Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.36 n.1 Texcoco Jan./Apr. 2018
https://doi.org/10.18781/r.mex.fit.1708-3
Phytopathological notes
In vitro antifungal activity of garlic essential oil (Allium sativum L.) against Alternaria tenuissima
1 Centro de Investigación en Alimentación y Desarrollo AC, unidad Culiacán, Carretera Eldorado Km 5.5, Colonia Campo El Diez, Culiacán, Sinaloa, CP 80110, México.
2 CONACYT-CIAD, Centro de Investigación en Alimentación y Desarrollo, A.C. Coordinación Culiacán, Carretera a Eldorado Km 5.5, Colonia Campo El Diez, Culiacán, Sinaloa, CP 80110 México.
Due to the environmental problems generated by the chemical control of phytopathogenic fungi, it is necessary to look for alternatives for their control. The essential oil of garlic may be a viable alternative for the control of A. tenuissima. In this study, the volatile compounds of garlic essential oil were analyzed by Gas Chromatography-Mass Spectrometry. Radial growth in vitro and biomass of A. tenuissima in presence of the garlic essential oil. Diallyl disulphide and diallyl sulphide were identified in 23.64 and 20.33%, respectively, among other compounds in a smaller proportion. At a concentration of 1000 ppm, the radial growth and biomass production were inhibited 100 and 86.20%, respectively, it compared with control PDA-Tween® 80 without oil. The medium and minimum inhibitory concentrations obtained for A. tenuissima were 229 and 1, 023 ppm, respectively. The spore germination of the fungus was inhibited 88.89% with medium minimum concentration and 94.17% with minimum inhibitory concentration. Garlic essential oil showed antifungal capacity against A. tenuissima, inhibiting spore germination, radial growth and biomass production.
Key words: control; spore germination; fungal biomass
Los problemas ambientales generados por el control químico de hongos fitopatógenos, requiere de la búsqueda de alternativas amigables con el medio ambiente para su control, como el aceite esencial de ajo (Allium sativum L.) para el control de Alternaria tenuissima. El objetivo del estudio fue caracterizar los volátiles del aceite esencial de ajo por cromatografía de gases acoplado a masas y evaluar el crecimiento radial y biomasa in vitro de A. tenuissima en presencia del aceite esencial. Se identificaron el dialil disulfuro y dialil sulfuro en un 23.64 y 20.33%, respectivamente, entre otros compuestos en menor proporción. Con la concentración de 1,000 ppm se inhibió el crecimiento radial y producción de biomasa en 100 y 86.20%, respectivamente, comparado con el testigo PDA-Tween® 80 sin aceite. Las concentraciones media y mínima inhibitorias obtenidas para A. tenuissima fueron 229 y 1,023 ppm, respectivamente. Se inhibió la germinación de esporas del hongo en 88.89 y 94.17% con las concentraciones media y mínima inhibitorias, respectivamente. El aceite esencial de ajo mostró capacidad antifúngica contra A. tenuissima inhibiendo la germinación de esporas, crecimiento radial y la producción de biomasa.
Palabras clave: control; germinación de esporas; biomasa fúngica
Alternaria tenuissima (Nees & T. Nees: Fr.) Wilshire is a fungus that causes losses in the production of crops such as apple (Malus x domestica Borkh.) and pear trees (Pyrus communis L.), broccoli (Brassica oleracea var. italica), and others. The problem becomes more important due to the production of toxins (Jones and Aldwinckle, 2002; Fraire-Cordero et al., 2010; Agamy et al., 2013). Alternaria is a widely distributed genus in agricultural areas and it has saprophytic, endophytic, and phytopathogenic species. Likewise, as a product of its activity, about 70 secondary metabolites have been identified, all toxic to plants, and some may affect human health, relating to cases of allergies and esophagus cancer (Pavón-Moreno et al., 2012; Woudenberg et al., 2015).
Traditionally, to prevent and control of the invasion of phytopathogenic fungi in agriculture, synthetic fungicides have been used (Campbell and López-Ortíz, 2014). The most widely used against Alternaria are azoxystrobin, difenoconazole, mancozeb, tebuconazole, and others (Malandrakis et al., 2015; Savitha and Ajithkumar, 2016). However, these fungicides have adverse effects on the environment and human health. It is therefore necessary to search for environmentally friendly alternatives for the control of phytopathogenic fungi (Blair et al., 2015).
Some compounds from botanical sources have the potential to be used for the control of plant pathogens in crops of agricultural interest. Considering that about 374,000 plant species have been described, there is a wide window of opportunity for the development of antifungal substances from the extracts of these plants (Cowan, 1999; Christenhusz and Byng, 2016). Among the compounds of plants with antifungal capabilities, a few that stand out are essential oils (Isman et al., 2011), some of which have proven antifungal capabilities, such as those based on lemon (Cymbopogon citratus (D.S.) Stapf.), eucaliptus (Eucalyptus spp.), cinnamon (Cinnamomum verum L.), clove teas (Syzygium aromaticum L.), and others (Calo et al., 2015). Garlic (Allium sativum L.) contains an essential oil composed of aromatics such as diallyl disulfide, diallyl trisulfate and other sulfurous compounds with antimicrobial activity with a potential to be used in fungal control (Casella et al., 2013; Kocić-Tanackov et al., 2012). The aim of this study was to evaluate the antifungal activity in vitro of garlic essential oil against A. tenuissima.
The study was conducted in the laboratories of the Centro de Investigación en Alimentación y Desarrollo, AC (Food and Development Research Center), Culiacán unit. The A. tenuissima strain was provided by the Department of Food Research and Postgraduate Studies of the University of Sonora, previously identified by Quintana-Obregón et al. (2013). It was reactivated and grown in a potato-dextrose-agar (PDA) medium at 25 °C with light periods of 12 h light-darkness for seven days. Later, spore suspensions were prepared with a Tween® 20 at 0.1% (v/v), and finally, the concentration was determined using a hematometer (Neubauer camera, BRAND ® Germany).
The garlic essential oil used was of an analytical grade (Sigma-Aldrich® lot MKBB8390V). Oil volatiles were identified using a GC-7890B (Agilent Technology®, USA) gas chromatographer linked to an Agilent 240 (CG-MS) selective mass detector with an electric ionization system of 70 eV. The capillary column used was DB-5 (50 m*0.25 mm) (J&C Scientific, Agilent Technologies®, Pennsylvania, USA) and it was conditioned at 60 °C for 10 min. The temperature was raised (20 °C/ min) up to 180 °C for 2 min and finally (4 °C/min) up to 250 °C for 4.5 min. Helium carrier gas was used at a flow of 2 mL min-1. The temperatures of the ionization chamber and of the line of transfer were 220 and 280 °C, respectively. The constituents were identified by comparing linear retention indices based on a mixture of n-alkanes and times of retention of the spectra obtained using database NIST 98 (National Institute of Standard and Technology, Maryland, EUA).
Radial Growth. The PDA with Tween® 80 (1% v/v) was sterilized for 15 min at 15 psi, then cooled at 45 °C and mixed with garlic essential oil at different concentrations (0, 10, 100, 500, 1,000 and 10,000 ppm). In addition, PDA was prepared without Tween® 80 under the same conditions, and without mixing it with garlic essential oil, each mixture was placed, 15 mL at a time, in Petri dishes with diameter of 50 mm. Later, using a sterilized glass Pasteur pipette, a 6 mm perforation was made in the center of the solidified culture medium and it was inoculated with 25 µL of the A. tenuissima spore solution (105 spores). Finally, the cultures were incubated at 25 °C with photoperiods of 12 h light-darkness and the radius of the fungal colony was measured every 24 h until it covered 95% of the control (PDA without Tween® 80) taken as a reference. The minimum inhibiting (CMI) and average inhibiting (CI50) conditions were determined at 120 h post-inoculation of the fungus with a Probit analysis with the statistical program NCSS 2000 (Number Cruncher Statistical Systems, Utah, USA).
Production of Biomass. The Petri dishes with oil concentrations described above were inoculated with 25 µL of the A. tenuissima spore suspension (105 spores) in the central section, spreading them by surface diffusion and kept in the same conditions as described above. After 120 h, the dry weight was taken of the cultures that grew in the culture medium, and for this, the mycelium was separated from the Petri dish and transferred to a precipitate beaker with 50 mL of distilled water; it was sterilized and the mycelium was separated with Whatman® # 2 filter paper. The filter paper, along with the mycelium, was dried in an air convection over at 105 °C for 2 h and collected in a desiccator. The dry weight was expressed in mg dish-1 (Larralde-Corona et al., 1997; López-Insunza et al., 1997).
Spore Germination. An evaluation was carried out on the effect of the CI50 and CMI of the essential oil obtained with a Probit analysis against the A. tenuissima spores. For this, 25 μL of the spore suspension (105 spores) were distributed on the surface of the culture medium in the Petri dish in controls and treatments with essential oils. The Petri dishes were incubated at 25 ºC with a photoperiod of 12 h light-darkness for up to 24 h. Random samples of each were taken every 4 h and the number of germinated and not germinated spores were counted, out of a total 200 spores, under a light microscope. The spore was considered germinated when the length of the germinative tube was at least 50% as long as the not germinated spore (Dantigny et al., 2006).
Experimental Design and Statistical Analysis. A randomized design was used in the experiment, in which the treatments were: PDA, PDA-Tween® 80 (controls) and concentrations of garlic essential oils in PDA-Tween® 80 (10, 100, 250, 500 and 1,000 ppm). The seven treatments were evaluated against A. tenuissima , where the experimental unit was a Petri dish. The response variables were radial growth and production of biomass. CI50 and CMI were evaluated in the spore germination stage. All experiments were carried out three times. Data were analyzed using the statistical program JMP version 5.0 (SAS, 2002) for the analysis of variance (ANOVA) and the averages of the treatments were separated using a Tukey test (p≤0.05).
Table 1 shows the tentative main volatile components of the garlic extracts obtained by CG-MS with relative proportions ≥1%. There is a predominance of compounds with sulfur in their structures, some of the most important of which are diallyl sulfate and diallyl disulfate, which derive from the activity of the enzyme allinase when released by rupture from the tissue during the oil extraction (Kocić-Tanackov et al., 2012).
Table 1 Main components of essential garlic oil identified by structural recognition using CG-MSx.
| Tiempo de Retención (min) | Identificación | Cantidad Relativa (%) |
| 6.867 | Dialil sulfuro | 20.33 |
| 8.885 | 1,3-Ditiano | 6.1 |
| 11.205 | Ciclopentano, 1-acetil-1,2-epoxi | 2.51 |
| 12.144 | [1,3] Ditiano-2-ona | 1.15 |
| 13.784 | Dialil disulfuro | 23.64 |
| 14.038 | 2-etileden [1,3] ditiano | 3.54 |
| 14.6893 | Hidroxiperóxido, 1,4-dioxan-2-il | 6.89 |
| 15.314 | 3-Vinil-1, 2-dithiacyclohex-4-ene | 2.02 |
| 19.526 | Cyclopenteno, 3-metil-3-(trimethylsily) acetil- | 1.94 |
x With relative proportions ≥ 1%.
The radial growth of A. tenuissima in the treatment with PDA medium only covered 95% of the Petri dish after 120 h, reaching a radius of 22 mm. There were no significant differences in the radial growth in treatments PDA and PDA-Tween® 80 after 120 h. The essential oil achieved a significant reduction of the radial growth of A. tenuissima with concentrations equal to or greater than 250 ppm, achieving, at 1,000 ppm, an inhibition of 100% in regard to the control PDA-Tween® 80 (Table 2).
Table 2 Anti-fungal effect of essential garlic oil at different concentrations on the production of biomass of Alternaria tenuissima after 120 h.
| Aceite esencial de ajo (ppm) | Crecimiento radial (mm) ±DE | Biomasa (mg caja-1) ±DE |
| 0 (PDA) | 22±0.0ax | 161.13±11.60cx |
| 0 (PDA-Tween® 80) | 22±0.0a | 522.60±83.01b |
| 10 | 22±0.0a | 692.35±16.47a |
| 100 | 20±2.17a | 148.10±19.74cd |
| 250 | 9.67±0.76b | 101.00±1.13cde |
| 500 | 2.83±0.56c | 68.03±1.86e |
| 1000 | 0±0.0d | 72.10±7.23de |
xDifferent letters between columns indicate statistical differences according to the Tukey test (p≤0.05). Average values of at least three replicas.
In the production of biomass, medium PDA-Tween® 80 with 10 ppm of essential oil showed a significant increase in regard to the PDA alone. However, when increasing the concentration of essential oil to 500 and 1,000 ppm, there were significant differences with the controls, presenting inhibition on the growth of A. tenuissima at these concentrations, with the inhibition of biomass production being of 86.20% at 1,000 ppm of oil in regard to the control PDA-Tween® 80 (Table 2). The increase in biomass in a culture medium with Tween® 80 at 10 ppm could be due to the interaction between Tween® 80 and the essential oil. Reportedly, Tween® 80 can increase cell permeability, favoring nutrient absorption; in Aspergillus fumigatus it has been suggested that the compound is used as a source of carbon (Inouye et al., 2001; Taoka et al., 2011). Since the concentration of Tween® 80 is greater than the essential oil (10 ppm) the surface of mycelial contact is competed for by Tween® 80, reducing the antifungal activity of the oil on its own (Inouye et al., 2001). When increasing the concentration of essential oil (500 and 1,000 ppm) the oil-mycelium interaction increases, favoring its bioactivity, shown by the significant reduction of biomass at 500 and 1,000 ppm in regard to the PDA-Tween® 80 control (Table 2).
The CI50 and CMI of 229 ppm and 1,023 ppm, respectively, were obtained from the radial growth. The kinetics of the germination of A. tenuissima spores exposed to the CI50 and CMI of garlic essential oil (Figure 1) showed significant differences with those of the control averages (PDA and PDA-Tween® 80) with spore germination inhibitors of 88.89 and 94.17% for CI50 y CMI, respectively.
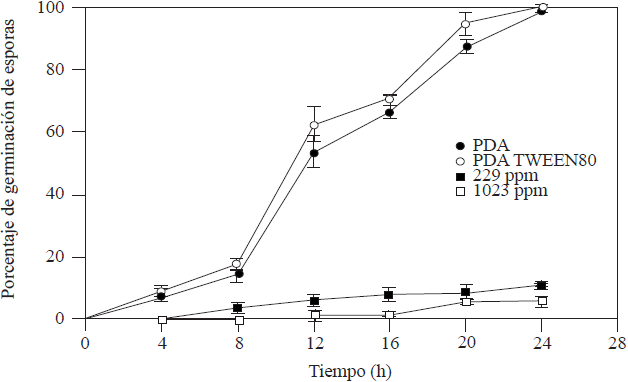
Figure 1 Kinetics of the germination of A. tenuissima spores at 25 °C and photoperiods of 12 h in PDA, PDA-Tween® 80, CI50 and CMI of essential garlic oil.
According to Inouye et al. (2001), the inhibiting capacity of the volatile compounds of essential oils stands out for having three stages of fungal development: spore germination, vegetative mycelia, and reproductive mycelia. This effect of garlic essential oil has been reported in growth stages of Aspergillus versicolor and Penicillium funiculosum (Kocić-Tanackov et al. 2012; Li et al., 2014). When evaluating the CMI of the essential oil versus A. tenuissima, a fungistatic effect was observed. It is possible that garlic essential oil interacts with the cell membrane, and when its concentration is reduced in time by the volatility of its components, the interaction diminishes and the fungus normalizes its metabolism. Tian et al. (2012) found alterations in the cell membrane of Aspergillus flavus, particularly ergosterol, when treated with dill (Anethum graveolens). The antimicrobial action mechanism of sulfured compounds has been related the interaction with hydrogen sulfide groups of the cell and the disulfide bonds that may form (El-Sayed et al., 2017); it is possible that the inhibition in A. tenuissima is due to this type of chemical interactions. However, additional studies are necessary to elucidate mechanisms of interaction of the garlic essential oil on the in vitro growth of A. tenuissima.
Literatura citada
Agamy R, Alamri S, Moustafa MFM and Hashem M. 2013. Management of tomato leaf spot caused by Alternaria tenuissima Wiltshire using salicylic acid and agrileen. International Journal of Agriculture and Biology 15: 266-272. Disponible en línea: https://www.researchgate.net/profile/M_Hashem/publication/286315953_Management_of_Tomato_Leaf_Spot_Caused_by_Alternaria_tenuissima_Wiltshire_using_Salicylic_Acid_and_Agrileen/links/570e369408aec783ddd1ba7b.pdf [ Links ]
Blair A, Ritz B, Wesseling C and Freeman LB. 2015. Pesticides and human health. Occupational and Environmental Medicine 72:81-82. http://dx.doi.org/10.1136/oe-med-2014-102454 [ Links ]
Calo JR, Crandall PG, O’Bryan CA, and Ricke SC. 2015. Essential oils as antimicrobials in food systems-A review. Food Control 54: 111-119. http://dx.doi.org/10.1016/j.fo-odcont.2014.12.040 [ Links ]
Campbell WB and López-Ortiz S. 2014. Sustainable Food Production Includes Human and Environmental Health, Issues in Agroecology -Present Status and Future Prospectus (vol.3). Springer Science and Business Media, New York London. 232p. [ Links ]
Casella S, Leonardi M, Melai B, Fratini F and Pistelli L. 2013. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytotherapy Research 27: 380-383. http://dx.doi.org/10.1002/ptr.4725 [ Links ]
Christenhusz MJ and Byng JW. 2016. The number of known plants species in the world and its annual increase. Phytotaxa 261: 201-217. http://dx.doi.org/10.11646/phyto-taxa.261.3.1 [ Links ]
Cowan MM. 1999. Plant products as antimicrobial agents. Clinical Microbiology Reviews 12: 564-582. Disponible en línea: http://cmr.asm.org/content/12/4/564.long [ Links ]
Dantigny P, Bensoussan M, Vasseur V, Lebrihi A, Buchet C, Ismaili-Alaoui M. and Roussos S. 2006. Standardisation of methods for assessing mould germination: A workshop report. International Journal of Food Microbiology 108: 286-291. https://doi.org/10.1016/j.ijfoodmicro.2005.12.005 [ Links ]
El-Sayed HS, Chizzola R, Ramadan AA, and Edris AE. 2017. Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and self-micro emulsifying water based delivery systems. Food Chemistry 221: 196-204. https://doi.org/10.1016/j. foodchem.2016.10.052 [ Links ]
Fraire-Cordero ML, Nieto-Ángel D, Cárdenas-Soriano E, Gutiérrez-Alonso G, Bujanos-Muñiz R.L. y Vaquera-Huerta H. 2010. Alternaria tenuissima, A. alternata y Fusarium oxysporum hongos causantes de la pudrición del florete de brócoli. Revista Mexicana de Fitopatología 28: 25-33. Disponible en línea: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0185-33092010000100003 [ Links ]
Inouye S, Tsuruoka T, Uchida K and Yamaguchi H. 2001. Effect of sealing and Tween 80 on the antifungal susceptibility testing of essential oils. Microbiology and Immunology 45: 201-208. http://dx.doi.org/10.1111/j.1348-0421.2001.tb02608.x [ Links ]
Isman MB, Miresmailli S and Machial C. 2011. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochemistry Reviews 10: 197-204. https://doi.org/10.1007/s11101-010-9170-4 [ Links ]
Jones AL and Aldwinckle HS. 2002. Plagas y enfermedades del manzano y del peral. APS - Ediciones Mundi-Prensa, Madrid, España. 99 p. [ Links ]
Kocić-Tanackov S, Dimić G, Lević J, Tanackov I, Tepić A, Vujičić B and Gvozdanović-Varga J. 2012. Effects of onion (Allium cepa L.) and garlic (Allium sativum L.) essential oils on the Aspergillus versicolor growth and sterigma-tocystin production. Journal of Food Science 77: M278-M284. https://doi.org/10.1111/j.1750-3841.2012.02662.x [ Links ]
Larralde-Corona C, López-Insunza F, and Viniegra-González G. 1997. Morphometric evaluation of the specific growth in agar plates at high glucose levels. Biotechnology Bioengineering 56: 287-294. https://doi.org/10.1002/(SICI)1097-0290(19971105)56:3<287::AID-BIT6>3.0.CO;2-F [ Links ]
Li WR, Shi QS, Liang Q, Huang XM and Chen YB. 2014. Antifungal effect and mechanism of garlic oil on Penicillium funiculosum. Applied Microbiology and Biotechnology 98: 8337-8346. https://doi.org/10.1007/s00253-014-5919-9 [ Links ]
López-Insunza F, Larralde-Corona CP, Viniegra-González G. 1997. Mass transfer and growth kinetics in filamentous fungi. Chemical Engineering Science 52: 2629-2639. https://doi.org/10.1016/S0009-2509(97)00078-X [ Links ]
Malandrakis A, Apostolidou ZA, Markoglou A, and Flouri F. 2015. Fitness and cross-resistance of Alternaria alternata field isolates with specific or multiple resistance to single site inhibitors and mancozeb. European Journal of Plant Patholology 142: 489-499. https://doi.org/10.1007/s10658-015-0628-5 [ Links ]
Pavón-Moreno MÁ, González-Alonso I, Martín-de Santos R. y García-Lacarra T. 2012. Importancia del género Alternaria como productor de micotoxinas y agente causal de enfermedades humanas. Nutrición Hospitalaria 27: 1772-1781. http://dx.doi.org/10.3305/nh.2012.27.6.6017 [ Links ]
Quintana-Obregón EA, Plascencia-Jatomea M, Burgos-Hernández A, Figueroa-López P and Cortez-Rocha MO. 2013. Isolation and identification of fungi from leaves infected with false mildew on safflower crops in the Yaqui Valley, Mexico. Revista Mexicana de Micología 37:19-27. Disponible en línea: http://www.scielo.org.mx/pdf/rmm/v37/v37a4.pdf [ Links ]
SAS. 2002. JMP Scripting Guide V.5. SAS Institute Inc. North Carolina, USA. 456p. [ Links ]
Savitha AS, and Ajithkumar K. 2016. Evaluation of Newer Combination of Azoxystrobin and Tebuconazole for the Management of Purple Blotch of Onion. Madras Agricultural Journal 103: 233-236. Disponible en línea: http://web.a.ebscohost.com/ehost/pdfviewer/pdfviewer?vid=0&sid=a17e817e-fded-4696-801c-92182ad395fb%40sessionmgr4008 [ Links ]
Taoka Y, Nagano N, Okita Y, Izumida H, Sugimoto S and Hayashi M. 2011. Effect of Tween 80 on the growth, lipid accumulation and fatty acid composition of Thraustochytrium aureum ATCC 34304. Journal of Bioscience and Bioengineering 111: 420-424. https://doi.org/10.1016/j. jbiosc.2010.12.010 [ Links ]
Tian J, Ban X, Zeng H, He J, Chen Y and Wang Y. 2012. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PloS One 7: e30147. https://doi.org/10.1371/journal.pone.0030147 [ Links ]
Woudenberg JHC, Seidl MF, Groenewald JZ, de Vries M, Stielow JB, Thomma BPHJ and Crous PW. 2015. Alternaria section Alternaria: Species, formae speciales or pathotypes?. Studies in Mycology 82: 1-21. https://doi.org/10.1016/j.simyco.2015.07.001 [ Links ]
Received: August 12, 2017; Accepted: October 06, 2017











 text in
text in 


