Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.35 n.3 Texcoco Sep. 2017
https://doi.org/10.18781/r.mex.fit.1705-2
Scientific articles
Genetic resistance to Sporisorium reilianum f. sp. zeae in selected maize (Zea mays L.) lines with white and yellow endosperm
1Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria, Unidad Integral de Diagnóstico, Servicios y Constatación. Carretera Federal México-Pachuca km. 37.5, Tecámac, Edo. Méx., C.P. 55740, México.
2Instituto de Fitosanidad, Colegio de Postgraduados, km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Edo. Méx., C.P. 56230, México.
Head smut (Sporisorium reilianum f. sp. zeae) of maize (Zea mays L.) is an important disease present in Mexico, including the Mezquital and Toluca Valleys. The objective of this research was to identify C2-S1 and C2-S2 maize lines adapted to Mexican highlands to be used in a breeding program for disease resistance and good agronomic characters. Seed of lines were inoculated with a suspension of 7x107 teliospores mL-1 of S. reilianum f. sp. zeae with 1% sodium carboximethyl cellulose and planted in a greenhouse. Plants were monitored until tassel stage, when disease incidence was recorded. In 258 C2-S1 lines with white endosperm showed an incidence of 92.3% and 41.7% in 71 lines with yellow endosperm. Based on disease incidence and agronomic characters, 38 and 24 C2-S1 lines with white and yellow endosperm, respectively, were selected and self-pollinated in the field. A total of 123 and 114 C2-S2 lines with white and yellow ensoperm, respectively, inoculated with teliospores of the pathogen showed incidence of the disease up to 42.8% and 28.5%, respectively. Inoculation with teliospores and planting in the greenhouse resulted in the identification of maize lines with different percentages of infection allowing the selection of resistant lines, proving the efficiency of the technique.
Key words: Sporisorium reilianum f. sp. zeae; Zea mays; head smut; seed inoculation; resistant lines
El carbón de la espiga (Sporisorium reilianum f. sp. zeae) del maíz (Zea mays L.) es una enfermedad de gran importancia que se presenta en zonas productoras de maíz, incluyendo los Valles del Mezquital y Toluca. El objetivo del presente estudio fue identificar líneas C2-S1 y C2-S2 de maíz adaptadas al Altiplano de México para ser utilizadas en un programa de mejoramiento de resistencia y buenas características agronómicas. Semillas de cada línea se inocularon con una suspensión de 1.7x107 mL-1 teliosporas de S. reilianum f. sp. zeae en 1% de carboximetilcelulosa de sodio y se sembraron en invernadero. Las plantas fueron monitoreadas hasta la formación de espiga, donde se evaluó la incidencia. En 258 líneas C2-S1 de endospermo blanco se presentó hasta 92.3% de incidencia y hasta 41.7% en 71 líneas de endospermo amarillo. Con base en la incidencia y características agronómicas, se seleccionaron y autofecundaron en campo 38 líneas C2-S1 de endospermo blanco y 24 de endospermo amarillo. Un total de 123 y 114 líneas C2-S2 de endospermo blanco y amarillo, respectivamente, fueron inoculadas con teliosporas del patógeno y presentaron una incidencia de la enfermedad de hasta 42.8% y 28.5%, respectivamente. La inoculación con teliosporas y siembra en invernadero permitió identificar líneas de maíz con diferente porcentaje de infección y seleccionar las líneas resistentes, probando la eficiencia de ésta técnica.
Palabras clave: Sporisorium reilianum f. sp. zeae; Zea mays; carbón de la espiga; inoculación de semilla; líneas resistentes
INTRODUCTION
Maize (Zea mays L.) is susceptible to diseases in different stages of its development. Head smut, caused by Sporisorium reilianum f. sp. zeae (Kühn) Langdon and Fullerton, synonym Sphacelotheca reiliana (Khün) Clint. (Matyac and Kommedahl, 1985a; Matyac and Kommedahl, 1985b) has been a serious problem since the 1970s in the U.S.A., Mexico, Australia, China, South Africa, and France (Stromberg, 1981; Bernardo et al., 1992; Jin et al., 2000).
The symptoms of head smut are visible in flowering stage, even when the pathogen infects the root during germination and in the first stages of seedling development (Martínez et al., 2000; Martínez et al., 2002; Zhao et al., 2015). The infective mycelium invades the tissues systematically until it reaches the apical meristem (Frederiksen and Reyes, 1980; Martínez et al., 1999; Zhao et al., 2015). The main damages are caused during flowering, when the mycelium established in the meristem produces sori that replace grains in the ear (female inflorescence) and anthers. When maturing, the sori release teliospores that fall to the ground and are carried short distances by the wind (Montes and Díaz, 2006; Ghareeb et al., 2011).
Because the inoculum is found in the soil, efforts to fight this disease are focussed on avoiding infection during seedling development. In order to do this, several strategies are used, such as treating seeds with fungicides (Stienstra et al., 1985; Martínez and Ledesma, 1990; Pradhanang and Ghimire, 1996; Wright et al., 2006), cultural practices (Mack et al., 1984; Matyac and Kommedahl, 1985a), and host resistance (Baggett and Koepsell, 1983; Stromberg et al., 1984; Song et al., 2000; Aquino et al., 2011). The latter is the most convenient technology, since it avoids environmental damages and reduces costs of production.
Among the studies carried out to identify genotypes resistant to head smut, some of the most important are those performed in South Africa (Krüger, 1962), the United States (Baggett and Koepsell, 1983), Kenya (Njuguna and Odhiambo, 1989), France (Lübberstedt et al., 1999), and China (Duan et al., 1992; Wang et al., 2008). In Mexico, despite the importance of this disease in Valles Altos (States of Hidalgo and Mexico), breeding programs do not contemplate the selection for resistance and have only focused on determining percentages of incidence in hybrids and maize varieties (Pérez and Bobadilla, 2002 and 2003; Aquino et al., 2011), under natural infection conditions (Quezada et al., 2013).
In 2006 and 2007, the “National Food Health, Safety and Quality Service” (Servicio Nacional de Sanidad, Inocuidad y Calidad - SENASICA), and the “General Board for Plant Health - DGSV” (Dirección General de Sanidad Vegetal), and the Colegio de Postgraduados (CP), created the genetic improvement programs PM0531 and PM0542, which included the selection of maize populations and lines with head smut resistance for Valles Altos. In the states of Mexico and Hidalgo, maize populations were formed with a wide genetic base of white or yellow endosperm, with the aim of selecting material resistant to S. reilianum f. sp. zeae implementing a S1 recurrent selection program.
The recurrent selection method involving S1 or S2 lines are the most widely used to improve resistance to pests and diseases; they also increase their efficiency by increasing the frequency of favorable genes of one or more agronomic characteristics under selection while maintaining genetic variability to continue the selection (Hallauer and Miranda, 1988; Hallauer, 1992). The genetic variation present in open-pollinated crops helps select different levels of susceptibility to the pathogen. Therefore, the aim of this study was to evaluate the level of resistance or susceptibility of maize C2-S1 lines artificially inoculated with teliospores of the pathogen.
MATERIALS AND METHODS
Host
The formation of base maize populations with white or yellow endosperms began (C0) in the Spring-Summer cycle 2007, by recombining several sources of desirable germplasm in a nursery established in the ejido of Cinta Larga, Hgo. (20° 13´ 47” N, 99° 12´ 52” O), selected due to its high incidence of head smut under natural conditions. In the Autumn-Winter cycle 2007/08, in a nursery established in a farmer’s plot in Valle de Bravo, Mex. (19° 11´ 42” N, 100° 07´ 52” O), the C0 seeds were planted, and best plants were self-pollinated obtaining the C0-S1 seed. The nursery in Valle de Bravo, Mex. was established due to the presence of head smut and to advance a planting season under subtropical conditions without frost.
From this cycle onwards, a S1 population improvement program was followed, recombining S1 families selected for agronomic characters and resistance to head smut in the Spring-Summer cycle in Hidalgo and generating new S1 lines in the Autumn-Winter cycle in Valle de Bravo, Mex.
In 2009, two breeding cycles were completed, resulting in C2-S1 seed with a total of 258 lines of white endosperm, and 71 lines of yellow endosperm. In these lines, the response to infection by S. reilianum f. sp. zeae was evaluated by artificial inoculation of seeds and crops in greenhouse conditions.
In 2010, in the state of Hidalgo, C2-S1 lines were planted and were simultaneaously recombined and self-pollinated, resulting in C3 and C2-S2 seeds. To obtain the C2-S2 seed, six or seven plants of the best artificially inoculated C2-S1 lines were selected based on agronomic traits with a pressure of selection to head smut with 0 to 7.7% of incidence in the lines of white endosperm and 0 to 12.5% in the lines of yellow endosperm. After harvest, seeds from 123 and 114 C2-S2 ears with white or yellow endosperm, respectively, were inoculated, planted in the greenhouse, and evaluated for resistance to the pathogen.
Pathogen
The study used S. reilianum f. sp. zeae teliospores obtained from tassels and ears of maize plants infected in natural conditions in the state of Hidalgo. The teliospores were disinfested in a 1% CuSO4 solution for 24 h, washed with three changes of sterile distilled water and recovered in filter paper for drying.
To determine the viability of the inoculum, a suspension of teliospores was prepared with a concentration of 50 000 teliospores mL-1 in sterile distilled water and 0.5 mL of this suspension was spread in Petri dishes with a PDA medium. The dishes were incubated at 25 °C in darkness. The percentage of germination was determined after 96 h counting 4 compound microscope fields (40X), and the percentage of germinated teliospores was recorded.
Inoculation of the host
A suspension of teliospores at a concentration of 1.7x107 spores mL-1 was prepared in sterile distilled water with sodium carboxymethylcellulose as a sticker (NaCMC; Droguería Metropolitana, Mexico, D. F.). Twenty two seeds of each maize line, were submerged for 1 min in this suspension of teliospores and dried at 22 °C during 48 h. As a control and source of comparison, seed of the susceptible hybrid AS-910 was used.
The inoculated seeds were planted in pots with sterilized soils at a depth of 2 cm. The soil had a pH of 8.0, electric conductivity of 5.08 dSm-1, 3.23% of organic matter and a sandy-loam texture (sand 75.6%, lime 14.5%, and clay 9.9%). The pots (40 cm wide x 40 cm high) were placed in a greenhouse and distributed in a completely randomized. The treatments were 123 lines of white endosperm and 114 of yellow endosperm. The experimental unit was a pot with 22 plants and 2 repetitions per treatment. After planting, the pots were watered at field capacity. Temperature and moisture in the greenhouse and soil were taken with a data recorder (WatchDog Micro Station, Spectrum Technologies Inc.).
Evaluation of the incidence of the disease
In C2-S1 and C2-S2 lines, resistance and susceptibilty were recorded during flowering by visual observation of the symptoms of smut, both in the ear and tassel of the plants of each line. A plant was considered as susceptible when presented sori in the ear, tassel, or both.
The percentage of incidence was determined as the quotient between plants with symptoms and the total of plants in the experimental unit, multiplied by 100; later, these values were transformed by arcsine to homogenize the variances. According to the incidence, the lines were classified according to the following scale: 0%=highly resistant, 11-25%= moderately resistant, 26-50%= moderately susceptible, 51-75%= susceptible, and 76-100%= highly susceptible. The data were anlyzed using the SAS analysis system and a mean comparison.
RESULTS AND DISCUSSION
The S. reilianum f. sp. zeae teliospores used as an inoculum presented a germination of 31% after 96 h of incubation in darkness at 25 °C. The infection by S. reilianum f. sp. zeae was identified by the presence of sori in the ears, where kernels were replaced by masses of teliospores. In infected plants, ears were small (Figure 1A), with the proliferation of bracts and presence of the black masses of teliospores. To observe the infection in the ear, it was necessary to cut them in half lengthwise; in infected ears, teliospores developed inside these sori, grouped in a black mass (Figure 1B). The symptoms and signs observed in the tassel and ear coincide with those reported by White (1999) and Baggett and Kean (1989).

Figure 1 Symptoms and signs of head smut present in maize plants after seeds were inoculated with a suspension of Sporisorium reilianum f. sp. zeae with 1.7x107 teliospores mL-1 in a 1% sodium carboxymethylcellulose and planted in the greenhouse. A) Formation of sori in the tassel. B) Formation of sori in the ear.
The plants developing symptoms and signs determined the percentage of incidence of the disease in the C2-S1 and C2-S2 lines evaluated.
Evaluation of incidence in lines C2-S1
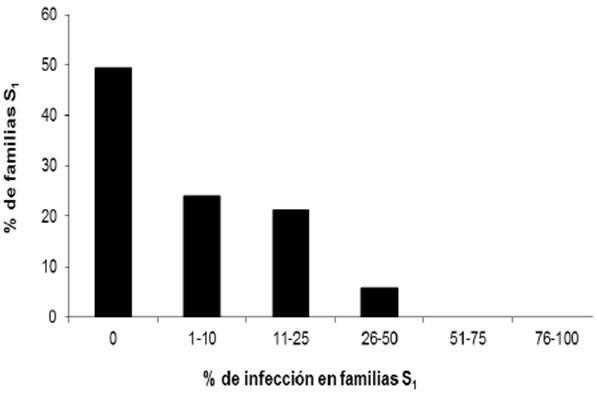
In the lines with white endosperm, only one was highly susceptible to the disease, with an incidence of 92.3%. In the yellow endosperm lines, the highest incidence values were moderately susceptible with 28.6, 30, 33.3, and 40%. The frequency of the disease in the 258 C2-S1 lines with white endosperm and 71 C2-S1 lines with yellow endosperm is shown in Figures 1 and 2, and the analysis of variance included in Table 1, showing that only one line with white endosperm presented an average incidence value of 92.3%, higher than the control, with incidence of 77.8% of the disease.
Table 1 Response of S1 lines with white or yellow endosperm to the infection of head smut (Sporisorium reilianum f. sp. zeae) with artificial seed inoculation.

In the greenhouse, average temperatures for soil and environment were 19.4 and 22.1°C, respectively, and a relative humidty of 70%, adequate conditions for the development of the disease.
Lines with the greatest susceptibility to the disease had up to 92.3 and 40% of incidence in white or yellow endosperm maize, respectively. These results suggest that maize with white endosperm is more susceptible than those with yellow endosperm.
The frequency of the disease in the 258 inoculated lines with white endosperm was: 106 lines (41.1%) did not show the disease, 47 (18.2%) had an incidence of 1-10%, 58 (22.5%) with an incidence of 11-20%, 46 (17.8 %) with an incidence of 21-70%, only one line had an average incidence value of 92.3%, greater than in the control which had a value of 77.8% of disease incidence.
In the case of the 71 S1 yellow endosperm lines, the distribution of the incidence was: 35 lines (49.3%) did not show the disease, 17 (23.9%) had an incidence of 1-10%, 13 (18.3%) had an incidence of 11-20%, and 6 (8.4%) had an incidence of 21-40%. In the greenhouse, the average temperature values were 19.4 and 22.1 °C for soil and environment, respectively, and a relative humidity of 70%. These values were favourable for disease development.
Evaluation of incidence in C2-S2 lines
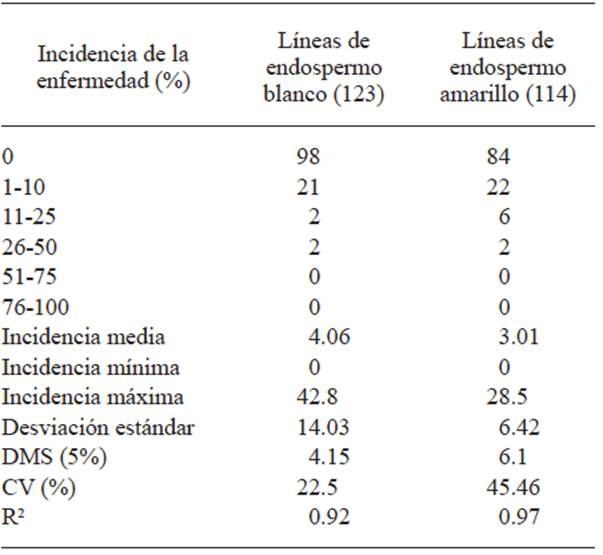
The highest head smut incidence in the tassel in C2-S2 lines with white endosperm was 42.8% and up to 28.5% in yellow endosperm lines, whereas in the control the incidence was 86.1%.
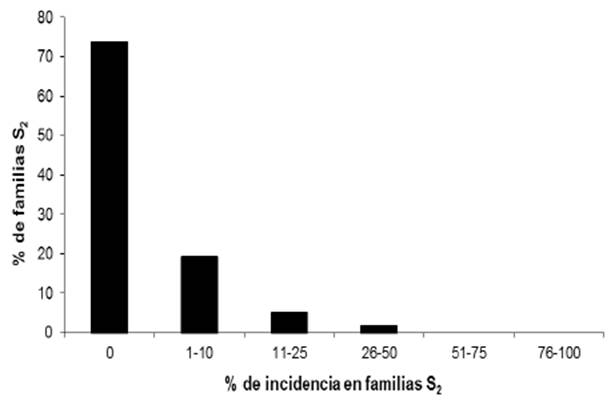
Out of the 123 lines with white endosperm, 98 (79.7%) did not show the disease and in the 114 yellow endosperm lines, 84 (73.7%) did not show the disease (Table 2). The frequency of the disease in C2-S2 lines with white and yellow endosperm is shown in Figures 3 and 4. The analysis of variance is shown in Table 2.
Table 2 Response of C2-S2 lines with white or yellow endosperm to the infection of head smut (Sporisorium reilianum f. sp. zeae) with artificial seed inoculation.
In the greenhouse, during the development of this study, average temperatures of 18.47 °C and 21.61 °C were recorded in the soil and in the environment, respectively, and an average relative humidity of 69.76%.
The response of maize plants to infection by S. reilianum f. sp. zeae has been evaluated with the aim of identifying resistant germplasm. To do this, different seed inoculation methods have been used. Njuguna and Odhianbo (1989) reported incidences of up to 62.8% when placing teliospores on the seeds at planting time. Stromberg et al. (1984), after inoculating the seeds with substrates contaminated with teliospores, obtained maximum incidences of 56% and 35.5% in maize lines and hybrids, respectively. In this study, seed inoculation was done with a 1% NaCMC solution and 1.7x107 teliospores mL-1 (Quezada et al., 2013), producing up to 92.3% incidences in C2-S1 lines with white endosperm and up to 40% in those with yellow endosperm. Using seed inoculation, it is possible to simulate the natural conditions of the infection, ensuring that the pathogen is in direct contact with the host during the seed germination process (Prom et al., 2011).
When advancing the C2-S1 lines to C2-S2, the proportion of lines with some degree of incidence decreased, and the proportion of lines with an incidence of 0% increased from 41.1 to 79.7% and from 49.3 to 73.7%, in maize with white or yellow endosperm, respectively. These results show that this method of inoculation leads to higher levels of infection and reliable selection in a program for the selection of germplasm resistant to head smut and helps in identifying lines with different response to infection. In this study, the presence or absence of sori in the ear or tassel was used as an indicator of the resistance or susceptibility. According to Zhao et al. (2015), resistance is determined by the suppression of the invasion of tissues inside the plant and not with the suppression of pathogen penetration.
Not only is important to rely in an efficient inoculation method, but temperature and soil humidity are also important factors in the epidemic. In this study, an excellent germination of teliospores was determined resulting in an infection at 18.47 and 19.4 °C, which differs with Matyac and Kommedahl (1985a), and Baier and Krüger (1962), who report that temperatures ranging between 23 and 30 °C are ideal for the germination of teliospores and seedling infection. In this study, the results obtained can be explained by the lower temperatures in the Central Mexican Highlands, where the disease is present at seed germination and first stages of development of the seedlings, the crucial stages for the infection, or by the presence of different genetic backgrounds with different behavior depending on the weather conditions and the genotype of the host (Pecina et al., 2004). The average soil humidity remained at 26.18 kPa, equivalent to 0.2618 bar, resulting in an incidence of up to 92.3% in the first cycle of evaluation. This level of humidity is different to reports by Matyac and Kommedahl (1985a), who stated that a soil humidity of 1.5 bar leads to a greater number of infected plants, with incidences of 32%.
In most maize genetic improvement programs, evaluations and selection against diseases are done under field conditions. This study showed that it is possible to combine the use of a greenhouse (greater selection pressure in controlled conditions) while perform breeding work in the field only with germplasm that has previously been evaluated and selected in the greenhouse.
Finally, the lines selected in this study have been used in the formation of synthetic varieties with a good agronomic behavior and resistance to head smut, as well as being incorporated into breeding programs for resistance to head smut in the states of Mexico and Hidalgo.
CONCLUSIONS
The S1 recurrent selection program proves to be efficient in generating and selecting lines with different degrees of susceptibility to head smut.
The technique of inoculation used was efficient in the discrimination of resistant and suscetible germplasm.
The selection of maize lines in the field with desirable agronomic characters and evaluation of infection with S. reilianum f. sp. zeae in the greenhouse are complementary methodologies for the generation of resistant lines in a genetic improvement program.
REFERENCES
Aquino-Martínez JG, Sánchez-Flores A, González-HuertaA y Sánchez-Pale JR. 2011. Resistencia de variedades e híbridos de maíz (Zea mays L.) a Sporisorium reilianum y su rendimiento de grano. Revista Mexicana de Fitopatología. 29:39-49. Disponible en línea: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0185-33092011000100004 [ Links ]
Baggett JR and Kean D. 1989. Reduction on plant height by head smut infection in sweet corn cultivars. HortScience 24:497-499. [ Links ]
Baggett JR and Koepsell PA. 1983. Field inoculation of sweet corn with the head smut pathogen (Sphacelotheca reiliana). HortScience 18:67-68. Disponible en línea: http://agris.fao.org/agris-search/search.do?recordID=US19840032591 [ Links ]
Baier W and Krüger W. 1962. Sphacelotheca reiliana on maize. II. Field studies on the effect of soil conditions. South African Journal of Agricultural Science. 5:183-190. Disponible en línea: http://hdl.handle.net/10520/AJA05858860_789 [ Links ]
Bernardo R, Bourrier M and Oliver JL. 1992. Generation means analysis of resistance to head smut in maize. Agronomie 12: 303-306. https://doi.org/10.1051/agro:19920403 [ Links ]
Duan YZ, Li XX, Ai FZ, Yang JH and Li FM. 1992. Selection and identification on resistance resource of corn head smut of Shanxi Province. Acta Agriculturae Boreali-occidentalis Sinica. 1:83-86. Disponible en línea: http://en.cnki.com.cn/Article_en/CJFDTOTAL-XBNX199204019.htm [ Links ]
Frederiksen RA and Reyes L. 1980. The head smut program at Texas A&M. p. 367-372. In: Williams, R. J., R. A. Frederiksen, L. K. Mughogho, and G. D. Bengston (eds). Sorghum Diseases: A World Review. ICRISAT. Patancheru, A. P. India. [ Links ]
Ghareeb H, Becker A, Iven T, Feussner I and Schirawski J. 2011. Sporisorium reilianum infection changes inflorescence and branching architectures of maize. Plant Physiology. 156: 2037-2052. DOI: 10.1104/pp.111.179499 [ Links ]
Hallauer AR. 1992. Recurrent selection in maize. Plant Breeding Reviews. 9:115-119. [ Links ]
Hallauer AR and Miranda JB. 1988. Quantitative genetics in plant breeding. 2nd ed. Iowa State Univ. Ames, IO. Disponible en línea: http://majidi.iut.ac.ir/sites/majidi.iut.ac.ir/files//files_course/quantitative_genetics_in_maize_breeding.pdf [ Links ]
Jin QM, Li JP, Zhang XW, Wang GX, Song SY, Liu YC, and Wang LX. 2000. Establishment IPM of system of corn diseases and pest insects in the spring corn belt. J. Maize Sci. 8:84-88. Disponible en línea: http://en.cnki.com.cn/Article_en/CJFDTOTAL-YMKX200002024.htm [ Links ]
Krüger W. 1962. Sphacelotheca reiliana on maize. I. Infection and control studies. South African Journal of Agricultural Science. 5:43-56. Disponible en línea: http://journals.co.za/docserver/fulltext/sajags/5/1/651.pdf?expires=1501296467&id=id&accname=guest&checksum=B06F86322D5C9D77F5A385962E0632DD [ Links ]
Lübberstedt T, Xia XC, Tan G, Liu X and Melchinger AE. 1999. QTL mapping of resistance to Sporisorium reiliana in maize. Theoretical and Applied Genetics. 99:593-598. Disponible en línea: https://link.springer.com/article/10.1007%2Fs001220051273 [ Links ]
Mack HJ, Baggett JR and Koepsell PA. 1984. Effects of cultural practices on the incidence of head smut in sweet corn. HortScience 19:77-78. Disponible en línea: http://agris.fao.org/agris-search/search.do?recordID=US19850004321 [ Links ]
Martínez CA, Roux C and Dargent R. 1999. Biotrophic development of Sporisorium reilianum f. sp. zeae in vegetative shoot apex of maize. Phytopathology 89:247-253. http://dx.doi.org/10.1094/PHYTO.1999.89.3.247 [ Links ]
Martínez CA, Jauneau C, Roux C, Savy C and Dargent R. 2000. Early infection of maize roots by Sporisorium reilianum f. sp. zeae. Protoplasma 213:83-92. Disponible en línea: https://link.springer.com/article/10.1007/BF01280508 [ Links ]
Martínez CA, Roux A, Jauneau A and Dargent R. 2002. The biological cycle of Sporisorium reilianum f. sp. zeae: an overview using microscopy. Mycologia 94:505-514. http://dx.doi.org/10.1080/15572536.2003.11833215 [ Links ]
Martínez-Ramírez JL y Ledesma-Medrano J. 1990. Control químico del carbón de la espiga Sphacelotheca reiliana (Kühn) Clint. del maíz, en el Valle de Zapopan, Jalisco. México. Revista Mexicana de Fitopatología. 8:68-70. [ Links ]
Matyac CA and Kommedahl T. 1985a. Factors affecting the development of head smut caused by Sphacelotheca reiliana on corn. Phytopathology 75:577-581. Disponible en línea: https://www.apsnet.org/publications/phytopathology/backissues/Documents/1985Articles/Phyto75n05_577.PDF [ Links ]
Matyac CA and Kommedahl T. 1985. Occurrence of chlorotic spots on corn seedlings infected with Sphacelotheca reiliana and their use in evaluation of head smut resistance. Plant Disease. 69:251-254. DOI: 10.1094/PD-69-251 [ Links ]
Montes GN y Díaz AF. 2006. Fitopatología. p. 192-213. In: Campo Experimental Río Bravo: 50 años de Investigación Agropecuaria en el Norte de Tamaulipas, Historia, Logros y Retos. Rodríguez del Bosque, L. A. (ed). Libro Técnico No. 1, INIFAP. Río Bravo, Tamaulipas, México. [ Links ]
Njuguna JGM. and Odhiambo RO. 1989. Head smut distribution, expression and genetic resistance of maize to Sphacelotheca reiliana in Kenya. East African Agricultural and Forestry Journal. 55:81-83. Disponible en línea: http://www.kalro.org:8080/repository/bitstream/0/2082/1/KARI%20EAAF%20JULY%20-%20OCTOBER1989%20VOL%20LV%20NOS%201%20-%202%20Split%2012.pdf [ Links ]
Pecina-Quintero V, Williams-Alanís H, Montes-García N, Rodríguez-Herrera R, Rosales-Robles E y Vidal-Martínez VA. 2004. Incidence of head smut Sporisorium reilianum (Kûhn) Langdon and Fullerton in sorghum [Sorghum bicolor (L.) Moench] hybrids with A1 and A2 cytoplasms. Revista Mexicana de Fitopatología. 22:315-319. Disponible en línea: https://www.researchgate.net/publication/237037476_Incidence_of_Head_Smut_Sporisorium_reilianum_Kuhn_Langdon_and_Fullerton_in_Sorghum_Sorghum_bicolor_L_Moench_Hybrids_with_A1_and_A2_Cytoplasms [ Links ]
Pérez-Camarillo JP y Bobadilla-Meléndez M. 2003. Carbón de la espiga de maíz. Síntesis de resultados del ciclo agrícola 2002. Valle del Mezquital, Hgo. Desplegable Téc. 6. CIRCE. INIFAP. [ Links ]
Pérez-Camarillo JP y Bobadilla-Meléndez M. 2004. Carbón de la espiga de maíz. Resultados de los ciclos agrícolas Pv 2002 y 2003. Foll. Informativo 1. CIRCE. INIFAP. [ Links ]
Pradhanang PM and Ghimire SR. 1996. Fungicide management of maize head smut (Sphacelotheca reiliana) by seed treatment. Trop. Agric. 73:325-328. Disponible en línea: http://agris.fao.org/agris-search/search.do?recordID=US201302866937 [ Links ]
Prom LK, Perumal SR, Erattaimuthu JE, Erpelding N, Montes GN, Odvody C, Greenwald Z, Jin R, Frederiksen R and Magill CW. 2011. Virulence and molecular genotyping studies of Sporisorium reilianum isolates in sorghum. Plant Disease. 95:523-529. http://dx.doi.org/10.1094/PDIS-10-10-0720 [ Links ]
Quezada S.A., De León-G. C., Hernández A.M. y Nava-D. C. 2013. Evaluación de métodos de inoculación de semillas de maíz con Sporisorium reilianum f. sp. zeae (Kûhn) Langdon &Fullerton. Revista Mexicana Fitopatologia. 31:80-90. Disponible en línea: http://www.scielo.org.mx/pdf/rmfi/v31n2/v31n2a1.pdf [ Links ]
Song SY, Sun XH, Guo WG and Liu JR. 2000. Identification of germplasm for resistance to head smut in maize. Agric. Sci. Jilin 25:32-33. [ Links ]
Stienstra WC, Kommedahl T, Stromberg EL, Matyac CA, Windels CE and Morgan F. 1985. Suppression of Corn head smut with seed and soil treatments. Plant Disease. 69:301-302. Disponible en línea: https://www.apsnet.org/publications/PlantDisease/BackIssues/Documents/1985Articles/PlantDisease69n04_301.pdf [ Links ]
Stromberg EL. 1981. Head smut of maize, a new disease in Minnesota. Phytopathology 71:906 [ Links ]
Stromberg EL, Stienstra WC, Kommedahl T, Matyac CA, Windels CE and Geadelman JL. 1984. Smut expression and resistance of corn to Sphacelotheca reiliana in Minnesota. Plant Disease. 68:880-884. Disponible en línea: https://www.apsnet.org/publications/PlantDisease/BackIssues/Documents/1984Articles/PlantDisease68n10_880.pdf [ Links ]
Wang ZH, Li XH, Xie CX, Li MS, Hao ZF, George MLC, Xiao MJ, Gao SR and Zhang SH. 2008. Genetic diversity in a collection of chinese maize inbred lines for resistance to head smut caused by Sporisorium reiliana. Maydica 53:47-54. Disponible en línea: http://www.maydica.org/articles/53_047.pdf [ Links ]
White DG. 1999. Smuts. In: Compendium of corn diseases. 3rd. Ed. APS Press. Saint Paul, MN. USA. 33 p. [ Links ]
Wright PJ, Fullerton RA and Koolaard JP. 2006. Fungicide control of head smut (Sporisorium reilianum) of sweetcorn (Zea mays). New Zealand Journal of Crop and Horticultural Science. 34:23-26. http://dx.doi.org/10.1080/01140671.2006.9514383 [ Links ]
Zhao X, Yea J, Weia L, Zhanga N, Xingc Y, Zuoa W, Chaoa Q, Tanc G, and Xua M. 2015. Inhibition of the spread of endophytic Sporisorium reilianum renders maize resistance to head smut. The Crop Journal. 3:87-95. https://doi.org/10.1016/j.cj.2015.02.001 [ Links ]
Acknowledgements
To the projects PM 0531 and PM 0542 “Development of maize (Zea mays) cultivars for the Mexican Highlands with high yield and resistance to head smut (Sporisorium reilianum f. sp. zeae)”, financed by the General Board for Plant Health of the National Food Health, Safety and Quality Service (SENASICA). To the National Science and Technology Council (CONACYT), for scholarship number 176171 for the main author.
Received: May 15, 2017; Accepted: July 28, 2017











 text in
text in