Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.36 no.1 Texcoco ene./abr. 2018
https://doi.org/10.18781/r.mex.fit.1707-5
Phytopathological notes
Differential gene expression of avocado defense genes in response to avocado sunblotch viroid infection
1 Posgrado de Recursos Genéticos y Productividad-Fisiología Vegetal, Colegio de Postgraduados, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Estado de México, CP 56230, México.
2 Posgrado en Fitosanidad-Fitopatología, Colegio de Postgraduados, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Estado de México, CP 56230, México.
Involvement of pathogenesis related proteins in defense against some of the most important avocado pathogens has been shown. However, infections caused by Avocado sunblotch viroid (ASBVd) require further research to elucidate the response components. Avocado response to ASBVd infections can be either asymptomatic or symptomatic, which further raises questions about plant defense mechanisms. This research analyzed the expression of genes PaNPR1, EREBP, PR-5 and PR-6, which encode defense proteins, in symptomatic and asymptomatic avocado leaves and fruits infected with ASBVd. Data analysis shows that ASBVd infections modify the expression of PR-5 and PR-6, while the expression of PaNPR1 and EREBP does not change. Expression differences on fruit were more evident with PR-5 on fruit from asymptomatic trees; PR-6 had higher expression on infected fruits but there were no differences between symptomatic and asymptomatic fruits. The coordinated expression of both genes on fruit suggest the activation of a synergistic defense mechanism in response to ASBVd infection.
Key words: Avocado sunblotch viroid; Persea americana; PR genes
En aguacate se ha demostrado la participación de proteínas relacionadas con patogénesis en la defensa contra algunos de sus patógenos más importantes. Sin embargo, en la enfermedad de la mancha de sol del aguacate causada por el Avocado sunblotch viroid (ASBVd) aún quedan algunos componentes de la respuesta de defensa por aclarar. En particular, la infección por ASBVd puede ser asintomática o causar síntomas, lo cual genera incógnitas sobre el mecanismo de defensa de la planta. En esta investigación se analiza la expresión de genes codificantes de proteínas de defensa, PaNPR1, EREBP, PR-5 y PR-6 en hojas y frutos de aguacate infectado por ASBVd, en condición sintomática y asintomática. El análisis de datos muestra que la infección de ASBVd modifica la expresión de los genes PR-5 y PR-6, mientras que la de PaNPR1 y EREBP no se modifica. Se encontraron diferencias en la expresión en frutos, siendo notable con PR-5 en frutos de árboles asintomáticos; a su vez, PR-6 se expresa más en frutos infectados pero sin diferencias entre sintomático y asintomático. La expresión coordinada de ambos genes en frutos sugiere la activación de un mecanismo sinérgico de respuesta de defensa a la infección de ASBVd.
Palabras clave: Avocado sunblotch viroid; Persea americana; genes PR
Avocado sunblotch is a disease caused by Avocado sunblotch viroid (ASBVd), one of the most persistent infections that affects avocado production in Mexico (Vallejo-Pérez et al., 2017). Avocado sunblotch causes cytopathic effects and physiological changes (Di Serio et al., 2013; Saucedo-Carabez et al., 2014; Vallejo-Pérez et al., 2014; Saucedo-Carabez et al., 2015; Vallejo-Pérez et al., 2015) that result in smaller fruit, low external and internal quality and reduced yield. Severe damages on fruit include depressed wounds on the surface with yellow to necrotic spots (Desjardins, 1987). In spite of its severity, a particular feature of ASBVd is that it causes asymptomatic infections in trees (Semancik and Szychowski, 1994), a fact that poses a high risk of non-intentional dispersion by plant material or seed. The first strategy to control this disease is the identification of infected trees, followed by identification of the defense mechanisms that allow a tree to show no symptoms.
Genes regulated during the defense response to pathogens in avocado have been found in gene expression profiles and have shown the molecular basis of the plant-pathogen interaction. These studies have mainly focused on infections by Colletotrichum gloeosporioides (Djami-Tchatchou et al., 2012) and Phytophthora cinnamomi (Mahomed and Van Den Berg, 2011). PR genes in avocado are particularly important because they favor an induced immune mechanism known as systemic acquired resistance. This study initiated with the hypothesis that the gene expression cascade in the avocado defense response to ASBVd (responsible for avocado sunblotch infection) is different between asymptomatic and symptomatic trees, possibly due to differential defensive capacity against the disease. Thus, this study described changes in the level of expression of defense genes selected in symptomatic and asymptomatic ASBVd-infected plants.
Mature leaves and unripe fruit from nine avocado trees (Persea americana Mill.) cv. Hass were collected in a commercial orchard (19º26’6” N 101º52’28” O, 1610 m altitude). Trees were previously classified as healthy, positive for ASBVd with visible symptoms, and positive for ASBVd without symptoms (Vallejo-Pérez et al., 2015), and with no visible symptoms from another disease. Three leaves and three fruits from three trees of each group were sampled.
Four genes associated with pathogen defense in avocado were selected: PaNPR1 (Backer et al., 2015), PR-5, PR-6 and EREBP (Djami-Tchatchou et al., 2013). The actin gene (Reeksting et al., 2014) was used as a reference gene. Sequences of the primers were taken from the mentioned references. ASBVd detection required primers designed by Schnell et al. (1997).
Total RNA extraction involved tandem use of PureLink® Plant RNA Reagent (Invitrogen) and Direct-zol® RNA MiniPrep (Zymo Research) as per manufacturer’s directions to deal with high phenol and polysaccharides content in sample tissue. RNA concentration and quality were measured in a Nanodrop 8000 (Thermo Scientific, USA), and the integrity of each sample was verified on a denaturing gel, according to the protocol by Aranda et al. (2012).
A mixture (10 μL final volume per reaction) containing the following substances was prepared to perform reverse transcription and amplification: 1X of Reaction Mix buffer (0.2 mM of each dNTP and 1.6 mM of MgSO4), 0.2 μM of sense primer, 0.2 mM of antisense primer, 0.4 μL of Super Script® III RT/Platinum® TaqMix (Invitrogen), and 100 ng of total RNA; the volume was completed by adding DEPC-treated water. PCR reactions were conducted in a thermocycler (Applied Biosystems 2720, EUA) under the following conditions: cDNA synthesis 1 cycle of 32 min at 50 °C; 1 cycle of 2 min at 94 °C, followed by 40 cycles of 15 s at 94 °C, 15 s using the calculated annealing temperature (TA) for each primer (Table 1), and 15 s at 68 °C. Finally, a 5 min elongation step at 68 °C was added. RT-PCR products were separated by electrophoresis in 2%/TAE agarose gel at 80 V for 60 min. Gels were exposed to UV light in a UV-transilluminator (Vilber Lourmat Infinity-1000/26MX, France) to capture images of the final products. Pathogen detection tests were carried out using single-step duplex RT-PCR, ASBVd and actin primers. The mixture used for a 10 μL final volume of reaction contained 1X Reaction Mix buffer, 0.2 μM of both primers (sense and antisense), 0.4 μL of SuperScript® III RT/Platinum® TaqMix, and 100 ng of total RNA; the volume was completed by adding DEPC-treated water. Amplification conditions were similar to those described previously. Defense genes and ASBVd viroid were detected in three samples of mature leaves and three samples of unripe fruit per group from healthy, symptomatic and asymptomatic trees. Nine viroid bands from leaf tissue, four from symptomatic trees and five from asymptomatic trees were sequenced (Macrogen Inc, Seul, Corea) and compared using BLASTn from the NCBI.
Table 1 Calculated annealing temperature (TA) for one-step RT-PCR, and size of the expected products in base pairs (pb).
| Iniciador | TA (°C) | Tamaño del producto (pb) |
| ASBVd | 52.0x | 250 |
| Actina | 57.1 | 103 |
| PaNPR1 | 56.3 | 119 |
| PR-5 | 55.6 | 171 |
| PR-6 | 52.0 | 158 |
| EREBP | 61.6 | 96 |
xAnnealing temperature to detect ASBVd through duplex RT-PCR.
Band density was compared using image processing with ImageJ® (Schneider et al., 2012). Band density values were normalized by data transformation √(x+1), compared with the actin density values and then plotted. The resulting graphs display a profile of the average relative intensity taken from a constant region of interest. Differences between tissue and trees were analyzed using Student’s t-test. Statistical significance was considered as p ≤ 0.05.
The detection test for ASBVd on tissue from infected trees amplified a band of approximately 250 pb, which coincides with the expected size of the studied pathogen; this band is not present in healthy trees (Figure 1). Amplification of the actin gene shows that a reaction occurred in all the analyzed samples. Sequencing of the bands highly matched three ASBVd isolates (KF562705.1, KF562706.1 and KF562704.1, greater than 92% identity, and E-value less than 7x10-67, data not shown) found in avocado production areas (Beltrán et al., 2014).
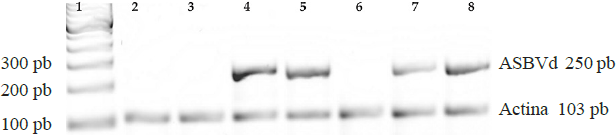
Figure 1 Detection of Avocado sunblotch viroid (ASBVd) in avocado. Amplified products using duplex one-step RT-PCR. Tissues that tested positive for ASBVd show two products, one that belongs to the 250 bp viroid, and one from the amplification of the 103 bp actin gene. Lane 1, 100 bp marker; Lanes 2 and 3, healthy leaf samples; Lane 4 and 7, leaf and fruit samples from asymptomatic trees; Lane 6, fruit sample from healthy tree; Lanes 5 and 8, leaf and fruit samples from trees with visible symptoms. The band corresponding to the viroid is expressed only in infected trees.
Amplified products separated by electrophoresis are shown in Figure 2. Healthy tree samples show basal expression of the tested genes and serve as expression reference to ASBVd infection between infected trees (symptomatic and asymptomatic) and healthy trees. Surface and percentage data calculated by ImageJ® were used to calculate relative density of each band; this relative density was adjusted to the level of expression of the actin gene reference (Figure 3).
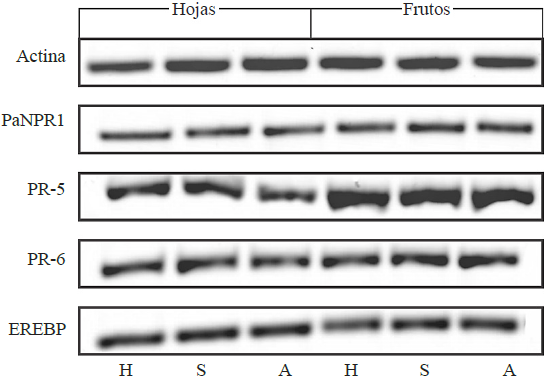
Figure 2 Amplification products from defense genes. The first three lanes correspond to leaf tissues, and the other three to fruit tissue. H, healthy; S, symptomatic; A, asymptomatic.
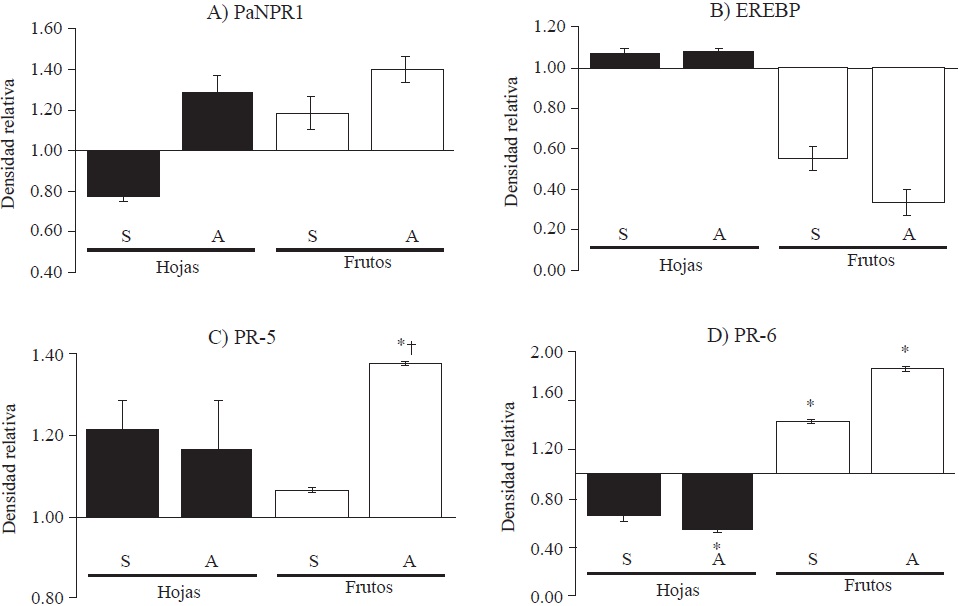
Figure 3 Quantitative analysis of band intensity from RT-PCR bands for defense-related genes. The average of the adjusted relative densities is shown; changes in intensity are negative or positive depending on the value of the basal expression assigned as 1. Vertical lines on each bar represent the standard error (n=3). S, tissue of trees with symptoms; A, tissue of asymptomatic trees; *, significant difference in relation to healthy tissue; †, significant difference between symptomatic and asymptomatic.
The PaNPR1 and EREBP genes did not show significant differences in differential expressions among infected, symptomatic or asymptomatic trees, nor regarding their basal expression on healthy tissue (Figures 3A and 3B). PR-5 expression on asymptomatic trees is over-expressed and significantly different from expression of symptomatic trees and basal expression, while fruits from symptomatic trees did not show any difference from basal expression (Figure 3C). No expression differences were found in leaves from symptomatic and asymptomatic trees, nor in the basal expression. The expression of PR-6 on fruit infected by ASBVd was up-regulated and, although it increased in asymptomatic fruits, no difference was observed on fruits from symptomatic trees (Figure 3D). As for leaf tissue, a significant down-regulation was observed on asymptomatic trees.
It is documented that changes in NPR1 gene expression induce changes in PR-5 expression (Shah et al., 2001). However, results in this research suggest that changes in PR-5 expression on tissue infected by ASBVd were not produced by changes in PaNPR1 expression because it did not change significantly from basal. Backer et al. (2015) reported that PaNPR1 constitutive expression was higher on leaves than on unripe fruits. Additionally, and contrary to documented reports on NPR1 activation by salicylic acid, they found PaNPR1 down-regulation 12 h after salicylic acid treatment; this behavior suggests an alternative function for defense responses.
On the other hand, it is known that viroids can inactivate some transcription factors, including EREBP, usually by suppressing regulation ways of mRNA associated with them (Matoušek et al., 2015; Katsarou et al., 2016). In this research, no differences were found in EREBP expression. Although ethylene is a common hormone that can simulate a pathogens attack and favor the expression of some PR proteins, it has a limited involvement in the development of symptoms induced by viroids (Hu et al., 2011), a fact that is consistent with the obtained results.
As for induction by PR-5 and PR-6 genes, the results in this research suggest that the mechanism that activates PR genes expression acts as a non-specific coordinated set of genes that varies in magnitude and can stop the primary infection (Fister et al., 2016). The expression of PR-5 on fruit from asymptomatic trees was high compared with that on other tissues, which suggest that this organ has a more active defense mechanism. The expression of PR-5 genes may occur by the action of abscisic acid, ethylene, salicylate, methyl jasmonate or any other elicitor (Velazhahan et al., 1999). Therefore, PR-5 induction in fruits from asymptomatic trees may have resulted from the activation of another signaling messenger different from ethylene, which would also explain why the EREBP transcription factor did not change its expression level (Figure 3B). However, the expression of PR-5 depends on the type of pathogen, since Colletotrichum gloeosporioides infection did not modify its expression level (Djami-Tchatchou et al., 2013).
PR-6 expression was up-regulated on fruits like PR-5. PR-6 proteins belong to the group of protease inhibitors reported to be present in an extensive range of plants that respond to diverse external stimuli, including wounds, insect-feed and microbial infections (Habib and Fazili, 2007; Turra and Lorito, 2011). The expression of PR-6 can be regulated by ethylene and activated in leaves during fruit ripening (Margossian et al., 1988); it can also be induced by exogenous applications of methyl jasmonate and abscisic acid (Wang et al., 2003). Given the poor EREBP expression on fruits from asymptomatic trees, the expression of PR-6 on them seems to be associated with induction by jasmonic acid or through an alternate route (Koiwa et al., 1997).
This study sets the basis to further explore diverse defense routes in avocado that are induced by ASBVd infection. The four studied genes showed differential expression, both in leaf tissue and fruit tissue from healthy trees and infected with ASBVd. The more significant changes in expression were recorded in fruits from asymptomatic trees with PR-5 and PR-6. In contrast, in leaf tissue from asymptomatic trees only the PR-6 basal expression was modified.
Literatura citada
Aranda PS, LaJoie DM and Jorcyk CL. 2012. Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis 33: 366-369. http://dx.doi.org/10.1002/elps.201100335 [ Links ]
Backer R, Mahomed W, Reeksting BJ, Engelbrecht J, Ibarra-Laclette E and van den Berg NN. 2015. Phylogenetic and expression analysis of the NPR1-like gene family from Persea americana (Mill.). Frontiers in Plant Science 6: 300. http://dx.doi.org/10.3389/fpls.2015.00300 [ Links ]
Beltrán-Peña H, Soria-Ruiz J, Téliz-Ortiz D, Ochoa-Martínez DL, Nava-Díaz C y Ochoa-Ascencio S. 2014. Detección satelital y molecular del viroide de la mancha de sol del aguacate (Avocado sunblotch viroid, ASBVd). Revista Fitotecnia Mexicana 37: 21-29. Disponible en línea: http://www.revistafitotecniamexicana.org/documentos/37-1/2a.pdf [ Links ]
Desjardins P. 1987. Avocado sunblotch. Pp. 299-313. In: Diener TO (eds.). The Viroids. Springer. Boston, MA., USA. 344p. http://dx.doi.org/10.1007/978-1-4613-1855-2_18 [ Links ]
Di Serio F, De Stradis A, Delgado S, Flores R and Navarro B. 2013. Cytopathic effects incited by viroid RNAs and putative underlying mechanisms. Frontiers in Plant Science 3: 288. http://dx.doi.org/10.3389/fpls.2012.00288 [ Links ]
Djami-Tchatchou AT, Straker CJ and Allie F. 2012. 454 sequencing for the identification of genes differentially expressed in avocado fruit (cv. Fuerte) infected by Colletotrichum gloeosporioides. Journal of Phytopathology 160: 449-460. http://dx.doi.org/10.1111/j.1439-0434.2012.01925.x [ Links ]
Djami-Tchatchou AT, Allie F and Straker CJ. 2013. Expression of defence-related genes in avocado fruit (cv. Fuerte) infected with Colletotrichum gloeosporioides. South African Journal of Botany 86: 92-100. https://doi.org/10.1016/j.sajb.2013.02.166 [ Links ]
Fister AS, Mejia LC, Zhang Y, Herre EA, Maximova SN and Guiltinan MJ. 2016. Theobroma cacao L. pathogenesis-related gene tandem array members show diverse expression dynamics in response to pathogen colonization. BMC Genomics 17: 363. http://dx.doi.org/10.1186/s12864-016-2693-3 [ Links ]
Habib H and Fazili KM. 2007. Plant protease inhibitors: a defense strategy in plants. Biotechnology and Molecular Biology Review 2: 68-85. Disponible en línea: http://www.academicjournals.org/journal/BMBR/article-abstract/8EB195410993 [ Links ]
Hu XX, Nie XZ, Song Y, Xiong XY and Tai H. 2011. Ethylene is involved but plays a limited role in tomato Chlorotic dwarf viroid-induced symptom development in tomato. Agricultural Sciences in China 10: 544-552. http://dx.doi.org/10.1016/S1671-2927(11)60035-7 [ Links ]
Katsarou K, Wu Y, Zhang R, Bonar N, Morris J, Hedley PE, Bryan GJ, Kalantidis K and Hornyik C. 2016. Insight on genes affecting tuber development in potato upon Potato spindle tuber viroid (PSTVd) infection. PLoS ONE 11: e0150711. http://dx.doi.org/10.1371/journal.pone.0150711 [ Links ]
Koiwa H, Bressan RA and Hasegawa PM. 1997. Regulation of protease inhibitors and plant defense. Trends in Plant Science 2: 379-384. http://dx.doi.org/10.1016/S1360-1385(97)90052-2 [ Links ]
Mahomed W and Van den Berg N. 2011. EST sequencing and gene expression profiling of defence-related genes from Persea americana infected with Phytophthora cinnamomi. BMC Plant Biology 11: 167. http://dx.doi.org/10.1186/1471-2229-11-167 [ Links ]
Margossian LJ, Federman AD, Giovannoni JJ and Fischer RL. 1988. Ethylene-regulated expression of a tomato fruit ripening gene encoding a proteinase inhibitor I with a glutamic residue at the reactive site. Proceedings of the National Academy of Sciences 85: 8012-8016. Disponible en línea: http://www.pnas.org/content/85/21/8012.full.pdf [ Links ]
Matoušek J, Piernikarczyk RJ, Týcová A, Duraisamy GS, Ko-cábek T and Steger G. 2015. Expression of SANT/HTH Myb mRNA, a plant morphogenesis-regulating transcription factor, changes due to viroid infection. Journal of Plant Physiology 183: 85-94. http://dx.doi.org/10.1016/j. jplph.2015.06.001 [ Links ]
Reeksting BJ, Coetzer N, Mahomed W, Engelbrecht J and Van Den Berg N. 2014. De novo sequencing, assembly, and analysis of the root transcriptome of Persea americana (Mill.) in response to Phytophthora cinnamomi and flooding. PLoS ONE 9: e86399. http://dx.doi.org/10.1371/journal.pone.0086399 [ Links ]
Saucedo-Carabez JR, Téliz-Ortiz D, Ochoa-Ascencio S, Ochoa-Martínez D, Vallejo-Pérez MR and Beltrán-Peña H. 2014. Effect of Avocado sunblotch viroid (ASBVd) on avocado yield in Michoacan, Mexico. European Journal of Plant Pathology 138: 799-805. http://dx.doi.org/10.1007/s10658-013-0354-9 [ Links ]
Saucedo-Carabez JR, Téliz-Ortiz D, Ochoa-Ascencio S, Ochoa-Martínez D, Vallejo-Pérez MR and Beltrán-Peña H. 2015. Effect of Avocado sunblotch viroid (ASBVd) on the postharvest quality of avocado fruits from Mexico. Journal of Agricultural Science 7: 85-92. http://dx.doi.org/10.5539/jas.v7n9p85 [ Links ]
Schneider CA, Rasband WS and Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671-675. http://dx.doi.org/10.1038/nmeth.2089 [ Links ]
Schnell R, Kuhn D, Ronning CM and Harkins D. 1997. Application of RT-PCR for indexing Avocado sunblotch viroid. Plant Disease 81: 1023-1026. http://dx.doi.org/10.1094/PDIS.1997.81.9.1023 [ Links ]
Semancik JS and Szychowski JA. 1994. Avocado sunblotch disease: a persistent viroid infection in which variants are associated with differential symptoms. Journal of General Virology 75: 1543-1549. http://dx.doi.org/10.1099/0022-1317-75-7-1543 [ Links ]
Shah J, Kachroo P, Nandi A and Klessig DF. 2001. A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. The Plant Journal 25: 563-574. http://dx.doi.org/10.1046/j.1365-313x.2001.00992.x [ Links ]
Turra D and Lorito M. 2011. Potato type I and II proteinase inhibitors: modulating plant physiology and host resistance. Current Protein and Peptide Science 12: 374-85. http://dx.doi.org/10.2174/138920311796391151 [ Links ]
Vallejo-Pérez MR, Téliz-Ortiz D, Colinas-León MT, De La Torre-Almaraz R, Valdovinos-Ponce G, Nieto-Ángel D and Ochoa-Martínez DL. 2015. Alterations induced by Avocado sunblotch viroid in the postharvest physiology and quality of avocado “Hass” fruit. Phytoparasitica 43: 355-364. http://dx.doi.org/10.1007/s12600-015-0469-y [ Links ]
Vallejo-Pérez MR, Téliz-Ortiz D, De La Torre-Almaraz R, Valdovinos-Ponce G, Colinas-León MT, Nieto-Ángel D and Ochoa-Martínez DL. 2014. Histopathology of avocado fruit infected by Avocado sunblotch viroid. Journal of Agricultural Science 6: 158-65. http://dx.doi.org/10.5539/jas.v6n9p158 [ Links ]
Vallejo-Pérez MR, Téliz-Ortiz D, De La Torre-Almaraz R, López-Martínez JO, Nieto-Ángel D. 2017. Avocado sunblotch viroid: pest risk and potential impact to Mexico. Crop Protection 99:118-127. http://dx.doi.org/10.1016/j.cropro.2017.05.015 [ Links ]
Velazhahan R, Datta SK and Muthukrishnan S. 1999. The PR-5 Family: Thaumatin-like Proteins. Pp107-129 In: Datta SK and Muthukrishnan S (eds.). Pathogenesis-Related Proteins in Plants. CRC Press. Boca Raton, FL., USA. 291p. http://dx.doi.org/10.1201/9781420049299.ch5 [ Links ]
Wang HY, Huang YC, Chen SF and Yeh KW. 2003. Molecular cloning, characterization and gene expression of a water deficiency and chilling induced proteinase inhibitor I gene family from sweet potato (Ipomoea batatas Lam.) leaves. Plant Science 165: 191-203. http://dx.doi.org/10.1016/ S0168-9452(03)00158-4 [ Links ]
Received: July 29, 2017; Accepted: October 09, 2017











 texto en
texto en 


