Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.36 no.2 Texcoco Mai./Ago. 2018
https://doi.org/10.18781/r.mex.fit.1711-2
Scientific articles
Effect of biocontrol and germinative inhibition of Bacillus spp. on zoospores of Phytophthora capsici
1Centro de Investigación en Alimentación y Desarrollo, A.C. Coordinación Culiacán. Km 5.5 Carretera Culiacán-El Dorado, Campo El Diez, CP. 80110. Culiacán, Sinaloa, México
2Facultad de Ciencias Biológicas y Agropecuarias. Universidad Veracruzana. Carretera Tuxpan-Tampico km. 7.5 Tuxpan, Veracruz, México.
The oomycete Phytophthora capsici is a pathogen of economic importance in tomato (Solanum lycopersicum L.) and chili (Capsicum annuum L.) crops. The objective of this work was to evaluate the effect of germinative inhibition and biocontrol of two isolates of the genus Bacillus on zoospores of P. capsici. The isolates were identified as Bacillus amyloliquefaciens and B. thuringiensis. In the in vitro tests of the germination of zoospores of P. capsici, the percentages of inhibition with cell suspension of B. amyloliquefaciens and B. thuringiensis were significant with 88.15 and 97.05% respectively, while, the filtrates showed 24.30% of inhibition. In the in vivo study, tomato seedlings treated with cell suspension of B. amyloliquefaciens and B. thuringiensis, showed lower severity of the disease caused by P. capsici with 22.22 and 27.78% respectively, compared to that observed in chili seedlings where values of 61% were obtained. The tomato and chili seedlings treated with bacterial filtrates showed up to 94% severity. With cell suspension of B. amyloliquefaciens and B. thuringiensis the efficiency of biocontrol was 72 and 77% respectively, which show that these microorganisms can be used as biocontrol agents of P. capsici in tomato and chili plants.
Key words: Bacillus amyloliquefaciens; Bacillus thuringiensis; phytopathogens; oomycete
El oomiceto Phytophthora capsici es un patógeno de importancia económica en los cultivos de tomate (Solanum lycopersicum L.) y chile (Capsicum annuum L.). El objetivo de este trabajo fue evaluar el efecto de inhibición germinativa y biocontrol de dos aislados del género Bacillus sobre zoosporas de P. capsici. Los aislados fueron identificados como Bacillus amyloliquefaciens y B. thuringiensis. En las pruebas in vitro de la germinación de zoosporas de P. capsici, los porcentajes de inhibición con suspensión celular de B. amyloliquefaciens y B. thuringiensis fueron significativos con 88.15 y 97.05% respectivamente, mientras que, los filtrados mostraron el 24.30% de inhibición. En el estudio in vivo, las plántulas de tomate tratadas con suspensión celular de B. amyloliquefaciens y B. thuringiensis, mostraron menor severidad de la enfermedad ocasionada por P. capsici con 22.22 y 27.78% respectivamente, respecto a lo observado en plántulas de chile donde se obtuvo valores de 61%. Las plántulas de tomate y chile tratadas con filtrados bacterianos, mostraron hasta 94% de severidad. Con suspensión celular de B. amyloliquefaciens y B. thuringiensis la eficacia de biocontrol fue 72 y 77% respectivamente, lo cual muestra que estos microorganismos pueden ser usados como agentes de biocontrol de P. capsici en plantas de tomate y chile.
Palabras clave: Bacillus amyloliquefaciens; Bacillus thuringiensis; fitopatógenos; oomiceto
Tomato (Solanum lycopersicum L.) and chili (Capsicum annuum L.) are economically important crops because of their high consumption in the human diet. Mexico ranks tenth in tomato production with 2,649,358 t and second in chili production with 2,294,400 t (SIAP 2016). However, the production of both crops is affected by diseases caused by different pathogens, the most important being the oomycete Phytophthora capsici Leonian, which causes serious economic damage to agriculture worldwide (Chen et al., 2016a). Phytophthora capsici causes “damping-off”, root rot, stem and leaf damage or fruit rot in more than 50 host plant species (Erwin and Riveiro, 1996; Khan et al., 2011). The pathogen has been reported to cause considerable yield losses (15 to 45%), and even total chili, tomato and eggplant crop losses (González et al., 2009; Wang et al., 2011). The high soil moisture levels and warm weather that prevail in tomato and chili farms favor the spread and survival of P. capsici (Erwin and Riveiro, 1996).
Several strategies have been used to manage and control the pathogen, including cultural practices, resistant varieties (Yang et al., 2015; Gómez-Rodríguez et al., 2017), fungicide applications (Lamour et al., 2012) and water management (Sanogo y Ji, 2013), but none of them individually have completely controlled the pathogen (Hausbeck and Lamour, 2004). Although several specific fungicides have quickly and effectively reduced disease severity, their indiscriminate use has led to the emergence of resistant isolates and environmental pollution (Qi et al., 2012). For this reason, new alternatives based on biological control are being considered for managing Phytophthora, an important strategy in soil-borne phytopathogen management, since they reduce the application of agrochemicals (Nguyen et al., 2012; Rios-Velasco et al., 2016).
Different microorganisms have been reported to suppress P. capsici growth, including Streptomyces spp. (Ko et al., 2010; Nguyen et al., 2012), Paenibacillus spp. (Naing et al., 2014), Trichoderma sp. (Segarra et al., 2013), Clitocybe nuda (Chen et al., 2012) Aspergillus sp. (Kang and Kim, 2004) and Bacillus spp. (Zhang et al., 2010). The Bacillus genus has been studied more as part of biological control, and species of this genus are considered ideal candidates for controlling diseases due to their antagonistic potential (Zhao et al., 2013; Torres et al., 2016). Control mechanisms include the production of antibiotic and lithic enzymes, physical and chemical interference, competition, host resistance induction, hyperparasitism and predation (Pal and Gardener, 2006).
The objectives of the present study were to determine how Bacillus spp. affects germinative inhibition by using cells and bacterial filtrates in vitro on P. capsici zoospores and they can be used as biocontrol, in tomato and chili plants, as well as to identify those microorganisms.
MATERIALS AND METHODS
Biological material
The microorganisms used in this study were two antagonistic bacterial isolates of the Bacillus genus coded as B17 and B32, and a Phytophthora capsici isolate from the isolate pool at the Phytopathology Laboratory of the Centro de Investigación en Alimentación y Desarrollo, Unidad Culiacán. Bacteria were isolated from the rhizosphere of tomato and chili crops at different geographical locations in Sinaloa, activated in nutrient agar (NA), incubated at 27°C for five days and preserved in a phosphate buffer at 4°C until they were used. The Phytophthora capsici pathogen was activated in V8 culture medium (10% V8 juice, 0.02% CaCO3 and 1.5% agar) and incubated at 27°C until it was used (Chen et al., 2016b).
Morphological and molecular identification
To confirm that the bacterial isolates belonged to the Bacillus genus, they were identified through colony morphology, cell, and Gram and flagella stains (Castillo et al., 2004), as well as molecular techniques in the Phytopathology Laboratory of CIAD Unidad Culiacán and Laboratorio Nacional de Genómica para la Biodiversidad (LANGEBIO) at CINVESTAV’s campus in Irapuato, Guanajuato, Mexico.
A purified colony with around 48-72 h of growth was used to identify the bacterial isolates. DNA extraction was carried out following the methodology of Heddi et al. (1999). Later, the 16S gene from DNAr was amplified using the polymerase chain reaction (PCR) technique and FD2 (5’-AGAGTTTGATCATGGCTCAG-3’) and RP1 (5’-TACCTTGTTACGACTTCACC-3’) universal primers that amplify a 1500 pb fragment. The amplification was performed in a Thermocycler 100 Thermal CyclerTM (Singapore). The time and temperature conditions were as follows: first, the enzyme was activated at 95 °C for 5 min, a second step consisted of 30 cycles including denaturation at 94°C for 1 min, an alignment step at 56°C for 1 min, an extension step at 72°C for 1 min and, when the cycles ended, a final extension step at 72°C for 10 min (McLaughlin et al., 2012).
The PCR amplified fragments were analyzed using 1% agarose gel electrophoresis in a PowerpacTM Basic chamber (BIO-RAD). Once detected, the expected fragment from the 16S region was purified and sequenced, and its nucleotidic sequence was compared to the sequences stored in the NCBI database (Altschul et al., 1990). Finally, the obtained sequences were deposited in the NCBI gene bank.
Inhibiting germination of P. capsici zoospores using a suspension of Bacillus spp. cells
The germination inhibition bioassay was conducted on concave slides. To induce P. capsici to produce sporangia, 5-mm agar disks with mycelium of the pathogen that had been growing for 5 days were taken and placed on Petri dishes to which 10 mL of distilled water were added, and incubated at 25-27°C for 48 h. To release the zoospores from the sporangia, the Petri dishes were incubated at 4°C for 30 min (Ko et al., 2010) and a concentration of 20 zoospores/µL was obtained. Each slide concavity was filled with 20 µL of the zoospore suspension to which 20 µL of bacterial cell suspension of B17 and B32 isolates at a concentration of 1 x 108 colony forming units (CFU) 1:1 v/v were added, covered with a slide to prevent evaporation and incubated at 25-27°C for 24 h (Ko et al., 2010). The zoospores that were not inoculated with the bacterial cell suspension were used as a control. To obtain the percentage of zoospore germination inhibition in the bioassays where cell suspension and bacterial filtrates were used, 100 zoospores from each concavity were counted using an optical microscope (Carl Zeiss AXIO Imager.A2) with an integrated camera (AxioCam ERc5s) and 10x and 40x objectives. Each treatment had seven replications and the experiment was conducted in duplicate.
Inhibition of zoospore germination using bacterial filtrates
The bacterial filtrate was made by placing in an Erlenmeyer flask 50 mL of nutrient broth and 200 µL of spore suspension of the B17 and B32 bacterial isolates at a concentration of 1 x 108 UFC. The flasks were incubated at 30°C in an orbital shaker (140 rpm) for 6 days. The cultures were filtered using 0.22 µm Millipore® filters to remove bacterial cells (Chen et al., 2016b). To inhibit germination, 20 µL of the zoospore suspension (20 zoospores/µL), mixed with 20 µL of bacterial filtrate (1:1 v/v), were placed in the slide cavity, covered with a slide to prevent evaporation and then incubated at 25-27°C for 24 h (Ko et al., 2010). Zoospores without bacterial filtrate were used as a control.
Antagonism of Bacillus spp. against P. capsici in vivo using cell suspension and bacterial filtrates in tomato and chili plants
For the present study we used Malinche hybrid tomato seedlings and SV3198HJL hybrid chili seedlings 3 three weeks after germination. The P. capsici pathogen was inoculated directly into the root of both crops using 1 mL of the zoospore suspension (1 x 103/mL) on both crops, followed by inoculation with 10 mL of the bacterial cell suspension (1 x 108/mL) or their filtrates. All the treatments were applied under the same conditions.
The level of disease severity or damage was measured based on a scale for root neck rot symptoms that was proposed by Segarra et al. (2013) and modified as follows: 0 = symptomless plants, 1 = damage symptoms ˂ 10 mm, 2 = 10-19 mm rotted, 3 = 20-30 mm rotted, and 4 = ˃ 30 mm rotted. The percentage of damage severity was calculated using the following formula:
where % DS= percentage of damage severity; GD= level of damage; NP= number of damaged plants; EM= maximum level of damage on the severity scale; and TP= number of plants included in the treatment (Shanmugam and Kanoujia, 2011; Li et al., 2012). The control effectiveness was calculated using the following formula:
where % EC = percentage of the control effectiveness; treatment SD = average damage severity per treatment; control SD = average damage severity on the control (Li et al., 2012). The experiment included three replications per treatment and was conducted in duplicate in the glasshouse. We used a pot with three seedlings as the experiment unit. This study was conducted on two different dates, and the analyzed data were reported as evaluation averages.
RESULTS
Morphological identification
The bacterial colonies of B17 and B32 were similar in shape and color. The colonies of the B17 isolate showed a mound of creamy and mucoid consistency (acuminate) in the middle compared to those of the B32 isolate, which were flat and of dry and lumpy consistency.
Both isolates were Gram positive and developed peritrichous flagella. Cells of the B17 isolate were between 2.0 and 3.2 µm long., while the cells of the B32 isolate were between 3.0 and 4.5 µm long. The latter also developed parasporal crystals (Cry protein), which are similar to the most important features of the bacteria of the Bacillus genus, which are bacillar in shape, 0.5-2.5 and 1.2-10 µm in cell size, Gram positive to stain and move through flagella inserted peritrichously.
Molecular identification
The PCR sequences of the B17 and B32 isolates were 99% to 100% identical to Bacillus amyloliquefaciens and Bacillus thuringiensis, respectively, when compared to the sequences already reported in the Gen Bank database (NCBI). The sequences were included in the database with Access number KX953161.1 for B17 and KX953162.1 for B32.
Inhibition of P. capsici zoospore germination using a Bacillus spp. cell suspension
The results show that both bacteria inhibit zoospore germination and deform the germinative tube after 24 h (Figures 1 and 2). In the control treatments, 24 h after being released from the sporangium, zoospores showed 100% germination. The B. amyloliquefaciens isolate caused 88.15% germinative inhibition, but the B. thuringiensis isolate was more effective and caused 97.05% inhibition (Table 1).
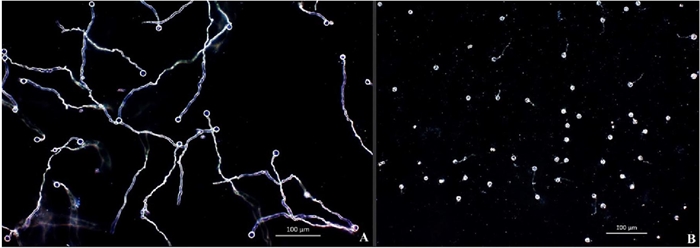
Figure 1 Effect of the cell suspension and filtrates of B. amyloliquefaciens and B. thuringiensis on P. capsici zoospores germination inhibition 24 h after inoculation. (A) zoospores germination without the treatment used as a control. (B) germination inhibition with the treatment. The photographs were taken under a darkfield microscope with objective (10x).
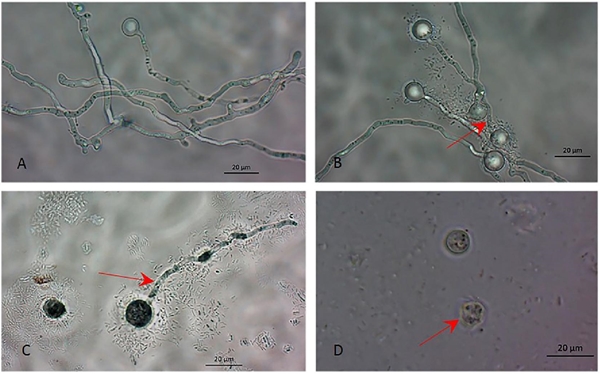
Figure 2 Morphology of a P. capsici germinated zoospore with and without B. amyloliquefaciens and B. thuringiensis bacterial treatment (cell suspension or filtrates). (A) Germinated zoospore without inoculation. (B y C) Unusual germinative tube and non-germinated zoospores, and (D) P. capsici zoospore cells became bare when treated with B. amyloliquefaciens and B. thuringiensis cell suspension. The photographs were taken under a bright-field microscope with objective (40x).
Table 1 Inhibition of (P. capsici) zoospores germination using B. amyloliquefaciens and B. thuringiensis cell suspension and filtrates 24 h after inoculation.
| Tratamientos | % de inhibición de la germinaciónx + DE | |
| Suspensión Cel. | Filtrados | |
| Control (Agua) | 0.00 ay | 0.00 a |
| B. amyloliquefaciens | 88.15±2.519 b | 24.30±6.280 b |
| B. thuringiensis | 97.05±1.191 c | 49.35±5.031 c |
xMeans with different letters are significantly different (ANOVA), according to Tukey’s test (P ≤ 0.05).
yGermination inhibition = (Number of germinated zoospores/Total of zoospores) x 100 (Chen et al., 2016b). SD= Standard deviation.
Inhibition of P. capsici zoospore germination using Bacillus spp. filtrates
Bacillus amyloliquefaciens bacterial filtrates inhibited 24.3% of P. capsici zoospore germination in vitro, while B. thuringiensis filtrates produced 49.35% inhibition. The control showed 100% germination (Table 1).
Severity of damage caused by P. capsici using cell suspension and bacterial filtrates in tomato and chili plants.
The results indicate that in plants treated with a B. thuringiensis cell suspension, the disease incidence was 38.89% in chili and 22.22% in tomato plants, while in plants treated with a B. amilolyquefaciens cell suspension, the incidence was 61.11% in chili and 27.78% in tomato. When the plants were inoculated with filtrates of B. amilolyquefaciens and B. thuringiensis, the level of severity of neck rot symptoms was greater than 90% and 70% in chili plants and 60% and 40% in tomato plants, respectively (Table 2).
Table 2 Severity of damage caused by P. capsici in chili and tomato seedlings treated with B. amyloliquefaciens and B. thuringiensis cell suspension and filtrates 7 d after inoculation.
| Tratamiento | Severidad de daño (%) x +DE | |||
| Chile | Tomate | |||
| S. celular | Filtrado | S. celular | Filtrado | |
| Control (Agua) | 0.00 cy | 0.00 c | 0.00 c | 0.00 c |
| P. capsici | 100 a | 100 a | 100 a | 100 a |
| B. amyloliquefaciens-P. capsici | 61.11±6.49 b | 94.44±2.26 ab | 27.78±4.18 b | 61.11±4.18 b |
| B. thuringiensis-P. capsici | 38.89±4.18 b | 72.25±418 b | 22.22±2.87 bc | 44.44±4.54 b |
xMeans that do not share a letter are significantly different (ANOVA), according to Tukey’s test (P ≤ 0.05).
ySeverity of damage = [Σ (extent of damage x number of damaged plants)/(extent of maximum damage in the severity scale x total of plants from the treatment)] x 100 . SD= Standard deviation.
Biological effectiveness of Bacillus spp. on P. capsici using cell suspension and bacterial filtrates in tomato and chili plants.
The obtained results show that in chili and tomato plants inoculated with a cell suspension of B. thuringiensis, the efficacy of the biocontrol was higher than 60 and 77%, respectively, while in the plants treated with bacterial filtrates, the efficacy was lower than 28 and 56%, respectively (Table 3). The biocontrol efficacy of the cell suspension of B. amyloliquefaciens in chili was only 38.89%, while in tomato it was significantly higher, more than 72%. However, the biocontrol efficacy of its bacterial filtrates was lower than 6 and 38% in chili and tomato plants, respectively (Table 3, Figure 3).
Table 3 Biological effectiveness of the suspension and filtrates of B. amyloliquefaciens and B. thuringiensis on P. capsici in chili and tomato seedlings.
| Tratamiento | Eficacia biológica (%)x +DE | |
| Chile | Tomate | |
| P. capsici | 0 cy | 0 d |
| S. Cel. B. amyloliquefaciens-P. capsici | 38.89±3.61 b | 72.25±2.20 a |
| S. Cel. B. thuringiensis-P. capsici | 61.11±5.03 a | 77.78±0.00 a |
| Filtrado B. amyloliquefaciens-P. capsici | 5.56±7.78 c | 38.89±3.61 c |
| Filtrado B. thuringiensis-P. capsici | 27.78±3.38 b | 55.56±0.00 b |
xMeans that do not share a letter are significantly different (ANOVA), according to Tukey’s test (P≤0.05).
yEffectivenes of the control = (100 - average severity of damage per treatment)/(average severity of damage from the control) x 100 (Li et al., 2012). SD= Standard deviation.

Figure 3 Effectiveness to control the disease caused by P. capsici in chili and tomato seedlings using B. amyloliquefaciens and B. thuringiensis cell suspension or filtrates. The seedlings were inoculated with 1 µL of 1x103 zoospores and 10 µL of cell suspension or filtrate. (A) damage caused by P. capsici in chili seedlings, (B) chili seedlings with P. capsici+cell suspension of B. thuringiensis, (C) damage caused by P. capsici in tomato, and (D) tomato seedlings using P. capsici+cell suspension of B. thuringiensis.
DISCUSSION
The disease caused by P. capsici is very important because it affects economically important crops such as tomato and chili (Agrios, 2005; Hansen et al., 2012). Considering this situation, and because chemical control and most crop management practices are not efficient tools to reduce the damage caused by P. capsici in tomato and chili seedlings, the use of biocontrol organisms could be a promising alternative (Bae et al., 2016; Thampi and Bhai, 2017).
Since the microorganisms of the Bacillus genus are found mainly in the soil, in this study we used two bacterial isolates whose morphological traits belong to that genus. Using molecular techniques, the B17 isolate was identified as B. amyloliquefaciens, and isolate B32 was in alignment with B. thuringiensis and B. cereus. However, different authors, such as Sánchez et al. (2016), mention that one of the main differences between B. thuringiensis and B. cereus is that they produce parasporal crystals (Cry protein), a characteristic of B. thuringiensis observed in the B32 isolate.
It was also demonstrated that they show the effect of biocontrol and P. capsici zoospore germination inhibition in tomato and chili seedlings, because P. capsici zoospore germination inhibition in vitro was 88.15±2.5 and 97.05±1.19% when we used cell suspensions of B. amyloliquefaciens and B. thuringiensis, respectively. Torres et al. (2016) reported 56.5±2.1% inhibition of Macrophomina phaseolina mycelial growth using the dual crop technique and cell suspension of B. amyloliquefaciens. According to Torres et al. (2016) and to the present study, it has been proven that B. amyloliquefaciens biocontrol capacity is affected by the pathogen’s nature, because when the cell suspension of B. amyloliquefaciens was mixed with P. capsici zoospores in a liquid culture medium, it caused 88.15% germination inhibition. On the other hand, Chen et al. (2016b) reported 100% P. capsici zoospore germination when they were mixed with filtrates from a Streptomyces plicatus culture whose ingredients were potato and sucrose. However, the results obtained using filtrates from a culture medium containing chitin, potato and sucrose show 100% zoospore germination, a fact that indicates that biocontrol agents are more effective when the pathogen to be controlled is present than under conditions that do not threaten its development. This was demonstrated by using bacterial filtrates of B. amyloliquefaciens and B. thuringiensis on a suspension of P. capsici zoospores, where 24.30±6.28 and 49.35±5.03% germination inhibition was observed, respectively, which is lower than that observed when a cell suspension was used.
While conducting in vivo experiments using cell suspensions of B. amyloliquefaciens and B. thuringiensis, damage severity was 61.11±6.49 and 38.89±4.18%, respectively, while the control showed 100% damage. Several Bacillus species are promising as part of the biocontrol strategy given their ability to produce a variety of antibacterial and antifungal metabolites (Zhi et al., 2017). Due to their versatile ability to produce bioactive compounds, different Bacillus species are being studied in order to use them in several applications (Torres et al., 2017). For example, many Bacillus isolates produce several antifungal cyclic lipopeptides (CLPs), including members of the surfactin, iturine and fengycin families (Torres et al., 2016). It has been demonstrated that lipopeptides belonging to the iturine, fengycin and surfactin families, are the most important compounds in the biocontrol activity of Bacillus isolates against different phytopathogenic fungi in different plant species (Masmoudi et al., 2017; Abdallah et al., 2017). Therefore, using Bacillus to control fungal diseases would be a great opportunity for agricultural biotechnology.
Several authors have reported B. amyloliquefaciens as a biocontrol option for different pathogens (Yu and Lee., 2013; Wei et al., 2015; Zhang et al., 2015; Torres et al., 2016; Chen et al., 2016b; Abdallah et al., 2017). However, there are no reports on P. capsici control of tomato and chili in which B. amyloliquefaciens shows effective biocontrol; therefore, based on the results obtained in the present study, more research on this microorganism must be conducted. Bacillus thuringiensis has also proven to be efficient mainly for controlling insects in different crops and fruits after harvest (Zheng et al., 2013; Deepak and Jayapradha 2015; Kim et al., 2017). Mojica-Marín et al. (2009) reported they controlled chili wilting using B. thuringiensis, but their study was directly conducted on germination of seeds inoculated with P. capsici, where 62 to 93% germination was observed on treated seeds. However, information on the use of B. thuringiensis for controlling P. capsici in tomato and chili seedlings is almost nil.
CONCLUSIONS
The cell suspensions of B. amyloliquefaciens and B. thuringiensis species produced significantly higher inhibition than their filtrates on P. capsici zoospores. They also showed biological control potential in tomato and chili crops against P. capsici. This result suggests that these microorganisms produce secondary metabolites that have biological activity and can thus be used as P. capsici biocontrol agents in tomato and chili plants. Therefore, more studies are required to identify secondary metabolites or bioactive compounds produced by these bacteria, as well as to assess their antifungal capacity.
LITERATURA CITADA
Abdallah RAB, Stedel C, Garagounis C, Nefzi A, Jabnoun-Khiareddine H, Papadopoulou KK and Daami-Remadi M. 2017. Involment of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Protection 99:45-58. http://dx.doi.org/10.1016/j.cropro.2017.05.008 [ Links ]
Agrios GN. 2005. Plant Pathology. Fifth Edition. Elsevier. Academic Press. USA. Pp. 305-592. [ Links ]
Altschul S, Gish W, Miller W, Myers EW and Lipman DJ. 1990. Basic Local Alignment Search Tool. Journal of Molecular Biology 215:403-40. Disponible línea: https://www.biostat.wisc.edu/bmi576/papers/blast.pdf [ Links ]
Bae SJ, Mohanta TK, Chung JY, Ryu M, Park G, Shim S, Hong SB, Seo H, Bae DW, Bae I, Kim JJ and Bae H. 2016. Trichoderma metabolites as biological control agents against Phytophthora pathogens. Biological Control 92:128-138. http://dx.doi.org/10.1016/j.biocontrol.2015.10.005 [ Links ]
Castillo C, Sosa B y Scorza J. 2004. Evaluación de la termorresistencia en metabolitos antifúngicos producidos por esporulados del género Bacillus. Revista de la Sociedad Venezolana de Microbiología [online]. Vol.24, n.1-2 [citado 2017-09-14], pp. 65-67. Disponible en línea: http://www.scielo.org.ve/scielo.php?script=sci_arttext&pid=S1315-25562004000100011&lng=es&tlng=es [ Links ]
Chen JT, Su HJ and Huang JW. 2012. Isolation and identification of secondary metabolites of Clitocybe nuda responsible for inhibition of zoospore germination of Phytophthora capsici. Journal of Agricultural and Food Chemistry 60:7341-7344. http://dx.doi: 10.1021/jf301570y [ Links ]
Chen X, Zhang Y, Fu X, Li Y and Wang Q. 2016 a. Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biology and Technology 115:113-121. https://doi.org/10.1016/j.postharvbio.2015.12.021 [ Links ]
Chen YY, Chen PCh and Tsay TT. 2016 b. The biocontrol efficacy and antibiotic activity of Streptomyces plicatus on the oomycete Phytophthora capsici. Biological Control 98:34-42. http://dx.doi.org/10.1016/j.biocontrol.2016.02.011 [ Links ]
Deepak R and Jayapradha R. 2015. Lipopeptide biosurfactant from Bacillus thuringiensis pak 2310: A potential antagonist against Fusarium oxysporum. Journal de Mycologie Médicale 25:15-24. https://doi.org/10.1016/j.mycmed.2014.10.011 [ Links ]
Erwin D and Ribeiro O. 1996. Phytophthora Diseases Worldwide. Minnesota. The American Phytopathological Society. 562 p. [ Links ]
González ChMM, Villordo PE, Pons HJL, Delgadillo SF, Paredes MR, Godoy HH, Anaya LJL, Gámez VFP, Medina CT, Rodríguez GR, Ruiz CE, Ruiz LA, Cárdenas BR, Cárdenas AJR, Torres PI, Rendón PE, Martínez SJ, Mojarro DF, Villaseñor EOM y Guerrero ABZ. 2009. Guía para el manejo de la marchitez del chile en Guanajuato. Primera Edición. Prometeo Editores, S. A. de C. V. CEPROCH- Guanajuato. México, D. F. 34 pp. [ Links ]
Gómez RO, Corona TT and Aguilar RVH. 2017. Differential response of pepper (Capsicum annuum L.) lines to Phytophthora capsici and root-knot nematodes. Crop Protection 92:148-152. http://dx.doi.org/10.1016/j.cropro.2016.10.023 [ Links ]
Hansen EM, Reeser PW and Sutton W. 2012. Phytophthora beyond agriculture. Annual Review of Phytopathology 50:359-378. https://doi.org/10.1146/annurev-phyto-081211-172946 [ Links ]
Hausbeck MK and Lamour KH. 2004. Phytophthora capsici on vegetable crops: research progress and management challenges. Plant Disease 88:1292-1303. http://dx.doi.org/10.1094/PDIS.2004.88.12.1292 [ Links ]
Heddi A, Grenier AM, Khatchadourian C, Charles H and Nardon P. 1999. Four intracellular genomes direct weevil biology: Nuclear, mitochondrial, principal endosymbiont and Wolbachia. Proceedings of the National Academy of Sciences of the United States of America 96:6814-6819. Disponible en línea: http://www.pnas.org/content/96/12/6814.full [ Links ]
Kang SW and Kim SW. 2004. New antifungal activity of Penicillium acid against Phytophthora species. Biotechnology Letters 26:695-698. https://doi.org/10.1023/B:BILE.0000024090.96693.a4 [ Links ]
Khan MA, Cheng Z, Xiao X, Khan AR and Ahmed SS. 2011. Ultrastructural studies of the inhibition effect against Phytophthora capsici of root exudates collected from two garlic cultivars along with their qualitative analysis. Crop Protection 30:1149-1155. https://doi.org/10.1016/j.cropro.2011.04.013 [ Links ]
Kim HS, Noh S and Park Y. 2017. Enhancement of Bacillus thuringiensis Cry1 Ac and Cry1 Ca toxicity against Spodoptera exigua (Hubner) by suppression of a chitin synthase B gene in midgut. Journal of Asia-Pacific Entomology 20:199-205. DOI: 10.1016/j.aspen.2016.12.015 [ Links ]
Ko WH, Tsou YJ, Lin MJ and Chern LL. 2010. Activity and characterization of secondary metabolites produced by a new microorganism for control of plant diseases. New Biotechnology 27:397-402. DOI: 10.1016/j.nbt.2010.05.014 [ Links ]
Lamour KH, Stam R, Jupe J and Huitema E. 2012. The oomycete broad-host-range pathogen Phytophthora capsici. Molecular Plant Pathology 13:329-337. DOI: 10.1111/j.1364-3703.2011.00754.x [ Links ]
Li CH, Shi L, Han Q, Hu HL, Zhao MW, Tang CM and Li SP. 2012. Biocontrol of verticillium wilt and colonization of cotton plants by an endophytic bacterial isolate. Journal of Applied Microbiology 113:641-651. DOI: 10.1111/j.1365-2672.2012.05371.x [ Links ]
Masmoudi F, Khedher SB, Kamoun A, Zouari N, Tounsi S and Trigui M. 2017. Combinatorial effect of mutagenesis and medium component optimization on Bacillus amyloliquefaciens antifungal activity andefficacy in eradicating Botrytis cinerea. Microbiological Research. 197:29-38. http://dx.doi.org/10.1016/j.micres.2017.01.001 [ Links ]
McLaughlin RW, Chen M, Zheng J and Wang D. 2012. Analysis of the bacterial diversity in the fecal material of the endangered Yangtze finless porpoise, Neophocaena phocaenoides asiaeorientalis. Molecular Biology Reports 39(5):5669-5676. DOI: 10.1007/s11033-011-1375-0 [ Links ]
Mojica-Marín V, Luna-Olvera HA, Sandoval-Coronado CF, Pereyra-Aferez B, Mrales-Ramos LH, Gonzalez-Aguilar NA, Hernandez-Luna CE y Alvarado-Gomez OG. 2009. Control biológico de la marchitez del chile (Capsicum annuum L.) por Bacillus thuringiensis. Revista Internacional de Botánica Experimental 78:105-110. Disponible en línea: http://www.scielo.org.ar/pdf/phyton/v78n2/v78n2a04.pdf [ Links ]
Naing KW, Anees M, Nguyen XH, Lee YS, Jeon SW, Kim SJ, Kim MH and Kim KY. 2014. Biocontrol of late blight disease (Phytophthora capsici) of pepper and the plant growth promotion by Paenibacillus ehimensis KWN38. Journal of Phytopathology 162:367-376. DOI: 10.1111/jph.12198 [ Links ]
Nguyen XH, Naing KW, Lee YS, Tindwa H, Lee GH, Jeong BK, Ro HM, Kim SJ, Jung WJ and Kim KY. 2012. Biocontrol potential of Streptomyces griseus H7602 against root rot disease (Phytophthora capsici) in pepper. The Plant Pathology Journal 28(3):282-289. DOI: 10.5423/PPJ.OA.03.2012.0040 [ Links ]
Pal KK and Gardener BM. 2006. Biological control of plant pathogens. The Plant Health Instructor. http://dx.doi.org/10.1094/PHI-A-2006-1117-02 [ Links ]
Qi R, Wang T, Zhao W, Li P, Ding J and Gao Z. 2012. Activity of ten fungicides against Phytophthora capsici isolates resistant to Metalaxyl. Journal Phytopathology 160:717-722. DOI:10.1111/jph.12009 [ Links ]
Rios-Velasco C, Caro-Cisneros JN, Berlanga-Reyes DI, Ruíz-Cisneros MF, Ornelas-Paz JJ, Salas-Marina MA, Villalobos-Pérez E and Guerrero-Prieto VM. 2016. Identification and antagonistic activity in vitro of Bacillus spp. and Trichoderma spp. isolates againts common phytopathogenic fungi. Revista Mexicana de Fitopatología 34:84-99. http://dx.doi: 10.18781/R.MEX.FIT.1507-1 [ Links ]
Sánchez J, Correa M y Castañeda-Sandoval LM. 2016. Bacillus cereus un patógeno importante en el control microbiológico de los alimentos. Revista Facultad Nacional de Salud Pública 34(2):230-242. DOI: 10.17533/udea.rfnsp.v34n2a12 [ Links ]
Sanogo S and Ji P. 2013. Water management in relation to control of Phytophthora capsici in vegetable crops. Agricultural Water Management 129:113-119. https://doi.org/10.1016/j.agwat.2013.07.018 [ Links ]
Segarra G, Aviles M, Casanova E, Borrero A and Trillas I. 2013. Effectiveness of biological control of Phytophthora capsici in pepper by Trichoderma asperellum strain T34. Phytopathology Mediterranea 52(1):77-83. http://hdl.handle.net/11441/30458 [ Links ]
Shanmugam V and Kanoujia N. 2011. Biological management of vascular wilt of tomato caused by Fusarium oxysporum f. sp. lycospersici by plant growth-promoting rhizobacterial mixture. Biological Control 57:85-93. https://doi.org/10.1016/j.biocontrol.2011.02.001 [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2016. Cierre de la producción agrícola por estado. www.siap.gob.mx. [ Links ]
Thampi A and Bhai RS. 2017. Rhizosphere actinobacteria for combating Phytophthora capsici and Sclerotium rolfsii, the major soil borne pathogens of black pepper (Piper nigrum L.). Biological Control 109:1-13. http://dx.doi.org/10.1016/j.biocontrol.2017.03.006 [ Links ]
Torres MJ, Brandan CP, Sabaté DC, Petroselli G, Erra-Balsells R and Audisio MC. 2017. Biological activity of the lipopeptide-producing Bacillus amyloliquefaciens PGPBacCA1 on common bean Phaseolus vulgaris L. pathogens. Biological Control. 105:93-99. http://dx.doi.org/10.1016/j.biocontrol.2016.12.001 [ Links ]
Torres MJ, Perez Brandan CP, Petroselli G, Erra-Balsells R and Audisio MC. 2016. Antagonistic effects of Bacillus subtilis subsp. subtilis and B. amyloliquefaciens against Macrophomina phaseolina: SEM study of fungal changes and UV-MALDI-TOF MS analysis of their bioactive compounds. Microbiological Research 182:31-39. https://doi.org/10.1016/j.micres.2015.09.005 [ Links ]
Wang CK, Liang CY, Chu CH and Lin MJ. 2011. Genetic comparison of sexual and asexual reproduction of Phytophthora capsici. Plant Pathology Bulleting 20:98-107. Disponible en línea: https://www.cabdirect.org/cabdirect/abstract/20123409244 [ Links ]
Wei Z, Huang J, Yang Ch, Xu Y, Shen Q and Chen W. 2015. Screening of suitable carriers for Bacillus amyloliquefaciens strain QL-18 to enhance the biocontrol of tomato bacterial wilt. Crop Protection 75:96-103. https://doi.org/10.1016/j.cropro.2015.05.010 [ Links ]
Yang R, Fan X, Cai X and Hu F. 2015. The inhibitory mechanisms by mixtures of two endophytic bacteria strains isolated from Ginkgo biloba against pepper phytophthora blight. Biological Control 85:59-67. http://dx.doi.org/10.1016/j.biocontrol.2014.09.013 [ Links ]
Yu SM and Lee YH. 2013. Effect of light quality on Bacillus amyloliquefaciens JBC36 and its biocontrol efficacy. Biological Control 64:203-210. https://doi.org/10.1016/j.biocontrol.2012.11.004 [ Links ]
Zhang JX, Gu YB, Chi FM, Ji ZR, Wu JY, Dong QL and Zhou ZS. 2015. Bacillus amyloliquefaciens GB1 can effectively control apple valsa canker. Biological Control 88:1-7. https://doi.org/10.1016/j.biocontrol.2015.04.022 [ Links ]
Zhang S, White TL, Martinez MC, Mclnroy JA, Kloepper JW and Klassen W. 2010. Evaluation of plant growth-promoting rhizobacteria for control of Phytophthora blight on squash under greenhouse conditions. Biological Control 53:129-135. https://doi.org/10.1016/j.biocontrol.2009.10.015 [ Links ]
Zhao P, Quan C, Wang Y, Wang J and Fan S. 2013. Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. Journal of Basic Microbiology 54:448-456. DOI: 10.1002/jobm.201200414 [ Links ]
Zheng M, Shi J, Shi J, Wanga Q and Li Y. 2013. Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the anthracnose pathogen in postharvest mangos. Biological Control 65:200-206. https://doi.org/10.1016/j.biocontrol.2013.02.004 [ Links ]
Zhi Y, Wu Q and Xu Y. 2017. Production of surfactin from waste distillers’ grains by co- culture fermentation of two Bacillus amyloliquefaciens strains. Bioresource Technology 235:96-103. http://dx.doi.org/10.1016/j.biortech.2017.03.090 [ Links ]
Received: November 07, 2017; Accepted: February 04, 2018











 texto em
texto em 


