strains represented by the surface, which is considered a function of pH and temperature. PY-79 strain negative control of antagonistic activity (a). Kodiak strain; positive control of antagonistic activity (b), BEB-8bs strain (c).
Table 1 Kinetic parameters of growth of Bacillus subtilis strains.
| A. Condiciones óptimas de desarrollo. | ||||||
| Cepa de Bacillus subtilis | I. No | II. Condiciones óptimas de desarrollo | III. Parámetros cinéticos: | |||
| K | μ | dN/dtmax | t dN/dt min | |||
| PY-79 | 2.8x105 | 28°C/pH 5 28°C/pH 8 | 1.97x105 1.88x105 | 0.444 0.498 | 2.19x105 2.36x105 | 4.90 12.21 |
| Kodiak | 1.2x106 | 28°C/pH 5 28°C/pH 6 28°C/pH 7 28°C/pH 8 | 0.804x106 0.806x106 0.752x106 0.816x106 | 0.808 0.779 0.713 0.701 | 1.62x106 1.57x106 1.34x106 1.43x106 | 6.32 6.08 5.65 6.65 |
| BEB-8b | 2.2x108 | 15°C/pH 5 15°C/pH 8 28°C/pH5 28°C/pH 8 37°C/pH 5 37°C/pH 8 | 3.14x108 3.43x108 2.35x108 1.16x108 2.43x108 2.62x108 | 0.535 0.439 0.633 0.608 0.442 0.602 | 4.20x108 3.77x108 3.37x108 3.30x108 3.98x108 3.41x108 | 8.93 9.20 6.36 6.02 5.66 5.95 |
| B. Condiciones subóptimas de desarrollo. | ||||||
| Cepa de Bacillus subtilis | I. No | II. Condiciones óptimas de desarrollo | III. Parámetros cinéticos: | |||
| K | μ | dN/dtmin | t dN/dt min | |||
| PY-79 | 2.8x105 | 37°C/pH 6 | 0.19x105 | 0.156 | 0.77x105 | 11.01 |
| Kodiak | 1.2x106 | 37°C/pH 5 | 0.65x106 | 0.459 | 0.74x106 | 5-78 |
| BEB-8b | 2.2x108 | 15°C/pH 6 | 2.48x108 | 0.319 | 1.19x108 | 8.71 |
xNo; initial inoculum (cfu /mL), K; carrying capacity of media (10n cfu /mL), usually interpreted as the amount of resources expressed in the number of organisms that can be supported by these resources. µ specific speed of growth / h, µ significantly different from µ = 0, to > 95% of confidence level, dN/dt; maximum rate of growth (10n cfu /ml/ h), tdN/dt; time in which the maximum or minimal rate of growth is reached the (h.min). The adjustment of r2 to the logistic model was > 95% of confidence level.
Effect of growth conditions and physiological stage of Bacillus strains on the production of antagonistic metabolites
For each of the strains, the kinetic parameters were determined in the optimal growth conditions (Table 1A) and sub-optimal conditions (Table 1B). Under sub-optimal conditions of growth, a significant decrease in the specific rate of growth (μ) of each strain was observed, which contrast with the conditions in which the rate dN/dt was maximum. Under suboptimal conditions they show faster growth rates than optimal conditions, however, under these conditions there is no inhibition of Rhizoctonia except for the BeB 8 strain. (Figure 3). However, the time required to reach the stationary phase was similar for the Kodiak and Beb-8 strains, in both conditions, while the PY-79 took almost 10 more hours in sub-optimal conditions (Table 1A-III and 1B-III). Nevertheless, the most significant effect was observed in the carrying capacity of media (K). Under sub-optimal growth conditions, a reduction of one logarithmic unit (1.97 to 0.19 x105 cfu /mL/ h) was detected for PY-79 strain, a slight reduction from 0.804 x 106 to 0.65 x 106 cfu /mL/ h corresponded to Kodiak strain, and a reduction from 3.14 x 108 to 2.48 x 108 cfu/ mL/ h for the BEB-8 strain was observed. The effect of the physiological stage of each strain on the antagonistic activity against Rhizoctonia solani AG-3, under optimal and sub-optimal conditions of growth is shown in Figure 3. Under optimal conditions of growth, the BEB-8bs strain showed a similar antagonistic activity to the Kodiak strain (positive control). In the exponential growth phase (log), they both caused 15 to 25% fungal growth inhibition, while in the stationary phase, they caused 64 to 72% of fungal growth inhibition. As expected, the PY-79 strain (negative control) showed no significant inhibition of fungal growth (Figure 3a). Under conditions of minimal growth, the positive control Kodiak strain in the different stages of development, showed no antagonistic activity, however, the BEB-8 strain in the stationary phase reduced fungal growth by 25%. These results indicate that the highest biocontrol activity is in the stationary phase.
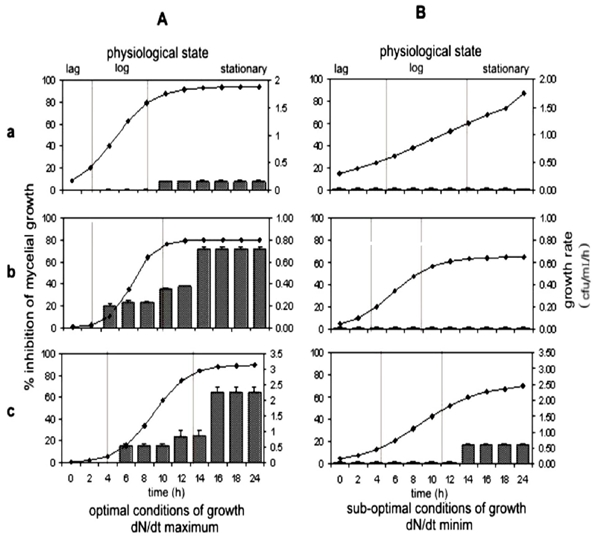
Figure 3 Effect of the physiological state and growth conditions of Bacillus subtilis strains on the antagonistic activity towards Rhizoctonia solani. AG-3. Bacterial growth under optimal conditions (A), and under sub-optimal conditions (B) of B. subtilis PY-79 (a), B. subtilis Kodiak (b) and B. subtilis BEB-8bs (c).
DISCUSSION
The application of microbial inoculants as biocontrol agents has largely been unsuccessful (Compant et al., 2005). This is due to plant protection being largely determined by an efficient colonization of the rhizosphere by inoculants and the competition with better adapted indigenous microflora (Castro-Sowinski et al., 2007; Conn y Franco 2004; Kozdrój y Van, 2000). These factors can greatly influence the establishment, proliferation, and activity of bacilli in a nutrient-limited environment. The utilization of a wide range of root exudates is correlated with the bacterial competitive ability and constitutes the nutritional basis of rhizosphere colonization (Bais et al., 2006; Haichar et al., 2008). In this study, the BIOLOG test indicated that the isolated BEB-8 was a strain of B. subtilis and confirmed the identities of PY-79 and Kodiak strains used as controls. Our results also confirmed the ability of the different strain of Bacillus to use a great variety of carbon sources. As indicated by Goddard et al. (2001) and Smalla et al. (1998) in yours studies, BIOLOG microtiter plates were originally developed for classification of bacterial isolates based on the ability of the isolates to oxidize 95 different carbon sources. However, this method has proved to be a useful tool for evaluating carbon source utilization patterns of microbial communities, potential metabolic differences between rhizosphere bacterial populations as well as for studying the effects of introduced inocula on soil microbial communities. In addition, although the metabolic profile of the three strains indicated that they were Bacillus subtilis, the AFLP analysis produced high-quality DNA fingerprints with detectable polymorphisms and revealed strain-level variations. This strain to strain variability confirms that they are different strains. Jones et al. (2005) described the individualization as the process by which morphological, biochemical or genetic characteristics are associated with a microorganism, such that a combination of characteristics is unique to that microorganism and exclude another microorganism. However, knowledge of the environmental niche where the biocontrol agent is able to grow is essential to establish the conditions for mass-production and also to become highly effective as a biocontrol agent. The most important environmental parameters are the water availability, temperature and pH of the rhizosphere (Costa et al., 2002). The physiological study of the Bacillus strains carried out in this work confirmed that environmental factors, such as pH and temperature, directly influence the capacity of growth and biocontrol activity. The findings reported in the present study clearly demonstrate that the environmental niche for the optimal growth of BEB-8 strain is at a temperature range of 15-37 °C and a pH between 5 and 8. Interestingly pH but not temperature influences growth rate in the ranges of the present study. However, the favorable environmental conditions where the Kodiak commercial preparation is able to grow are 28 °C in a pH range of 5-8, as was indicated by the significance of the effect of interaction between temperature and pH on dN/dt. A microbial species usually has a fairly wide range of environmental conditions in which it will grow. However, the growth rate is the variable physiological microbial initially affected by changes in the environment. The lack of direct measurements of this factor has been a major drawback in understanding the rhizosphere effects in soil. Based on this, the biological and physiological characterization of Bacillus subtilis with antagonistic activity carried out in the present work is of great importance. In a recent report, Costa et al. (2002) defined the range of environmental conditions over which CPA-2 strain of Pantoea agglomerans may be developed for biological control. They determined the effect of water availability, temperature, pH, and their interactions on the growth of the bacteria under in vitro conditions. Nevertheless, in contrast to our results, Costa et al. (2000) did not correlate the growth conditions or physiological status of the bacteria with the antagonistic activity of P. agglomerans. The results shown in Table 1 and Figure 3 emphasize the effects of proliferation, kinetics parameters and physiological status for each strain on antagonistic activity. Under optimal growth conditions, the CFS obtained from the exponential growth phase of the Kodiak and BEB-8 strains showed mycelial growth inhibition of R. solani. However, a greater inhibitory activity was achieved using CFS obtained during the stationary phase of either strain. On the contrary, altering pH and temperature to obtain a minimal growth rate caused the antagonistic capacity to be reduced or lost independently of the physiological state of the bacterium.
The relationship between bacterial growth rate and environmental conditions involves different response mechanisms. In this context, it has been reported that production of many secondary metabolites is regulated through a two-component system that is activated by an external or internal signal (Grahovac et al., 2015). The signals that activate the system are pH, temperature, osmolarity, and bacterial density. This regulatory system has been identified in different bacteria and is involved in the regulation of many different bacterial traits such as motility, pili formation, pathogenicity, siderophores and secondary metabolites production. (Cosby et al., 1998; Horswill, 2007). Ahlem et al. (2012) show that biosynthesis of the antagonistic factor, as well as the effectiveness of biological control strictly depend on the growth conditions (pH, temperature) this supports our results. Likewise, the physiological status should also be considered when thinking about the life of the bacteria when it is applied to the soil. This depends on the prevailing conditions in the soil, which represents a crucial factor in establishing and maintaining bacterial inocula in natural environments. This hypothesis is also supported by the results of Vandenhove et al. (1993), who demonstrated that exponentially growing Azospirillum cells inoculated into soil lead to a higher and more stabilized population than those than those inoculated during stationary phase. The authors concluded that the physiological state of an inoculum greatly influences its survival. In addition, Selim et al. (2005), reported that production of the antagonistic factor by B2 strain of Paenibacillus sp was detected in the middle of the exponential growth phase, but the maximum production was reached at the end of the stationary phase. These results are similar to those reported in the present work (Figure 3). These observations are particularly relevant since most of the literature describes the production of antagonistic metabolites by B. subtilis at the early stages of the stationary phase, which coincides with the beginning of the sporulation process. (Leclére et al., 2005; Savluchinske et al., 2004). The enzymes required for production of these metabolites begin in early stages of population growth, but it is not until the end of the exponential phase when the biofilm begins to form when its activity is greater as shown by the transcriptomic studies conducted by Kröber et al. (2016). The subsequent enzymatic activity can cease within a few hours. However, this is not a general rule, since according to the results shown in Figure 3, there is still antagonistic activity on Rhizoctonia solani by strains BEB-8 and Kodiak.
Finally, each metabolite may require a specific pH-range and temperature for its activity. This study reveals that all the examined factors influenced the antifungal activity against Rhizoctonia solani and that interactions between the factors were also important. In conclusion, studies focused on the physiological characteristics and kinetic parameters of growth of Bacillus subtilis strains that allow to better understand bacterial behavior in the biological control of Rhizoctonia solani.
Acknowledgements
We are grateful to Biol. Fernando Hernández Godínez for a technical assistance and Dra. June Simpson for a critical review of this manuscript. The research was carried out in the Department of Biotechnology and Biochemistry Cinvestav-Irapuato. This work received financial support from the Consejo Nacional de Ciencia y Tecnología; CONACyT, Mexico (Postdoctoral Research). Support for this research also came from CONACYT-SEP-106401 y 61238.
REFERENCES
Ahlem H, Mohammed E, Badoc A and Ahmed L. 2012. Effect of pH, temperature and water activity on the inhibition of Botrytis cinerea by Bacillus amyloliquefaciens isolates. African Journal of Biotechnology 11:2210-2217. Disponible en línea: http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.645 [ Links ]
Ashlee M, Losick ER and Kolter R. 2008. Ecology and genomics of Bacillus subtilis. Trends in Microbiology 16:269-275. https://doi.org/10.1016/j.tim.2008.03.004 [ Links ]
Ashlee M, Losick ER and Kolter R. 2007. Bacillus subtilis Genome Diversity. Journal. Bacteriology 189:1163-1170. Disponible en línea: http://jb.asm.org/content/189/3/1163.short [ Links ]
Bais HP, Fall R and Vivanco JM. 2004. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiology 134:307-319. https://doi.org/10.1104/pp.103.028712 [ Links ]
Bais HP, Weir TF, Perry LG, Gilroy S and Vivanco JM. 2006. The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology 57:233-266. https://doi.org/10.1146/annurev.arplant.57.032905.105159 [ Links ]
Bapat S and Shah AK. 2000. Biological control of fusarial wilt of pigeon pea by Bacillus brevis. Canadian Journal of Microbiology 46:25-132. https://doi.org/10.1139/w99-109 [ Links ]
Bernal G, Illanes A and Ciampi L. 2002. Isolation and partial purification of a metabolite from a mutant strain of Bacillus sp. with antibiotic activity against plant pathogenic agents. Electronic Journal of Biotechnology 5:12-20. Disponible en línea: http://www.scielo.cl/pdf/ejb/v5n1/04.pdf [ Links ]
Bloemberg G and Lugtenberg B. 2001. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Current Opinion Plant Biology 4:343-350. https://doi.org/10.1016/S1369-5266(00)00183-7 [ Links ]
Castro SS, Herschkovitz Y, Okon Y and Jurkevitch E. 2007. Effects of inoculation with plant growth-promoting rhizobacteria on resident rhizosphere microorganisms. Microbiology Letters 276:1-11. https://doi.org/10.1111/j.1574-6968.2007.00878.x [ Links ]
Compant S, Duffy B, Nowak J, Clement C and Ait Barka E. 2005. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology 71:4951-4959. https//doi.org/10.1128/AEM.71.9.4951-4959.2005 [ Links ]
Conn VM and Franco CMM. 2004. Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Applied and Environmental Microbiology 70:6407-6413. https://doi.org/10.1128/AEM.70.11.6407-6413.2004 [ Links ]
Cosby WM, Vollenbroich D, Lee OH and Zuber P. 1998. Altered srf expression in Bacillus subtilis resulting from changes in culture pH is dependent on the Spo0k oligopeptide permease and the ComQX system of extracellular compounds. Journal of Bacteriology 180:1438-1445. Disponible en línea: http://jb.asm.org/content/180/6/1438.short [ Links ]
Costa E, Usall J, Teixido N, Delgado J and Viñas I. 2002. Water activity, temperature and pH effects on growth of the biocontrol agent Pantoea agglomerans CPA-2. Canadian Journal of Microbiology 48:1082-1088. https://doi.org/10.1139/w03-001 [ Links ]
Fujinami S and Fujisawa M. 2010. Industrial applications of alkaliphiles and their enzymes-past, present and future. Environmental Technology 31:845-856. http://dx.doi.org/10.1080/09593331003762807 [ Links ]
Goddard VJ, Bailey MJ, Darrah P, Lilley AK and Thompson IP. 2001. Monitoring temporal and spatial variation in rhizosphere bacterial population diversity: a community approach for the improved selection of rhizosphere competent bacteria. Plant and Soil 232:181-193. https://doi.org/10.1023/A:1010302607616 [ Links ]
Gong M, Wang JD, Zhang J, Yang H, Lu XF, Pei Y and Cheng JQ. 2006. Study of the antifungal ability of Bacillus subtilis strain PY-1 in vitro and identification of its antifungal substance (Iturin A). Acta Biochimica et Biophysica Sinica 38:233-240. https://doi.org/10.1111/j.1745-7270.2006.00157.x [ Links ]
Grahovac JA, Rončević ZZ, Tadijan IŽ, Jokić AI and Dodić JM. 2015. Optimization of media for antimicrobial compounds production by Bacillus subtilis. Acta Alimentaria 44:427-435. DOI: 10.1556/066.2015.44.0014 [ Links ]
Haichar FZ, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J, Heulin T and Achouak W. 2008. Plant host habitat and root exudates shape soil bacterial community structure. The ISME Journal 2:1221-1230. https//doi.org/10.1038/ismej.2008.80 [ Links ]
Horswill AR, Stoodley P, Stewart PS and Parsek MR. 2007. The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Analytical and Bioanalytical Chemistry 387:371-380. https://doi.org/10.1007/s00216-006-0720-y [ Links ]
Johnson LF and Curl EA. 1972. Culture media. In Methods for research on the ecology of soil borne plant pathogens. Burgges Publishing Company Auburn, Alabama 16:187-208. Disponible en línea: https://www.cabdirect.org/cabdirect/abstract/19731903549 [ Links ]
Jones SW, Dobson ME, Francesconi SC, Schoske R and Crawford R. 2005. DNA assays for detection, identification, and Individualization of select agent microorganisms. Croatian Medical Journal 46:522-529. Disponible en línea: http://neuron.mefst.hr/docs/CMJ/issues/2005/46/4/16100754.pdf [ Links ]
Kamnev AA. 2008. FTIR spectroscopic studies of bacterial cellular responses to environmental factors, plant-bacterial interactions and signalling. Journal of Spectroscopy 22:83-95. http://dx.doi.org/10.3233/SPE-2008-0329 [ Links ]
Kim PLL, Ryu J, Kim YH and ChI YT. 2010. Production of biosurfactant lipopeptides Iturin A, fengycin, and surfactin a from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. Journal Microbiology and Biotechnology 20:138-145. http//doi.org/10.4014/jmb.0905.05007 [ Links ]
Kozdrój J and Van Elsas JD. 2000. Response of the bacterial community to root exudates in soil polluted with heavy metals assessed by molecular and cultural approaches. Soil Biology and Biochemistry 32:1405-1417. https://doi.org/10.1016/S0038-0717(00)00058-4 [ Links ]
Kröber M,Verwaaijen B, Wibberg D, Winkler A, Pühler A and Schlüter A. 2016. Comparative transcriptome analysis of the biocontrol strain Bacillus amyloliquefaciens FZB42 as response to biofilm formation analyzed by RNA sequencing. Journal Biotechnology 231:212-223. DOI: 10.1016/j.jbiotec.2016.06.013 [ Links ]
Leclére V, Béchet M, Adam A, Guez JS, Wathelet B, Ongena M, Thonart P, Gancel F, Chollet-Imbert M and Jacques P. 2005. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Applied and Environmental Microbiology 71:4577-4584. https://doi.org/10.1128/AEM.71.8.4577-4584.2005 [ Links ]
Matarante A, Baruzzi F, Cocconcelli PS and Morea M. 2004. Genotyping and toxigenic potential of Bacillus subtilis and Bacillus pumilus strains occurring in industrial and artisanal cured sausages. Applied and Environmental Microbiology 70:5168-5176. https://doi.org/10.1128/AEM.70.9.5168-5176.2004 [ Links ]
Nagórska K, Bikowski M and Obuchowski M. 2007. Multicellular behaviour and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol agent. Acta Biochimica Polonica 54:495-508. Disponible en línea: https://www.ncbi.nlm.nih.gov/pubmed/17882321 [ Links ]
O´Callagman M, Gerrard EM and Johnson VW. 2001. New Zeland Plant Protection 54:128-135. Disponible en línea: https://nzpps.org/journal/54/nzpp_541280.pdf [ Links ]
Ongena M and Jacques P. 2008. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiology 16:116-125. DOI: 10.1016/j.tim.2007.12.009 [ Links ]
Pal KK and B. Mc Spadden Gardener. 2006. Biological Control of Plant Pathogens. The Plant Health Instructor. DOI: 10.1094/PHI-A-2006-1117-02 [ Links ]
Rey MW, Ramaiya P, Nelson BA, Brody-Karpin SD, Zaretsky EJ, Tang M, López de León A, Xiang H, Gusti V, Clausen IG, Olsen PB, Rasmussen MD, Andersen JT, Jørgensen L, Larsen TS, Sorokin A, Bolotin A, Lapidus A, Galleron N, Ehrlich SD and Berka RM. 2004. Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. Genome Biology 5:r77. https://doi.org/10.1186/gb-2004-5-10-R77 [ Links ]
Savluchinske FS, Barbosa A, Cabrita M, Nunes L, Esteves A, Roseiro JC and Curto MJ. 2004. Antifungal activity of Bacillus subtilis 355 against wood-surface contaminant fungi. Journal of Industrial Microbiology and Biotechnology 31:199-203. https://doi.org/10.1007/s10295-004-0133-x [ Links ]
Schallmey M, Singh A and Ward OP. 2004. Developments in the use of Bacillus species for industrial production. Canadian Journal of Microbiology 50:1-17. https://doi.org/10.1139/w03-076 [ Links ]
Selim S, Negrel J, Govaerts C, Gianinazzi S and Van Tuinen D. 2005 Isolation and partial characterization of antagonistic peptides produced by Paenibacillus sp. strain B2 isolated from the sorghum mycorrhizosphere. Applied and Environmental Microbiology 71:6501-6507. https://doi.org/10.1128/AEM.71.11.6501-6507.2005 [ Links ]
Shelburne CE, An FY, Dholpe V, Ramamoorthy A, Lopatin DE and Lantz MS. 2007. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. Journal of Antimicrobial Chemotherapy 59:297-300. https://doi.org/10.1093/jac/dkl495 [ Links ]
Slater J. 1985. Microbial growth dynamics. In: Comprehensive biotechnology, Mac-Young, M. (eds). Oxforf Pergamosm, p. 184-213. [ Links ]
Smalla K, Wachtendorf U, Hever H, Liu WT and Forney L. 1998. Analysis of BIOLOG GN substrate utilization patterns by microbial communities. Applied and Environmental Microbiology 64:1220-1225. Disponible en línea: http://aem.asm.org/content/64/4/1220.short [ Links ]
Stein T. 2005. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Molecular Microbiology 56:845-857. https://doi.org/10.1111/j.1365-2958.2005.04587.x [ Links ]
Tamehiro N, Okamoto-Hosoya Y, Okamoto S, Ubukata M, Hamada M, Naganawa H and Ochi K. 2002. Bacilysocin, a novel phospholipid antibiotic produced by Bacillus subtilis 168. Antimicrobial Agents and Chemotherapy 46:315-320. https://doi.org/10.1128/AAC.46.2.315-320.2002 [ Links ]
Vandenhove H, Merck R, Van Steenbergen M and Vlassak K. 1993. Microcalorimetric characterization, physiological stages and survival ability of Azospirillum brasilense. Soil Biology and Biochemistry 25:513-519. https://doi.org/10.1016/0038-0717(93)90077-O [ Links ]
Veith B, Herzberg C, Steckel S, Feesche J, Maurer KH, Ehrenreich P, Bäumer S, Henne A, Liesegang H, Merkl R, Ehrenreich A and Gottschalk G. 2004. The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential. Journal of Molecular Microbiology and Biotechnology 7:204-211. https://doi.org/10.1159/000079829 [ Links ]
Vos P, Hoger R, Bleker M, Reijans M, Van de Lec T, Hones M, Frijters A, Pot J, Peleman J, Kaiper M and Zabeau M. 1995. AFLP: A new technique for DNA fingerprinting. Nucleic Acid Research 23:4407-4414. https://doi.org/10.1093/nar/23.21.4407 [ Links ]
Zhang X, Zhanng B, Zhang Z, Shen W, Yang C, Yu J and Zhao Y. 2005. Survival of the biocontrol agents Brevibacillus brevis ZJY-1 and Bacillus subtilis ZJY-116 on the spikes of barley in the field. Journal Zhejiang University SCIENCE. B. 6:770-777. https://doi.org/10.1631/jzus.2005.B0770 [ Links ]
Received: November 15, 2017; Accepted: April 30, 2018











 texto en
texto en 


