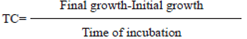Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.35 no.3 Texcoco Set. 2017
https://doi.org/10.18781/r.mex.fit.1703-6
Scientific articles
Etiology of the rotting of gladiolus corms in storage in Cuautla, Morelos, Mexico
1Instituto Politécnico Nacional-Centro de Desarrollo de Productos Bióticos, Carretera Yautepec-Jojutla km. 6, Colonia San Isidro, CEPROBI 8, C.P. 62531, Morelos, México.
2Departamento de Biología Molecular y Biotecnología, Instituto de Investigaciones Biomédicas, UNAM, Tercer circuito exterior s/n Ciudad Universitaria, Coyoacán, C.P. 04510, México, D.F.
3Centro de Investigación en Biotecnología Aplicada, Carretera Estatal Santa Inés Tecuexcomac Tepetitla Km 1.5 Tepetitla, C.P. 90700, Tlaxcala, México.
The advantages of Mexico as a floricultural producer lie in its diversity of climates and its proximity to the North American market. Currently the export of flowers and plants has been consolidated, gladiolus free of tariff. The main producing states are Puebla, State of Mexico and Morelos. Commercial production of the gladiolus cut flower is initiated through the planting of the corm; Which is affected by various pathogens that cause its rot, such as Penicillium that attacks during storage, causing limited root development and floral abortion. This causes infections through wounds and rupture sites; Is present in 90% of apparently healthy plant material. The objectives of this work were to isolate and to identify cultural, morphological and molecularly, as well as to realize tests of pathogenicity to determine the species of Penicillium that cause the rot. From the variety GrandPrix® corps (10/12) with different levels of morphological deterioration and according to visual hedonistic scale of seven-point. P. verrucosum Dierckx and P. rugulosum Thom were isolated and identified as the cause corm rot, the latter reported for the first time as a phytopathogen in gladiolus.
Key words: Penicillium verrucosum; Penicillium rugulosum; molecular characterization; pathogen; cultural characterization
Las ventajas de México como productor florícola recaen en su diversidad de climas y su cercanía al mercado norteamericano. Actualmente la exportación de flores y plantas ha quedado consolidada, tal como es el caso del gladiolo al estar libre de arancel. Los principales estados productores son Puebla, Estado de México y Morelos. La producción comercial de la flor de corte de gladiolo se inicia a través de la plantación del cormo; el cual es afectado por diversos patógenos que ocasionan su pudrición, como los del género Penicillium que ataca durante el almacenamiento, provocando desarrollo limitado de raíces y aborto floral. Éste provoca infecciones a través de heridas y sitios de ruptura; se encuentra presente en un 90% de material vegetal aparentemente sano. Los objetivos de este trabajo fueron aislar e identificar cultural, morfológica y molecularmente, así como realizar pruebas de patogenicidad para determinar las especies de Penicillium causantes de la pudrición. A partir de cormos variedad GrandPrix® (10/12) con diferentes niveles de deterioro morfológico y de acuerdo a una escala hedónica visual de siete puntos. Se aisló e identificó a P. verrucosum Dierckx y P. rugulosum Thom como causantes de la pudrición de cormos, este último reportado por primera vez como fitopatógeno en gladiolo.
Palabras clave: Penicillium verrucosum; Penicillium rugulosum; caracterización molecular; patógeno; caracterización cultural
INTRODUCTION
Because of its diversity of climates and its proximity to the North American market, Mexico has a great potential as a producer of flowers; however, its flower production has been catalogued as a sector with low quality and productivity levels (Claridades Agropecuarias, 2006). FUNPROVER (2008) mentions that Mexico is fifth worldwide in terms of the surface dedicated to flower production, and when comparing commercial values, Mexico is widely surpassed by countries such as Colombia and Ecuador, the surfaces of which are not small. Regardless of this, in the period between 2000 and 2011, the value of Mexican flower production increased by an annual average of 9.5%, and reached a value of over 5.6 billion pesos (DISEMINA, 2012).
The main flower-producing states are the State of Mexico, Puebla, and Morelos, which provide 48, 22, and 12% of the country’s production, respectively (CESAVEM, 2011). The economically important crops in the country are chrysanthemums, roses, carnations, and gladiolus. In terms of planted areas and demand, gladiolus is the main flower, since it adapts easily to technological (limited phytosanitary control, inadequate postharvest management) and weather limitations (extreme temperatures, high luminosity). In the state of Morelos, the crop is concentrated in the municipal areas of Cuautla, Yautepec, and Ayala.
The quality of the gladiolus, as a cut flower, depends largely on the corm, which is the plant reproductive structure. The countries specialized in its distribution are the Netherlands, France, Chile, and the United States (Claridades Agropecuarias, 2006). When farmers want to renew the cultivar they go to marketing venues or to farmers of the same area to acquire the new material. However, the health of the corm is not guaranteed, presenting one of the main limitations, known as “corm rotting,” which is related to fungi of the genus Penicillium (Pataky, 1983; González-Pérez et al., 2009; IFBC, 2012).
In storage, the agent related to corm rotting in the gladiolus is Penicillium gladioli, described by McCulloch and Thom (1928). Currently, there are reports of other species of Penicillium that affect the corms, causing losses calculated in 50% in storage, and 15% on the field (Singh, 1970; González-Pérez et al., 2009; González-Pérez, 2011). The aims of this study were a) to isolate and characterize culturally, morphologically, and molecularly the species of Penicillum that cause the rotting of gladiolus corms in storage, and b) to determine the pathogenicity of the isolations of Penicillium.
MATERIALS AND METHODS
Isolations
In August 2012, isolations were obtained from pathogenic fungi from GrandPrix commercial variety gladiolus corms, with a circumference of 10/12 cm (Grupo Nedermex, S.A. de C.V. del Estado de México) that presented severe damage caused by Penicillium. In a laminar flow cabinet, the characteristic dust of this type of rotting was placed in Petri dishes with potato dextrose agar (PDA) medium and incubated for 10 d at 27 °C in complete darkness. The diverse cultures were isolated and purified until monosporic cultures were obtained in water-agar medium using the decimal serial dilution method (Villanueva Arce et al., 2005), and incubated under environment conditions for 36 h. Some germinated spores were transferred to Petri dished with PDA. The fungal strains were conserved using the silica-gel technique (Chan and Elander, 1986).
Pathogenicity tests
Four healthy corms were used for every isolation, which presented no visible damages, of the variety GrandPrix 10/12, and four more without inoculating as a control treatment. The tunics were removed from the corms, which were washed with a soapy solution, and rinsed with tap water. In a laminar flow bell, the corms were disinfected with a sodium hypochlorite solution at 1.5% for 2 min, rinsed three times and placed on sterile drying paper contained in new and disinfected polystyrene trays. Once the surface water was discarded, each tray was placed in airtight bags with jagged zippers. The corms were inoculated with 10 d old Penicillium cultures and incubated for 8 d at 8 °C plus 22 d at room temperature. The inoculation methods used were two: by the infiltration of a suspension of spores following the method proposed by González-Pérez (2011), which consists in adjusting the suspension to a concentration of 1x105 spores mL-1 plus a Tween 20 aliquot, adjusting to a concentration of 0.1%. Later, 2 mL were infiltrated, one in the apical bud and another in the basal disc; by inoculation with mycelia, the latter was placed in an incision made with a hole puncher, 3 mm in diameter near the apical bud. The evaluation was based on a visual appreciation using a seven-point scale (Table 1). This evaluation was complemented by performing a vertical cut in the center of each corm. The total exposed surface of each half was measured in mm2 along with the damaged surface with the aid of images and the program ImageJ (Rasband, 2006). Values were reported as a percentage of tissue affected in respect to the total area.
Cultural and morphological characterization
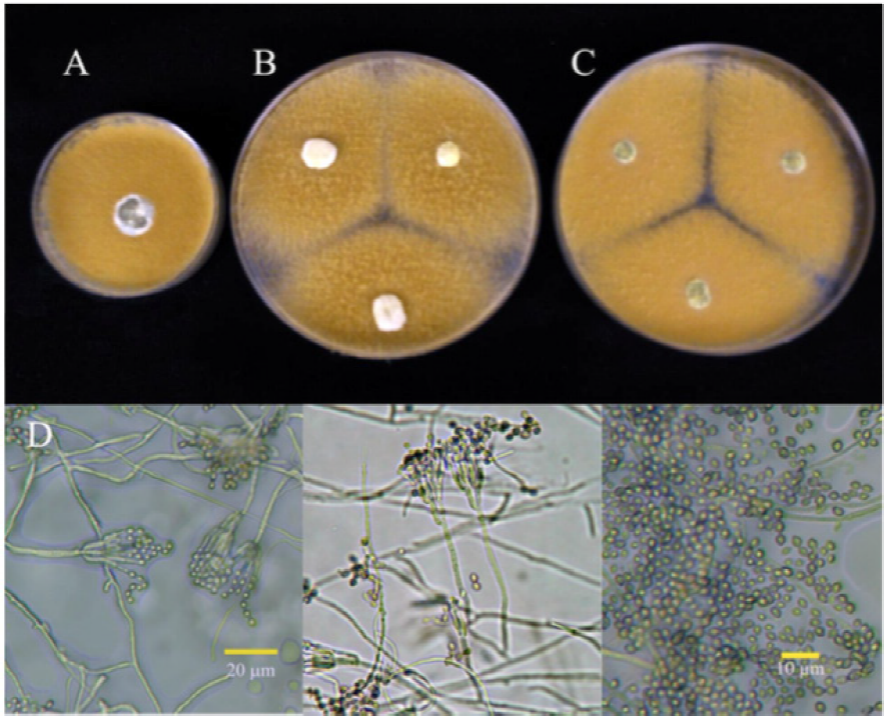
In malt extract agar (MEA) and czapek agar (Cz’) we observed color, texture, exudates, and maximum culture growth from a saturated spore solution from the monosporic cultures as recommended by Frisvad and Samson (2004). For morphometric measurements, growth microchambers were set up (Moreno et al., 2005), and from there, assemblies were prepared to observe the mycelium, conidophora, and conidia in retail under the compound microscope (Olympus CX31, lens 40X). The preparations were made with lactophenol, measuring an average of 50 fruit bodies and 100 spores per isolation. We considered the length and width of the stipe, metula, and phialide, as well as the diameter of the conidium; we also determined the shape, texture and ramification pattern of the conidiophore. To evaluate the growth rate (TC), as a physiological and differential characteristic between species, 5 mm discs from monosporic Penicillium cultures were used to place them in Petri dishes, 50 mm in diameter, with PDA, and incubated at room temperature until the mycelium reached its maximum growth area. Mycelial growth considered the evaluation of the maximum daily area using images and the program ImageJ (Rasband, 2006); finally, the following equation was used to determine the TC:
The cultural and morphological characterization of each isolation was compared with the referent species obtained from molecular characterization using the taxonomic codes described by Frisvad and Samson (2004) and Pitt and Hocking (2009).
Extraction and purification of genomic DNA
Based on the protocol proposed by Doyle and Doyle (1990) with some modifications, in order to obtain the fungal mycelium PDA discs, 5 mm in diameter, from monosporic discs, were used to plant in 50 mL of potato dextrose broth medium, which was incubated for 4 d at 27 °C. The mycelium recovered with sterile filter paper was macerated and crushed with liquid nitrogen in a porcelain mortar. In Eppendorf tubes, 0.2 g of pulverized mycelia were added along with 600 mL of lysis buffer solution, it was stirred in a vortex and incubated for 30 min at 65 °C in a thermomixer (Thermomixer Compact-Eppendorf®). Next, 250 mL of potassium acetate 5 M were added and stirred until homogenized in a vortex and a centrifuge. This one, as all the following, were carried out for 5 min, 13000 rpm, 4 °C. The supernatant was recovered and placed in a new tube with a volume of pnenol:chlorophorm:isoamylic acid (25:24:1 v/v/v), then mixed and centrifuged. Once the supernatant was separated again and placed in a new tube, 1 mL of RNAse was added and incubated for 30 min at 37 °C. After the 30 min, one volume of chlorophormo: isoamylic acid (24:1 v/v) was added, mixed and centrifuged. Once again, the supernatant was taken and placed in another tube, 2/3 volume of cold isopropanol were added, then left to rest for 2 h at -20 °C. It was then centrifuged and the supernatant was discarded. The tablet was washed with 1000 mL of ethanol at 70%, and left to dry until the alcohol evaporated completely. The samples were resuspended in 50 mL of sterile distilled water and stored at -20 °C. The purity of the DNA obtained was verified by electrophoresis in an agarose gel at 1% stained with 1 mL of GelRed 10,000x (0.1 mg mL-1) and observed in a molecular photodocumenter (Imager®).
DNA Quantification
The DNA concentration was determined in the Nano Drop® (ND-1000), to later adjust to 50 ng mL-1, as established by the method for quantification and sequencing.
Amplification by PCR
The primers used to amplify the ITS region of the ribosomal DNA were ITS1, with the sequence 5’TCCGTAGGTGAACCTGCGG3’, and ITS4 5’TCCTCCGCTTATTGATATGC3’ (White et al., 1990). The Polymerase Chain Reaction (PCR) was carried out with a final volume of the mixture of 50 µL and run in a thermocycler (Bio Rad DNA engine®). The reaction mixture was composed of: 5.0 µL of buffer solution for the PCR, 3.0 µL of MgCl2 (50 mM), 2.0 mL of the mixture of dNTPs (10 mM), 1 mL of each one of the ITS’s (50 pmol µL-1) and 0.5 µL of Taq-DNA polymerase (Invitrogen® 94 kDa). The program for the PCR consisted of 30 cycles: initial denaturalization 95 °C for 5 min; denaturalization 95 °C for 1 min; alignment at 57 °C for 40 s; extension 72 °C for 1 min. The amplified fragments were verified by gel electrophoresis in agarose gel at 1% stained with 1 µL of GelRed 10, 000x (0.1 µg mL-1) and compared with the molecular weights with the marker TriDye 1kb DNA. The products of the amplified PCR were purified with the kit GeneJet ™ (Fermentas, USA).
Sequencing
The DNA was quantified and sequenced in both ways to avoid assembly and comparison errors in the Institute of Biotechnology of the National Autonomous University of Mexico (Instituto de Biotecnología de la Universidad Nacional Autónoma de México), Cuernavaca, Morelos. The sequences were aligned and compared with the National Center for Biotechnology Information (Centro Nacional para la Información de Biotecnológica) using BLAST.
RESULTS AND DISCUSSION
Isolations
Out of the isolations obtained from the corms with symptoms of rotting in storage, four cultures, representative of Penicillium, were selected according to their cultural behavior in PDA; the cultures were labeled as 1P, 2P, 3P, and 4P.
Pathogenicity tests
The evaluation of the percentage of the surface infected and of the visual damage indicated that the four Penicillium isolations, inoculated in gladiolus corms of the variety GrandPrix 10/12, were pathogenic with different degrees of virulence, and isolations 1P and 4P were the most aggressive (Table 2). Differences were noticed between inoculation of spores and of mycelia. Visual damage was greater in the inoculation with mycelia. The evaluation of external damage reported the necrosis of anchor roots, spots and brown-colored collapses, with the presence of mycelia, although the presence of mycelia was greater in roots. In general, internal damage presented tissue discoloring. In both cases, the fungus was reisolated and its characteristics and cultural behavior were verified to comply with the postulated by Koch. Despite the aggressiveness of Penicillium as a phytopathogen being considered low, the pathogenicity tests carried out in this study show that the infection level increases depending on the places with lesions and the natural rupture of the corm. González-Pérez et al. (2009) reported an isolation of Penicillium affecting the production area of Puebla with a aggressiveness that mainfests itself in only 2.7% of the exposed area of the corms. The behavior of the isolations found in this investigation resemble descriptions by Overy et al. (2005), who point out that in their pathogenicity test, corms presented spots, external discoloring, root necrosis, and in their case, internal damage.
Table 2 Pathogenicity of four Penicillium sp. isolations in gladiolus corms, var. GrandPrix® 10/12.

Values with the same letter represent statistically equal values. One-way test of analysis of variance with one F = 4.206, F = 8.55, gl (4, 35) y P ≤ 0.007 determined with a Tukey comparison of averages (P≥0.05). The value shown for visual damage corresponds to the average.
Cultural and morphological characterization of isolations
The cultural behavior and the morphological characteristics of the Penicillium isolations are summarized in Tables 3 and 4. We can observe that the characteristics described coincide with most of the points referred to by the bibliography for the species reported in the molecular identification (Crous et al. 2004; Pitt and Hocking, 2009; Frisvad and Samson, 2004). The morphological characteristics of the conidiophore and the behavior of the cultures in specific media have been acknowledged as the main taxonomic characteristics (Comerio, 2000). Figures 1, 2, 3, and 4 show the cultural behavior in media Cz’, MEA, and PDA. Isolations 1P, 2P, and 4P belong to the same species P. verrucosum, although the cultural characteristics differe between isolation 1P and isolations 2P y 4P, probably considering the presence of two varieties, since they presented lower growth rates (.12 to .55 cm2 d-1 ) and this physiological characteristic helps separate individuals of the same species. On the other hand, the ITS region and the subunit 5.8s of the rDNA used in this type of analyses are very preserved instraspecifically, and they are therefore not the most adequate to carry out studies of subspecific groups. Regarding isolation 3P, it belongs to the species P. rugulosum. The difference lies in the maximum growth of the culture. The bibliography reports behavior characteristics for the reference species in a czapek yeast agar (CYA) medium and the medium used for this characterization was czapek agar medium (Cz’). However, the behavior displayed in malt extract agar (MEA), reported in the bibliography, also differs. Therefore, the use of culture media is not considered a part of the explanation in the discrepancy in the size of the cultures. This discrepancy may also be justified by the difference in growth rates.
Table 3 Comparison of the cultural and micromorphologic behavior of Penicillium isolations in gladiolus var. GrandPrix® corms in their time of storage with the species P. verrucosum (KC009832).
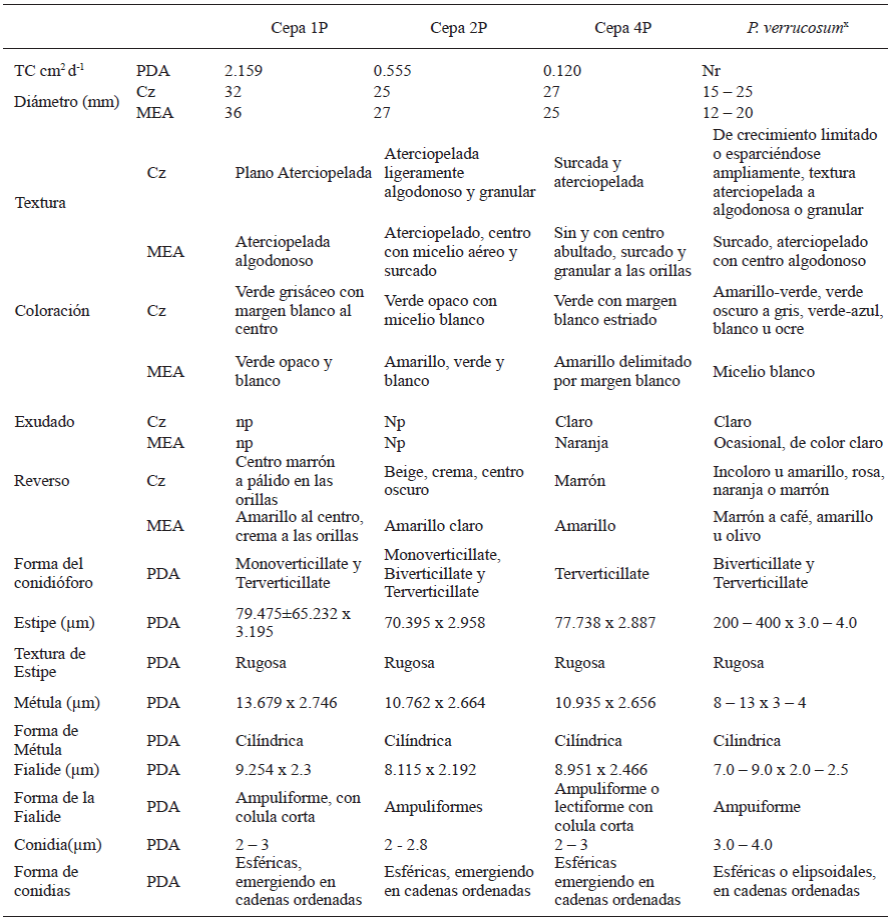
Characteristics in Czapek (Cz) agar medium and malt extract agar (MEA) at 25 °C 7 days in darkness. Presence of exudates at 25 °C 14 days in darknes in Cz medium and MEA; Np: not present; Nr: not reported. Mounted on potato dextrose agar (PDA), observations at 3 - 5 days under Olympus CX31 compound microscope, lens 40X. x Crous et al.2004; Pitt and Hocking, 2009; Frisvad and Samson, 2004.
Table 4 Comparison of the cultural and micromorphologic behavior of Penicillium isolations in gladiolus var. GrandPrix® corms in their time of storage with the species P. rugulosum (GU566230.1).


Figure 1 Cultures of isolate 1P identified as 7-day old P. verrucosum, at 25 °C in (A) PDA, (B) Cz and (C) MEA. (D) Conidiophora and conidia.
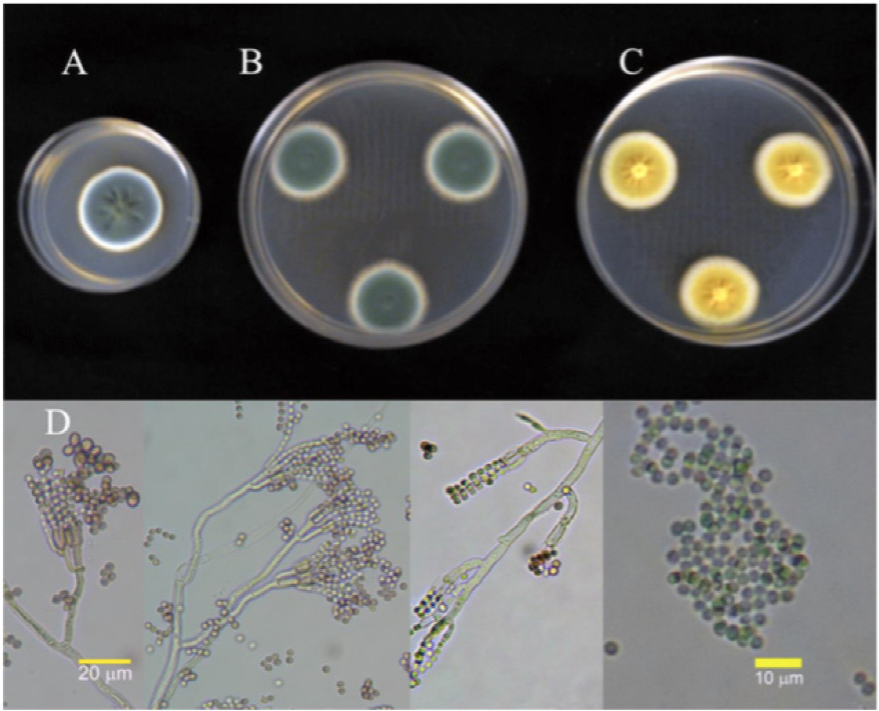
Figure 2 Cultures of isolate 2P identified as 7-day old P. verrucosum, at 25 °C in (A) PDA, (B) Cz and (C) MEA. (D) Conidiophora and conidia.
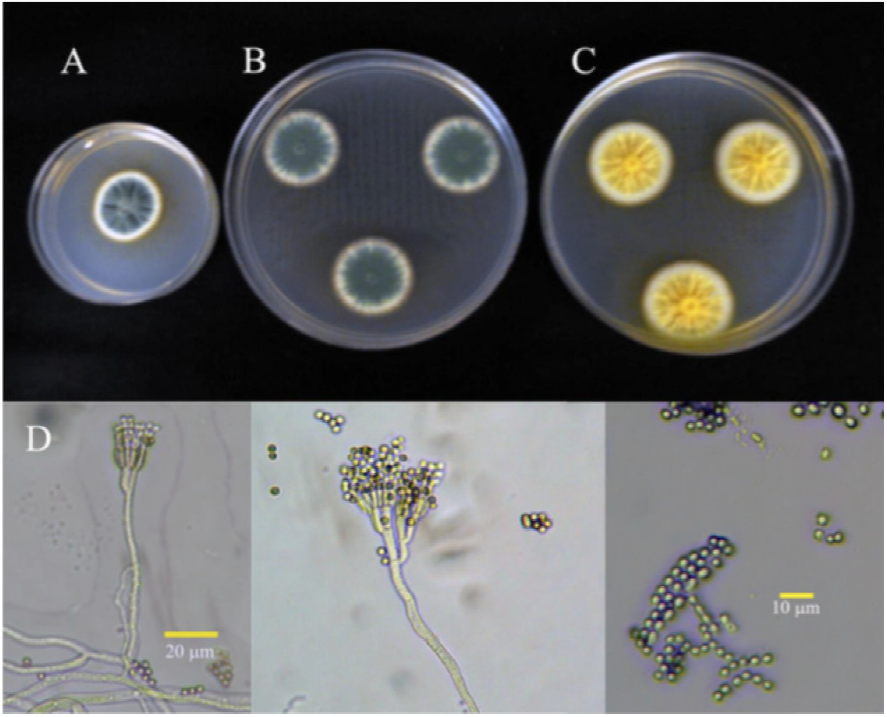
Figure 3 Cultures of isolate 4P identified as 7-day old P. verrucosum, at 25 °C in (A) PDA, (B) Cz and (C) MEA. (D) Conidiophora and conidia.
Molecular characterization of isolations
Table 5 shows the results of the amplification and sequencing in both directions of the rDNA of the four Penicillium isolations. This teste reported that the causal agents of the rotting of gladiolus agents in storage are P. verrucosum and P. rugulosum. Traditionally, this pathology is related to P. gladioli (McCulloch and Thom, 1928). However, Frisvad and Samson (2004) mention that this species could be extinct due to the chemical management given to plants. On the other hand, there are reports of other species of Penicillium related to the rottinf of gladiolus corms: P. funiculosum (Singh, 1970), P. hirsutum, P. venetum and P. tulipae (Overy et al., 2005), Penicillium sp. (González-Pérez et al., 2009) and P. brevicompactum (González-Pérez, 2011). Crous et al. (2004) mention in the mycobank database, that the species P. verrucosum, reported in the molecular analysis, is related to P. hirsutum and P. verrucosum var. corymbiferum, and therefore associated as being one of the main agents of the rotting of flower bulbs (Chauhan and Saaltink, 1969; Smid et al., 1995; Overy et al., 2005). On the other hand, P. rugulosum is known as a widely distributed species; isolated from diseased or healthy tissues of diverse products. Prince et al. (1988), isolated this species from tulip bulbs; however, to date there are no reports found of its presence in gladiolus corms.
CONCLUSIONS
Penicillum verrucosum and Penicillum rugulosum were identified as causal agents of rotting of the gladiolus corm during storage. The first species identified was considered the most aggressive in the pathogenicity tests. Penicillum rugulosum is reported for the first time as a phytopathogenic agent for gladiolus.
REFERENCES
CESAVEM. Comité Estatal de Sanidad Vegetal del Estado de México. 2011. Manejo fitosanitario de ornamentales. Disponible en línea: http://www.cesavem.org/?accion=ornamentales [ Links ]
Chang LT, Elander RP. 1986. Long-term preservation of industrially important microorganism. Manual of industrial Microbiology and Biotechnology. In: Demian AL, Solomon NA (eds). American Society for microbiology. USA. pp. 49-55. http://doi.org/10.1002/food.19880320124 [ Links ]
Chauhan SK, Saaltink GJ. 1969. A Penicillium attack on hyacinth bulbs as affected by temperature and humidity. Netherlands Journal of Plant Pathology 75: 197-204. http://doi.org/10.1007/BF01981990 [ Links ]
Claridades Agropecuarias. 2006. La floricultura mexicana, el gigante que está despertando. La Floricultura mexicana; flores de corte 154: 3-38. Disponible en línea: http://www.infoaserca.gob.mx/claridades/revistas/154/ca154.pdf#page=3 [ Links ]
Comerio RM. 2000. Nefrotoxinas y especies nefrotóxicas del género Penicillium Link. Revista Iberoamericana de Micología 17: 82-89. Disponible en línea: http://www.reviberoammicol.com/2000-17/082089.pdf [ Links ]
Crous PW, Gams W, Stalpers JA, Robert V and Stegehuis G. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19-22. Disponible en línea: http://www.westerdijkinstitute.nl/images/ResearchGroups/Phytopathology/pdf/PDF%20OP%20NUMMER/203.pdf [ Links ]
DISEMINA. Estadísticas del Sector Agroalimentario y Pesquero. 2012. La floricultura en México. Número 89. SIAP (Servicio de Información Agroalimentaria y Pesquera). Disponible en línea: http://infosiap.siap.gob.mx/aagricola_siap_gb/icultivo/ [ Links ]
Doyle JJ, Doyle JL. 1990. A rapid total DNA preparation procedure for fresh plant tissue. Focus 12:13-15. Disponible en línea: https://www.scienceopen.com/document?vid=6769c281-ab3f-4e1d-b01e-0414f9915561 [ Links ]
Frisvad JC and Samson RA 2004. Polyphasic taxonomy of Penicillium subgenus Penicillium: a guide to identification of food and air borne terverticillate Penicillia and their mycotoxins. Pp: 1-174 p. In: Samson RA, Frisvad JC. (eds.). Penicillium subgenus Penicillium: new taxonomic schemes, mycotoxins and other extrolites. Studies in Mycology No. 49. Utrecht, The Netherlands. 251 p. Disponible en línea: www.cbs.knaw.nl/publications/1049/voorwerk.pdf [ Links ]
FUNPROVER. 2008. Cadena productiva de horticultura ornamental en el Estado de Veracruz. Disponible en línea: http://www.funprover.org/formatos/PLANES%20ESTRATEGICOS/Cadena%20horticultura%20ornamental.pdf [ Links ]
González-Pérez E, Yáñez-Morales MJ, Ortega-Escobar HM, Velázquez-Mendoza J. 2009. Comparative analysis among pathogenic fungal species that cause gladiolus (Gladiolus grandiflorus Hort.) corm rot in Mexico. Revista Mexicana de Fitopatología 27:45-52. Disponible en línea: http://scielo.unam.mx/pdf/rmfi/v27n1/v27n1a6.pdf [ Links ]
González-Pérez E. 2011. Fenología, propagación In vitro y enfermedades del gladiolo en San Martín Texmelucan, Puebla. Tesis de Doctorado. Colegio de Postgraduados. Postgrado de Recursos Genéticos y Productividad, Producción de Semillas. Montecillos, Texcoco, Edo. de México. Disponible en línea: http://hdl.handle.net/10521/598 [ Links ]
IFBC. International Flower Bulb Centre. 2012. Gladiolus as cut flowers. Guidelines for cut flower production. Disponible en línea: http://edepot.wur.nl/167428 [ Links ]
McCulloch L and Thom C. 1928. A rot of gladiolus corms caused by Penicillium gladioli, McC. and Thom. Journal of Agricultural Research 36:217-224. http://doi.org/10.1126/science.67.1730.216-a [ Links ]
Moreno VM, Yáñez MMJ, Rojas MRI, Zavaleta ME, Trinidad SA. 2005. Diversidad de Hongos en Semilla de Amaranto (Amaranthus hypochondriacus L.) y su Caracterización molecular. Revista Mexicana de Fitopatología 23: 111-118. Disponible en línea: http://www.redalyc.org/pdf/612/61223201.pdf [ Links ]
Overy DP, Karlshj K and Due MJ. 2005b. Low temperature growth and enzyme production in Penicillium ser. Corymbifera species, casual agents of blue mold storage rot in bulbs. Journal of Plant Pathology 87: 57-63. http://doi.org/10.1139/b05-110 [ Links ]
Pataky NR. 1983. Gladiolus corm rots. University of Illinois. Extension. Report on plant disease. RPD No. 651. 6 p. Disponible en línea: https://ipm.illinois.edu/diseases [ Links ]
Pitt JI and Hocking AD. 2009. Fungi and Food Spoilage. Third Edition. Springer. Pp. 169-273. http://doi.org/10.1007/978-0-387-92207-2_7 [ Links ]
Prince TA, Stephens Ct and Herner RC. 1988. Pathogenicity, fungicide resistance, and ethylene production of Penicillium spp. isolated from tulip bulbs. Phytopathology 78: 682-686. http://doi.org/10.1094/Phyto-78-682. [ Links ]
Rasband W. 2006. ImageJ for microscopy. Image processing and analysis in Java. Disponible en línea: http://rsb.info.nih.gov/ij/docs/index.html [ Links ]
Singh RN. 1970. Penicillium rots of gladiolus in India. Plant and Soil 33: 249-250. http://doi.org/10.1007/BF01378215 [ Links ]
Smid EJ, de Witte Y and Gorris LGM. 1995. Secondary plant metabolites as control agents of postharvest Penicillium rot on tulip bulbs. Postharvest Biology and Technology 6: 303-312. https://doi.org/10.1016/0925-5214(95)00010-4 [ Links ]
Villanueva-Arce, R., A. M. Hernández-Anguiano, M. J. Yáñez-Morales, D. Téliz-Ortíz, A. Mora-Aguilera, E. Cárdenas-Soriano, y A. Castañeda-Vildózola. 2005. Caracterización e identificación de Colletotrichum fragariae en frutos de chirimoya. Agrociencia 39: 93-106. Disponible en línea: http://www.redalyc.org/articulo.oa?id=30239109 [ Links ]
White TJ, Bruns T, Lee S and Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, In: Innes MA, Gelfand DH, Sninsky JJ, White TJ. (eds.). PCR Protocols: a Guide to Methods and Applications. New York: Academic Press. Pp. 315-322. http://dx.doi.org/10.1016/B978-0-12-372180-8.50042-1 [ Links ]
Acknowledgements
Received: March 16, 2017; Accepted: August 03, 2017











 texto em
texto em