Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.35 n.3 Texcoco Sep. 2017
https://doi.org/10.18781/r.mex.fit.1704-3
Scientific articles
Incidence and causal agents of root diseases and its antagonists in apple orchards of Chihuahua, México
1Centro de Investigación en Alimentación y Desarrollo, A.C., Campus Cuauhtémoc, Chihuahua, Avenida Rio Conchos S/N, Parque Industrial. C.P. 31570, Cuauhtémoc, Chihuahua, México.
2Universidad de Ciencias y Artes de Chiapas, Facultad de Ingenierías, Unidad Académica Villacorzo. Km 3.0 Carretera Villacorzo-Ejido Monterrey. C.P. 29000, Villacorzo, Chiapas, México.
3Departamento de Biotecnología y Bioquímica, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Campus Irapuato, Km 9.6 Libramiento Norte, C.P. 36821, Irapuato, Guanajuato, México.
4Instituto de Investigaciones Agropecuarias y Forestales, Universidad Michoacana de San Nicolás de Hidalgo, Km 9.5 Carretera Morelia-Zinapecuaro, Campus Posta Zootécnica, C.P. 58880, Michoacán, México.
The incidence of root diseases was estimated in apple orchards from Chihuahua, Mexico. Three hundred isolates (fungi and Oomycetes) were identified in samples of root tissue and soil of trees with infectious damage symptoms and disease symptom-free trees. At the same time, putatively antagonistic agents were isolated and subsequently identified. The pathogenicity of fifteen selected isolates (eight fungi and seven Oomycetes), was tested in twelve apple rootstocks under greenhouse conditions. In addition, the in vitro antagonistic activity of Trichoderma and Bacillus species was evaluated against seven of the selected Oomycetes. The incidence of infection damage of roots in 20 tested orchards was 17% (1-40%). Fusarium was the most widely distributed fungi (67.8%). The distribution of the other organisms was variable. Four species of Trichoderma were identified, with T. gamsii being the most widely distributed (72.5%). Bacillus spp. substantially reduced the radial growth (>90%, p=0.05) of Phytophthora cactorum. Eleven rootstocks were susceptible to Pythium ultimum y Phytophthora cactorum C3. The G.935, Standard and M.25 rootstocks were the most resistant with 0% incidence. The antagonistic species of both genera inhibited the in vitro growth of P. cactorum (86.4-93.8%, p= 0.05), respect to Pythium species, and therefore, they might be used as biological control agents.
Key words: Bacillus spp.; Fusarium spp.; Phytophthora cactorum; Trichoderma spp.; ITS region; molecular identification
Se estimó la incidencia de enfermedades de raíz en manzanos de Chihuahua, México, y se identificaron trescientos aislados (hongos y Oomicetos) tomados del tejido de raíz y suelo de la rizosfera de árboles con síntomas de infección y libres de síntomas de enfermedad. Paralelamente, se aislaron e identificaron hongos putativamente antagonistas. Se determinó la patogenicidad de quince aislados seleccionados (ocho hongos y siete Oomicetos) sobre doce portainjertos de manzano bajo condiciones de invernadero. Adicionalmente, se evaluó la actividad antagónica in vitro de especies de Trichoderma y Bacillus contra siete de los Oomicetos seleccionados. La incidencia de daños infecciosos en raíz en 20 huertos analizados fue del 17% (1-40%). Fusarium fue el hongo más ampliamente distribuido (67.8%). La distribución del resto de los organismos fue variable. Se identificaron cuatro especies de Trichoderma, siendo T. gamsii la más ampliamente distribuida (72.5%). Bacillus spp., redujo sustancialmente el crecimiento radial (>90%, p=0.05) de Phytophthora cactorum. Once portainjertos fueron susceptibles al menos a Pythium ultimum y Phytophthora cactorum C3. Los portainjertos G.935, Standard y M.25 fueron los más resistentes con 0% de incidencia. Las especies antagonistas de ambos géneros inhibieron in vitro significativamente el crecimiento de P. cactorum (86.4-93.8%, p= 0.05), respecto a especies de Pythium, por lo que podrían ser utilizados como agentes de control biológico.
Palabras clave: Bacillus spp.; Fusarium spp.; Phytophthora cactorum; Trichoderma spp.; región ITS; identificación molecular
Apples (Malus x domestica Borkh.) are one of the most important temperate crops in Mexico (Ramírez-Legarreta et al., 2011). Apple trees are affected by several biotic and abiotic factors that limit their production, especially root diseases. In agroecosystems, the increased diversity of pathogenic microorganisms (Oomycetes, fungi, bacteria, nematodes, among others) can lead to root rot diseases. The root diseases caused by fungi and Oomycetes in apple trees, represent one important economic problem worldwide. These microorganisms destroy and collapse the root system of susceptible rootstocks, reducing the absorption of nutrients and water, blocking vascular bundles and, eventually, causing the death of apple trees (Rumberger et al., 2007; Samaniego-Gaxiola, 2007; Tewoldemedhin et al., 2011a, b). The main pathogens of apple trees include Phytophthora spp., Pythium spp., Phymatotrichopsis omnivora, Fusarium spp., Rhizoctonia spp., among others (Latorre et al., 2001; Samaniego-Gaxiola, 2007). However, there still are in many apple producing areas pathogens that have not been identified as disease agents for apple trees. The major diseases of apple trees, including crown-rot root diseases, result from a complex of pathogens (Lamichhane and Venturi, 2015). The incidence, causal agents and pathogenicity of causal agents of crown-rot root diseases have scarcely been studied during the last three decades in apple orchards from Northern Mexico. Information in this regard is needed in this particular apple producing area to develop a better-integrated disease management in order to reduce the excessive use of broad-spectrum chemical pesticides to control these diseases. Undoubtedly, this improved integrated disease management include the use of microorganisms with antagonistic activity, which are efficient colonizers, produce metabolites that inhibit the growth of phytopathogens and act as plant growth promoters (Ezziyyani et al., 2004). Thus, the aim of the present study was to estimate the incidence of root pathogens in apple orchards from Chihuahua, Mexico, identifying the fungi and Oomycetes probably involved in the diseases and evaluating their pathogenicity in commercial apple rootstocks. The antagonistic effect of some microorganisms against some of the identified Oomycetes was also determined in vitro.
MATERIALS AND METHODS
Experimental site and estimation of infection damage incidence
The incidence of roots with infection damage was determined on time in five orchards from the main apple-producing regions of Chihuahua, Mexico (Cuauhtémoc, Bachiniva, Namiquipa and Guerrero, Table 1) during June and July of 2013. The climate conditions and technification level varied among tested areas. Five hundred trees, distributed in 10 rows, were evaluated in each orchard (10,000 trees total) in a randomize design (Table 1). Root infection damage symptoms considered were wilting, poor shoot growth pale or yellow foliage, necrotic sparse foliage, premature defoliation, regressive death of leaves and terminal shoots, black or brown spots on the stems, cankers formation and irregular growth.
Table 1 Apple orchards sampled, geographical location, rootstock planted, age of the trees, and incidence of apple trees with putative root disease in four localities in the state of Chihuahua, Mexico.
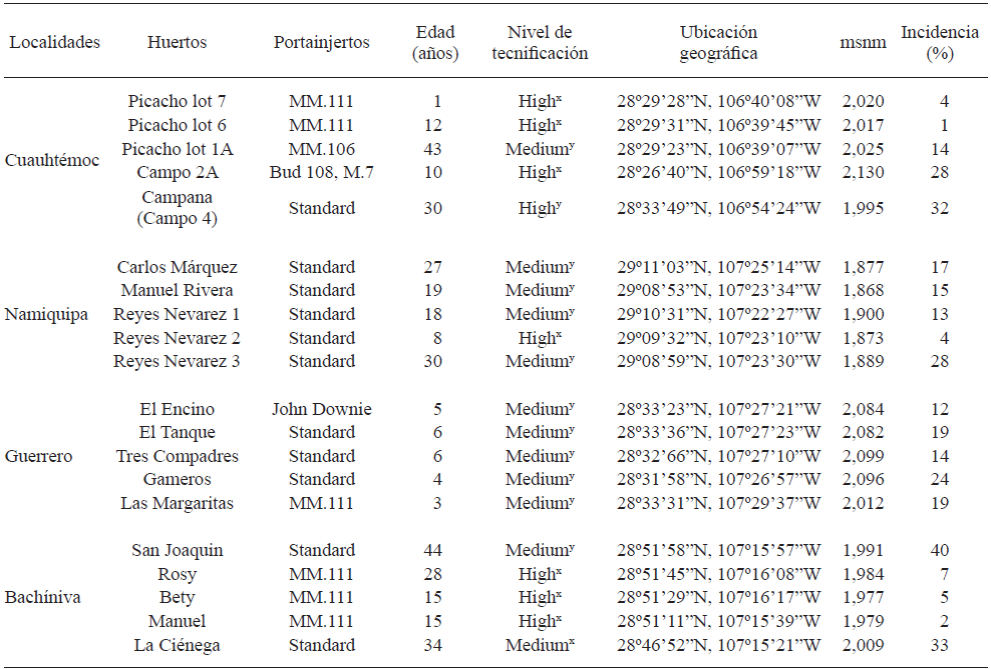
masl: meters above sea level.
x High: Micro-sprinkler irrigation, heating, hail mesh; y Medium: Rolled irrigation, heating, without hail mesh, without limiting the use of resources and casual technical assistance (Ramírez-Legarreta et al., 2004).
Soil and tissue samples
Diseased tissue (root and stem samples of 10-20 cm of length) and soil near the rhizosphere (500-600 g) of ten apple trees with apparent root infection damage and free of infection damage were collected from each orchard to isolate fungal and Oomycetes.
Isolation and purification of microorganisms
Three to five pieces (10-20 cm of length) of diseased tissue were washed with tap water to eliminate the excess of soil and subsequently cut into 1 cm pieces. The pieces were washed for 1 min with 0.5% sodium hypochlorite (NaOCl) solution, rinsed three times with sterile distilled water and dried on sterile brown wrapping paper in a biosafety hood (Envirco Corporation, Albuquerque, New Mexico, USA). Tissue subsamples (5 pieces each of 1 cm of length) were placed in triplicate in humid chambers (90-mm Petri dishes with moistened cotton). On the other hand, tissue subsamples (five pieces of 3-5 mm of length) were placed in triplicate on semi-selective media V8-agar [V8 juice, calcium carbonate (CaCO3; Sigma-Aldrich) - agar (Sigma-Aldrich)] and potato-dextrose-agar (PDA; BD Bioxon). These two media contained antibiotics [pimaricin 0.1 g/L (Sigma-Aldrich, USA), rifampicin 0.01 g/L (Sigma, USA), oxytetracycline 0.03 g/L (Sigma, Germany)] (Tewoldemedhin et al., 2011a). Thus, 15 subsamples of 1 cm and 15 subsamples of 3-5 mm from each tree with and without infection damage symptoms were evaluated. All assays were performed at 28 ºC for 5 d in an environmental chamber without light (Precision Scientific, Winchester, VA, USA). The fungi were purified on PDA medium, using a monosporic culture technique, followed by incubation at 28 ºC for 72-96 h. PARPH medium [17 g/L of corn meal agar medium (Analytical Fluka, Sigma); 0.10 g/L pentachloronitrobenzene (PCNB), 0.27 g/L ampicillin (Sigma) and 0.01 g/L rifampicin (Sigma)] was used to isolate Oomycetes. These microorganisms were incubated under the same conditions than fungi.
The Trichoderma species were isolated by serial dilutions (1:10) of sieved soil (1 g) from diseased and disease symptom-free trees in test tubes containing 9 mL of sterile peptone water (0.1% peptone and 0.85% NaCl in distilled water). Aliquots (50 µL) of the prepared dilutions (104, 105, and 106) were spread in triplicate by diffusion technique on 90-mm Petri dishes containing PDA medium added of the antibiotics previously indicated (Schoenborn et al., 2004).
Morphological and molecular identification of microorganisms
Fungi and Oomycetes were purified on PDA medium without antibiotics (Tewoldemedhin et al., 2011a) and identified at genus level using taxonomic keys (Barnett and Hunter, 1972; Dugan, 2006; Watanabe, 2010), according to their morphological characters observed in an optical microscope (Carl Zeiss, Jena, Germany). The identity of these microorganisms was confirmed molecularly.
The genomic DNA (gDNA) was extracted and used to perform the identity analysis of the fungal isolates based on their molecular characters. For this purpose, an explant of the purified fungi was placed on a Petri dish containing PDA medium, covered with sterile cellophane and incubated at 28 ºC for 7 d. The mycelia was collected and placed in a porcelain mortar with a buffer (200 mM Tris-HCl (pH = 8), 250 mM NaCl, 25 mM EDTA, 0.5% SDS) at 70 ºC. The mycelia was macerated according to Raeder and Broda (1985). The obtained gDNA was visualized by electrophoresis on a 1% agarose gel and subsequently used to amplify the internal transcribed spacers (ITS 4 and 5) of the rDNA, using the universal primers ITS5 (5’-GGAAGTAAAAGTCGTAACAAGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (White et al., 1990). The amplification conditions included an initial denaturation step at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, extension at 72 °C for 45 s, and a final extension step at 72 °C for 10 min. The PCR products were examined by electrophoresis on a 1% agarose gel and were subsequently purified using the DNA Clean & Concentrator kit (Zymo Research, California, USA), according to the manufacturer’s instructions. These products were sequenced at Macrogen (Rockville, MD, USA). The obtained sequences were compared against the NCBI database using the BLAST algorithm (Altschul et al., 1990) to verify the percent identity corresponding to the identified species. The nucleotide sequences obtained in the present study have not been deposited in NCBI.
Pathogenicity tests in apple rootstocks
The Koch’s postulates were performed under greenhouse conditions using 15 pathogens (eight fungi and seven Oomycetes) isolated from the rhizosphere of apple trees with and without root infection damage symptoms. One year old trees of the 12 most planted rootstocks in Mexico (G.30, G.41, G.202, G.222, G.935, M.7, M.25, MM.106, MM.111, Bud 118, Bud 9 and Standard) were purchased from two nurseries in Cuauhtemoc, Chihuahua (“Viveros Sacramento” and “Vivero Los Cienes”). Five trees of each rootstock were inoculated with each pathogen. Additionally, five trees of each rootstock were used as control group (without inoculum).
The trees were planted in dark polyethylene bags (5 L) containing substrate previously sterilized at 121 °C and 15 psi. The substrate was composed by loam soil, vermiculite and peat moss (1: 1: 1). Two months after planting, the trees were inoculated with 10 mL of unquantified fungi or Oomycetes inoculum (Hantula et al., 2000). The inoculum was 3 d old and grown in vegetable broth V8 [V8 juice (Campbell´s ™) and calcium carbonate (CaCO3; Sigma Aldrich)] at 28 °C under orbital shaking at 140 rpm (Orbit 1900, Labnet International Inc.). The inoculated rootstocks were maintained for another two months under greenhouse conditions (Uthkede and Smith, 1991; Latorre et al., 2001). During this time, the characteristic symptoms resulting from the fungal and Oomycete infections were monitored weekly. The Oomycetes and fungal isolates causing the death of trees or severe symptoms of damage in roots, stems, leaves and shoots after two months of the inoculation were considered as pathogenic.
In vitro antagonistic activity
Four fungal isolates and two antagonistic bacteria isolates were confronted against seven Oomycetes isolates. The antagonists included a Trichoderma asperellum and two Bacillus species previously identified (Rios-Velasco et al., 2016).
The activity of Trichoderma spp. and Bacillus isolates was evaluated in vitro against the Oomycetes obtained from apple orchards. For this purpose, confrontations were performed (Trichoderma spp. vs Oomycetes) using a dual culture technique in Petri dishes (90 mm) containing Potato-Dextrose-Agar (PDA). A paper filter disc (6 mm) containing the mycelium and conidia unquantified of the pathogen was placed on one side of the dish and another filter paper disc containing the mycelium and conidia unquantified of the antagonist was placed on the opposite side of the dish. The Bacillus spp. vs Oomycetes confrontations were performed by placing one filter paper circle of 6 mm in diameter of the pathogen (mycelium and conidia unquantified) in the center of a Petri dish containing PDA, whereas the bacteria were inoculated on the cardinal points of the Petri dish using filter paper circles (6 mm in diameter with the 48 h old inoculum), the same day that the phytopathogens (Figure 1).
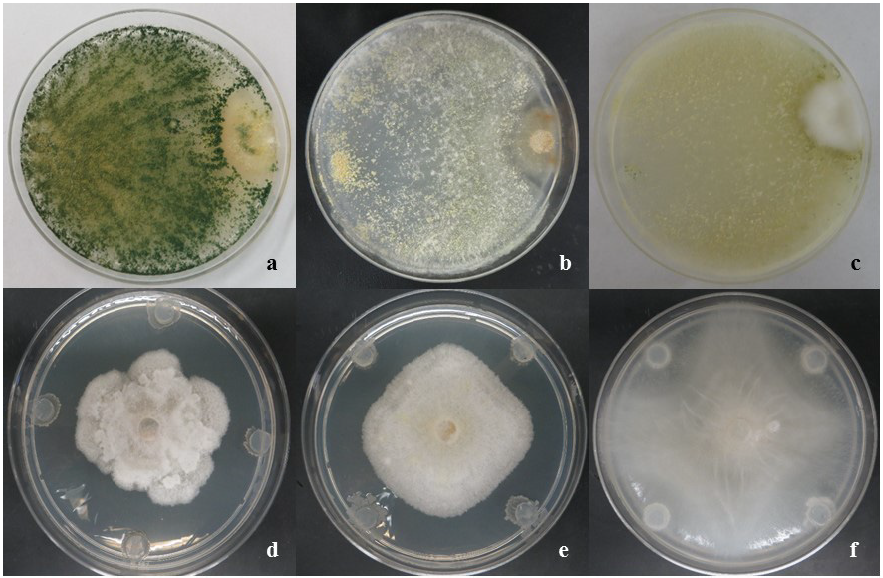
Figure 1 Mycoparasitism of Trichoderma species (a-c); a) T. asperellum vs Pythium ultimum; b) T. harzianum vs Pythium sp.; c) T. atroviride vs Phytophthora cactorum; and inhibition halos of Bacillus species (d-f); d) B. amyloliquefaciens vs Phytophthora cactorum; e) B. amyloliquefaciens vs Phytophthora cactorum; f) B. methylotrophicus vs Pythium ultimum at 6th d post-inoculation in vitro.
Controls, both antagonist and pathogen, were placed individually in the center of the Petri dish, and were performed in triplicate with ten experimental units each. The dishes were incubated at 28 °C in a Precision Scientific incubator (Model 31534), and systematic measurements of the radial growth of the fungal colonies confronted with antagonistic fungi and bacteria, and the control dishes, respectively, were performed every 24 h for eight days.
The possible antagonistic activity of Trichoderma spp. and Bacillus spp., was evaluated in terms of radial growth of the pathogen (RGP), radial growth of the antagonist (RGA), and percentage inhibition of radial growth (PIRG). The PIRG was evaluated according to Ezziyyani et al. (2004) using the formula PIRG = (R1 - R2) / R1 × 100, where R1 is the radial growth of the control pathogen, and R2 is the radial growth of the pathogen in the confrontation, while that of the antagonist type was measured according to Bell’s scale (Bell et al., 1982).
Statistical analysis
Both bioassays were performed in triplicate, with ten Petri dishes per replicate for each bacterium-fungus in vitro evaluation, using a completely randomized design with the following six treatments: four antagonistic fungal isolates and two antagonistic bacteria isolates confronted against seven Oomycetes isolates, with one control for each microorganism, where each treatment was an antagonist microorganism with 30 Petri dishes. The PIRG data and inhibition halo were analyzed using the Statistical Analysis System version 9.0 (SAS, 2002) for a balanced analysis of variance (ANOVA), and the means were separated using Tukey’s test (p = 0.05).
RESULTS AND DISCUSSION
Incidence of roots infection damage
The total incidence of root disease in sampled orchards was 17%. This incidence ranged from 1 to 32% and from 4 to 28% in the orchards from Cuauhtémoc and Namiquipa respectively, with an average incidence of 16% for both areas. In Guerrero and Bachíniva, the incidence ranged from 12 to 24% and from 2 to 40%, respectively, with an average incidence of 17% for both areas (Table 1). The highest incidence was observed in “San Joaquin” and “La Ciénega” orchards, located in Bachíniva, probably as a consequence of the advanced age of the trees (44 and 34 years old, respectively) and the medium level of technification making them more susceptible to the attack of the phytopathogens. The climate conditions of this region, which were better for the growth of the microorganisms might also be involved in this high incidence of infection damage of roots.
The incidence values probably depended of the technification level (mainly the irrigation method), climate conditions, soil type, unrecognized interactions among phytopathogens, rootstocks, differences in horticultural practices and age of the trees in each orchard. It is also important to consider that in some orchards, farmers apply organic matter to the soil, which could favor the establishment of antagonistic microorganisms, improving their development and thus avoiding the growth of phytopathogens (Kamal et al., 2010). The variability of incidence values for each orchard and locality might be consequence of the pathogenicity and virulence of the microorganism (Wilcox, 1993). Manici et al. (2013) also observed that the incidence of root diseases varied considerably among regions and the severity of the disease was attributed to the fungi composing the microorganism complex.
The crown and root rot diseases in apple orchards are important problems worldwide. Manici et al. (2013) observed a high incidence of infections caused by Pythium spp. and that infections caused by Fusarium species were highly associated to replanting diseases. This problem was also observed in the present study for 1-year-old apple rootstocks (MM.111) in “El Picacho Lot 7” orchard from Cuauhtémoc, with a high technification level. According to Ramírez-Legarreta et al. (2004), the level of technification (medium and high) in apple orchards directly affect the disease incidence primarily through micro-sprinkler type or rolled irrigation but heating type, use of hail mesh, use of inputs and ongoing or casual technical assistance are also involved, among other factors. However, most of the orchards sampled in the present study with a medium technification level showed the presence of P. cactorum and Pythium spp. and particularly the fungus F. oxysporum, which, in some cases, could be attributed to the type of orchard management being a potential determining factor in the incidence of pathogens, including the causal agents of crown and root rot diseases. Importantly, the orchards with a high level of technification have permanent technical assistance, which helps in the early detection of diseases for their prevention and management.
Morphological and molecular identification of the microorganisms
Microorganisms associated with root disease symptoms in apple trees were the Oomycetes Phytophthora spp. and Pythium spp. and the fungi Fusarium spp., Gibberella spp. (Fusarium spp. teleomorph), Bionectria ochroleuca, Clonostachys sp., Alternaria spp., and Phymatotrichopsis sp. These microorganisms were identified according to their morphological characters and genomic sequences. The genus Fusarium spp. was the most frequently observed (67.3%, 202 isolates), with 32.3% (97 isolates) of this genus belonging to F. oxysporum. The remainder species of this genus were F. solani, F. subglutinans, F. sacchari, F. succisae, F. tricinctum, F. fujikuroi, F. proliferatum and F. equiseti. This distribution of species of the genus Fusarium is similar to that found in other geographic locations worldwide (Serdani et al., 2002; Tewoldemedhin et al., 2011a). Leslie et al. (2007) found that F. succisae is phylogenetically closely related to F. proliferatum, which has been associated with apple seedlings in China (Ju et al., 2014). Both F. fujikuroi and F. sacchari were associated with rice, sugarcane and corn plots from Peninsular Malaysia (Hsuan et al., 2011), while F. equiseti was associated with apple tree rhizosphere in Chihuahua, Mexico (Pérez-Corral et al., 2015). In addition, Carreri et al. (2013) isolated F. tricinctum from onion roots and bulbs in southern Italy. However, an association between the Fusarium species and apple tree rhizosphere has not been reported. Thus, F. fujikuroi, F. sacchari, F. tricinctum and F. subglutinans are considered for the first time as pathogenic for apple roots.
The second most frequently observed genus was Fusarium teleomorph (Gibberella spp.), with 15% (45 isolates belonging G. moniliformis anamorph: Fusarium verticillioides and G. intermedia anamorph: Fusarium proliferatum), followed by Bionectria ochroleuca (12%, 36 isolates), Alternaria spp. (17%, 5 isolates) comprising mainly A. brassicae and A. alternata, Clonostachys sp. (1.3%, 4 isolates), Phytophthora cactorum (1.3%, 4 isolates), Pythium spp. (0.7%, 3 isolates), Phymatotrichopsis sp. (0.3%, 1 isolate) (Table 2). Scherm et al. (2013) demonstrated that the teleomorph of Fusarium species (Gibberella spp.) can be found in other crops. In addition, the Oomycetes P. cactorum and Pythium spp. found in this study were differentially distributed among the orchards. These species have been reported as the main causal agents of crown, collar and root rot in apples in other countries (Roiger and Jeffers, 1991; Yao et al., 2006). Other species, such as B. ochroleuca, A. alternata and A. brassicae, have also been associated with core rot in apple trees in South Africa (Serdani et al., 2002). Moreover, P. omnivora, the causal agent of Texas root rot, has been reported as a pathogen that causes important economic losses in different crops in northern Mexico and southern USA, particularly in cotton and walnut trees, reflecting its adaptability to a wide range of climatic conditions (Samaniego-Gaxiola, 2007) and being a potential causal agent of root diseases in other fruit trees. The present study reports for the first time the fungus Phymatotrichopsis sp. as a pathogenic agent for roots in apple orchards from Mexico. In addition, four Trichoderma species were obtained from soil samples, being Trichoderma gamsii the most frequently observed antagonist, with 72.5%, followed by T. hamatum, T. harzianum and T. atroviride with 15, 7.5, and 5%, respectively (Table 2).
Table 2 Fungi and Oomycetes isolates and putative fungal antagonists, molecularly identified, associated to apparently root diseased and diseased symptom-free apple trees from four localities in the state of Chihuahua, Mexico, and their occurrence rate (%).
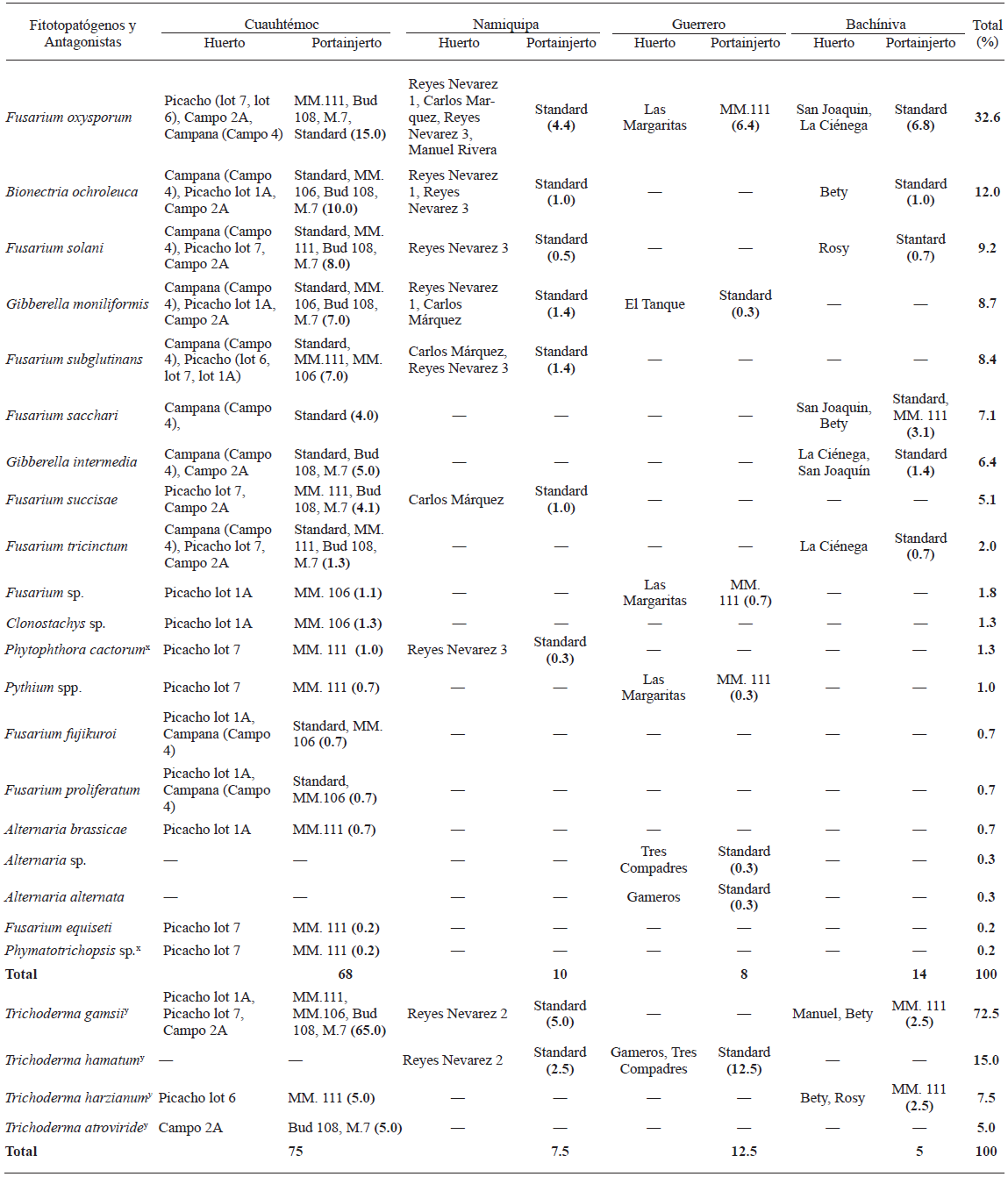
x Oomycete and fungal isolates identified according to their morphological characters.
y Putative antagonistic fungi.
Pathogenicity tests
The susceptibility of the rootstocks to tested fungi and Oomycetes is shown in Table 3. All rootstocks were susceptible to at least two of the isolates, except G.935, which was resistant to the tested isolates of fungi and Oomycetes. The G.30, G.202 and G.41 rootstocks were the most susceptible to 13, 10 and 9 of the tested pathogens, respectively. Pythium ultimum was the most pathogenic, causing diseases in 100% of the tested trees (Table 3). Although the G.30 and MM.111 were highly susceptible rootstocks (more than 50-75%), they are widely planted in the apple producing areas of Chihuahua, Mexico. These rootstocks showed symptoms of infection with five Oomycete isolates, with P. cactorum C1 and Pythium ultimum isolates being the most pathogenic. These rootstocks along with MM.106 have been reported as the most susceptible to these pathogens (Roiger and Jeffers, 1991; Uthkede and Smith, 1991; Latorre et al., 2001). Similar results were found by Latorre et al. (2001) after inoculation of Phytophthora isolates, with P. cactorum resulting the most virulent and pathogenic to M.9, M.25, MM.106 and MM.111 apple rootstocks. The Standard rootstock was only susceptible to two Pythium species, P. irregulare and P. ultimum with 80 and 60%, respectively (Table 3). This finding is contrary to that was observed in tested orchards, where the highest incidence of infection damage of roots was found on Standard rootstocks. However, this could be related to the age of the trees (30-40 years old), making them more susceptible to the attack of phytopathogens. Most of tested rootstocks were susceptible to P. cactorum and Pythium spp. isolates, as previously demonstrated by others (Yao et al., 2006). Roiger and Jeffers (1991) found that P. cactorum was highly responsible for crown and root rot in apple trees worldwide. However, Tewoldemedhin et al. (2011a-b) reported that the etiological agent of root diseases is a complex of multiple fungal and Oomycete species belonging to the genera Phytophthora, Pythium, and Rhizoctonia (Manici et al., 2013). In the present study, P. cactorum, P. ultimum, F. sacchari and F. oxysporum were the most pathogenic species. These species have previously been reported in other studies as causal agents of root rot diseases in apple trees of major importance worldwide (Uthkede and Smith, 1991; Latorre et al., 2001).
The most pathogenic Fusarium isolate was F. sacchari, which infected 100% of the M.25, Bud.9 and G.30 trees in spite of the fact that this isolate had low consistency (7.1%) as compared to the rest of the identified microorganisms. This effect of F. sacchari might be consequence of its low growth rate or to the presence of some antagonistic isolates coexisting in the same microenvironment (Table 3). In contrast, F. tricinctum and F. solani resulted non-pathogenic in seven rootstocks while in the rest of the Fusarium isolates showed low pathogenicity. Manici et al. (2003) also observed non-pathogenic of Fusarium isolates in apple trees.
Table 3 Pathogenicity percentage of Oomycetes and fungi isolates when tested under greenhouse conditions against twelve 1-year-old rootstocks predominantly planted in the state of Chihuahua, Mexico, after two months of being inoculated.
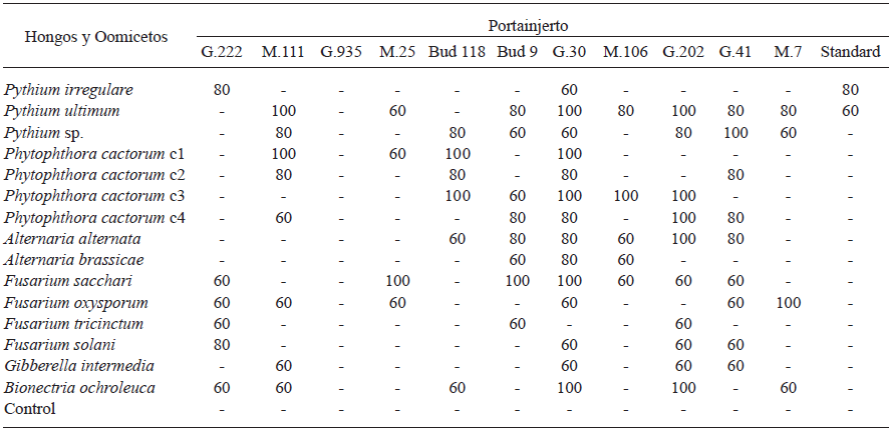
Only Oomycetes and fungal isolates that caused ≥ 60% symptoms in each rootstock, after two months of being inoculated, were considered pathogenic
(-) Without symptoms.
Alternaria alternata was the most pathogenic in G.202 rootstock. Control rootstocks remained free of diseased during the experiment. The main symptoms observed were malformations, gradual wilting in diseased rootstocks, defoliation, senescence, necrotic tissues, chlorosis, rigid leaves, leaves with dark brown round spots with concentric yellowish rings, young shots withered, root and neck rot, collapsed stems with black and oval spots, canker, discoloration of brown to reddish-brown and gummy exudates.
Antagonistic activity in vitro of Trichoderma spp. vs Oomycetes
The four species of Trichoderma showed similar antagonistic behavior when they were confronted against Pythium spp. and P. cactorum isolates. However, the PIRG was different for isolates of Pythium spp. (1.6-31.4%, p=0.05) and P. cactorum (8.1-92.1%, p=0.05). The overgrowth (total invasion over the pathogens) of the four species of Trichoderma was observed for all isolates of both Oomycetes after eight days post-confrontation (Table 4). Gajera and Vakharia (2010) also observed the highest growth inhibition of phytopathogenic fungi with some Trichoderma species. Gajera and Vakharia (2010) demonstrated that these species competed against phytopathogenic fungi and produced extracellular enzymes (i.e. chitinase, β-1,3 glucanase, and proteases), besides of being antifungal, growth promoters, and induced the resistance of plants to pathogens. Among testes Trichoderma species, T. asperellum showed a low PIRG (15.5-31.4%, p=0.05) in the three species of Pythium, with the highest inhibition being observed with P. irregulare at the fifth day post-confrontation. In confrontations with P. cactorum isolates, the PIRG was >84% (p=0.05) at the eighth day post-confrontation (Table 4; Fig. 1a). Similarly, Rios-Velasco et al. (2016) also observed significant inhibition of radial growth in confrontations of T. asperellum against Aspergillus nidulans and Penicillium crustosum, showing the potential of this strain to be used as biological control agent of different types of phytopathogens. In our study, T. harzianum showed the same trend in PIRG as compared with T. asperellum; when it was confronted against Pythium spp., the incidence ranged from 1.6 to 7.9% (p=0.05) but in confrontations with P. cactorum isolates, the incidence ranged from 86.6 to 92.1% (p=0.05) (Table 4; Figure 1b). This difference could be attributed to the ability of T. harzianum to produce the antibiotic 6-pentyl-α-pyrone and regulate genes involved in the biosynthesis of trichothecenes and mycotoxins with broad spectrum of antimicrobial activities (Cooney et al., 2001). Additionally, this antagonist completely filled the Petri dish after three days.
Trichoderma gamsii showed values of PIRG from 15.5 to 29.7% (p=0.05) in Pythium spp. isolates and >81% in P. cactorum isolates. These results were similar to those obtained with T. asperellum (Table 4). Moreover, T. atroviride showed the same trend, with a PIRG values from 11.8 to 26.3% (p=0.05) in Pythium spp. isolates and >89% (p=0.05) in P. cactorum isolates at the fifth and sixth day post-confrontation, respectively (Table 4; Figure 1c). In this study, T. atroviride was the best control agent for the inhibition of the growth of P. cactorum in PDA medium. Gajera and Vakharia (2010) also observed that T. atroviride was effective to reduce the incidence of collar rot, reducing pathogen virulence. Roiger and Jeffers (1991) suggested that Trichoderma species are promissory as biological control agents to P. cactorum, because its use is compatible with management practices and other root diseases, including the use of pesticides. Trichoderma species also showed plant growth promoter activities, suggesting another potential benefit for their use. According to Bell’s scale, an antagonism Type 1 was found for the four Trichoderma species, where the antagonist (Trichoderma spp.) overgrew completely on pathogen’s colony and completely covered medium’s surface after six days post-inoculation against Phytophthora isolates.
Table 4 Growth inhibition of Oomycetes, when confronted in vitro against Trichoderma species 8 d post-inoculation and by Bacillus species at sixth day post-inoculation.
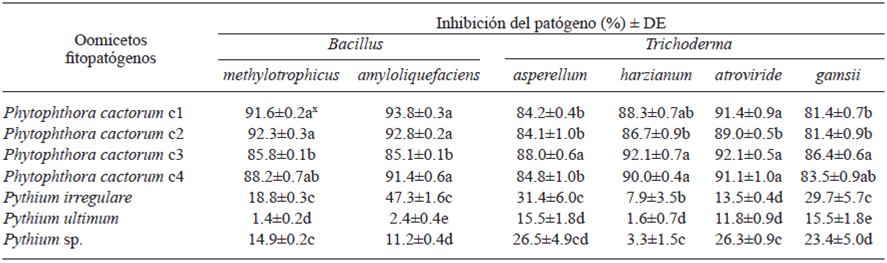
x Letters in the same column indicate significant differences in the treatments where the means followed by the same letter are not significantly different (ANOVA) according to Tukey’s test (p=0.05).
Antagonistic activity in vitro of Bacillus spp. vs Oomycetes
The bacteria B. amyloliquefaciens and B. methylotrophicus inhibited the growth of P. cactorum isolates more than 85% (p=0.05) when evaluated in vitro, and both antagonists showed PIRGs values lower than 50 and 20% (p=0.05), respectively, when they were confronted against Pythium spp. Bacillus amyloliquefaciens showed a PIRG ranging from 85.1 to 93.8% when it was confronted against P. cactorum and from 2.4 to 47.6% (p=0.05) against Pythium spp. at the sixth day post-confrontation. Bacillus methylotrophicus showed the same trend, with a PIRG of 1.4 to 18.8% (p=0.05) when it was confronted against Pythium spp. and a PIRG value higher than 85% (p=0.05) in P. cactorum isolates (Table 4; Fig. 1d-f). Both bacteria showed high PIRG in confrontations with P. cactorum isolates, reflecting probably the production of antibiotics, enzymes that attack the cell components of the pathogens or volatile compounds that inhibit the growth of the pathogen. However, a dissimilar behavior was observed when they were confronted with Pythium species exhibiting fast growth, and the rates of the PIRG were low compared with the P. cactorum isolates when confronted in vitro against Bacillus spp. (Table 4). Jeyaseelan et al. (2012) evaluated the antagonistic effect of some Bacillus species against Pythium aphanidermatum and also observed a low inhibition effect by some antagonistic species such as those tested in the present study, reflecting the inability of Bacillus species to produce highly effective antimicrobial compounds against some Pythium species. According to Guillén-Cruz et al. (2006), the Bacillus genus showed antagonistic effects in vitro against Phytophthora spp. with B. amyloliquefaciens being the most efficient and showing a plant growth promoter effect, likely contributing to the setting of macronutrients and the solubilization of phosphates and other micronutrients.
Notably, Bacillus species showed an inhibition halo >29 mm when it was confronted against P. cactorum, at 6 d post-confrontation and there was no contact day (Table 5). Some of the most important mechanisms of antagonistic bacteria such as Bacillus are the antibiosis, consisting on the production of antibiotic compounds and the inhibition of other microbes (Intana et al., 2008). Souto et al. (2004) demonstrated that B. amyloliquefaciens exerted antifungal properties and that it might be considered as a good biological control agent. Dev-Sharma et al. (2013) obtained >90% inhibition in phytopathogenic fungi when they were confronted in vitro against B. methylotrophicus. Silo-Suh et al. (1994) reported that some Bacillus species have the ability to produce effective and broad-spectrum antibiotics, such as peptides, lipopeptides, aminoglycosides and aminopolyols. Moreover, Wheatley (2002) referred to the volatile organic compounds produced by different bacteria and fungi as ideal in microbial interactions, reflecting efficacy over a wide range of spatial scales, inhibiting the growth of many pathogenic fungi, as these compounds have direct or indirect effects on the activity of specific fungal enzymes.
Table 5 Inhibition halo showed when confronted in vitro Bacillus species against Oomycetes at six days after inoculation.
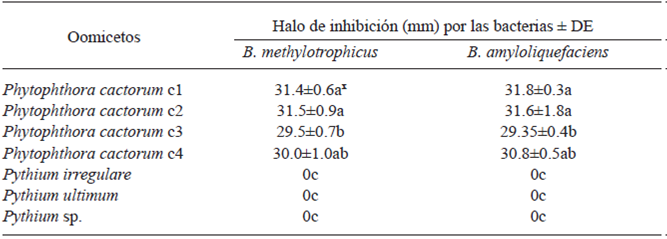
x Letters in the same column indicate significant differences in the treatments where the means followed by the same letter are not significantly different (ANOVA) according to Tukey’s test (p=0.05).
CONCLUSIONS
Fungi and Oomycetes were the most abundant in collected isolates, with Fusarium oxysporum being the most representative species. The 1-year-old G.935, Standard and M.25 rootstocks were the most resistant to tested isolates, which could be attributed to the age and genotype of the rootstocks. Our results demonstrated that both antagonistic groups could be considered as candidates to be used as biological control agents for P. cactorum, which is important causal agent of root diseases in apple rootstocks. Further in vitro and in vivo studies are required to confirm the effectiveness of Trichoderma and Bacillus isolates against various causal agents of root diseases in apple orchards in Chihuahua.
REFERENCES
Altschul SF, Gish W, Miller W, Myers EW y Lipman DJ. 1990. Basic local alignment search tool. Molecular Biology 15: 403-410. https://dx.doi.org/10.1016/S0022-2836(05)80360-2 [ Links ]
Barnett, H.L., y Hunter, B.B. 1998. Illustrated genera of imperfect fungi (3rd ed.). The American Phytopathological Society. U.S Department of Agriculture, Agricultural Research Service, Washington State University, Pullman. APS Press. USA. St. Paul, Minnesota USA. 218p. [ Links ]
Bell D, Well H y Markham C. 1982. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology 72: 379-382. Disponible en línea: https://www.apsnet.org/publications/phytopathology/backissues/Documents/1982Articles/Phyto72n04_379.PDF [ Links ]
Carreri R, Raimo F, Pentangelo A y Lahoz E. 2013. Fusarium proliferatum and Fusarium tricinctum as causal agents of pink rot of onion bulbs and the effect of soil solarization combined with compost amendment in controlling their infections in field. Crop Protection 43: 31-37. http://doi.org/10.1016/j.cropro.2012.09.013 [ Links ]
Cooney JM, Lauren DR y di-Menna ME. 2001. Impact of competitive fungi on trichothecene production by Fusarium graminearum. Journal of Agricultural and Food Chemistry 49: 522-526. http://dx.doi.org/10.1021/jf0006372 [ Links ]
Dev-Sharma SC, Shovon MS, Sarowar-Jahan MG, Asaduzzaman AK, Rahman Md.A, Biswas KK y Roy N. 2013. Antibacterial and cytotoxic activity of Bacillus methylotrophicus SCS 2012 isolated from soil. Journal of Microbiology, Biotechnology and Food Sciences 2(4): 2293-2307. Disponible en línea: http://www.jmbfs.org/wp-content/uploads/2013/02/jmbfs_0247_devsharma.pdf [ Links ]
Dugan FM. 2006. The Identification of fungi. An illustrated introduction with keys, glossary, and guide to literature. The American Phytopathological Society. U.S Department of Agriculture, Agricultural Research Service, Washington State University, Pullman. APS Press. USA. St. Paul, Minnesota USA. 176p. [ Links ]
Ezziyyani M, Sánchez C, Requena ME, Rubio L y Candela ME. 2004. Biocontrol por Streptomyces rochei -Ziyani-, de la podredumbre del pimiento (Capsicum annuum L.) causada por Phytophthora capsici. Anales de Biología 26: 69-78. Disponible en línea: https://www.um.es/analesdebiologia/numeros/26/PDF/08-BIOCONTROL.pdf [ Links ]
Gajera HP y Vakharia DN. 2010. Molecular and biochemical characterization of Trichoderma isolates inhibiting a phytopathogenic fungi Aspergillus niger Van Tieghem. Physiological and Molecular Plant Pathology 74: 274-282. http://dx.doi.org/10.1016/j.pmpp.2010.04.005 [ Links ]
Guillén-Cruz R, Hernández-Castillo FD, Gallegos-Morales G, Rodríguez-Herrera R, Aguilar-González CN, Padrón-Corral E y Reyes-Valdés MH. 2006. Bacillus spp. como biocontrol en un suelo infestado con Fusarium spp., Rhizoctonia solani Kühn y Phytophthora capsici Leonian y su efecto en el desarrollo y rendimiento del cultivo de Chile (Capsicum annuum L.). Revista Mexicana de Fitopatología 24(2): 105-114. Disponible en línea: http://www.redalyc.org/pdf/612/61224204.pdf [ Links ]
Hantula J, Lilja A, Nuorteva H, Parikka P y Werres S. 2000. Pathogenicity, morphology and genetic variation of Phytophthora cactorum from strawberry, apple, rhododendron, and silver birch. Mycological Research 104(9): 1062-1068. https://doi.org/10.1017/S0953756200002999 [ Links ]
Hsuan HM, Salleh B y Zakaria L. 2011. Molecular identification of Fusarium species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. International Journal of Molecular Sciencies 12: 6722-6732. http://dx.doi.org/10.3390/ijms12106722 [ Links ]
Intana W, Yenjit P, Suwanno T, Sattasakulchai S, Suwanno M y Chamswarng C. 2008. Efficacy of antifungal metabolites Bacillus spp. for controlling tomato damping-off caused by Pythium aphanidermatum. Walailak Journal Science and Technology 5(1): 29-38. Disponible en línea: http://wjst.wu.ac.th/index.php/wjst/article/view/108/92 [ Links ]
Jeyaseelan EC, Tharmila S y Niranjan K. 2012. Antagonistic activity of Trichoderma spp. and Bacillus spp. against Pythium aphanidermatum isolated from tomato Damping Off. Archives of Applied Science Research 4(4): 1623-1627. Disponible en línea: http://www.scholarsresearchlibrary.com/articles/antagonistic-activity-of-trichoderma-spp-and-bacillus-spp-against-pythium-aphanidermatum-isolated-from-tomato-damping-of.pdf [ Links ]
Ju R, Zhao Y, Li J, Jiang H, Liu P, Yang T, Bao Z, Zhou B, Zhou X y Liu X. 2014. Identification and evaluation of a potential biocontrol agent Bacillus subtilis against Fusarium sp. in apple seedlings. Annals of Microbiology 64: 377-383. http://dx.doi.org/10.1007/s13213-013-0672-3 [ Links ]
Kamal S, Prasad R y Varma A. 2010. Soil microbial diversity in relation to heavy metals Soil Heavy Metals. Springer Berlin Heidelberg. 19:31-63. https://doi.org/10.1007/978-3-642-02436 [ Links ]
Lamichhane JR y Venturi V. 2015. Synergisms between microbial pathogens in plant disease complexes: a growing trend. Frontiers in Plant Science 6(385): 1-12. https://doi.org/10.3389/fpls.2015.00385 [ Links ]
Latorre BA, Rioja ME y Wilcox WF. 2001. Phytophthora species associated with crown and root rot of apple in Chile. Plant Disease 85: 603-606. http://dx.doi.org/10.1094/PDIS.2001.85.6.603 [ Links ]
Leslie JF, Anderson LL, Bowden RL y Lee YW. 2007. Inter-and intra-specific genetic variation in Fusarium. International Journal of Food Microbiology 119: 25-32. http://doi.org/10.1016/j.ijfoodmicro.2007.07.059 [ Links ]
Manici LM, Ciavatta C, Kelederer M y Erschbaumer G. 2003. Replant problems in South Tyrol: role of fungal pathogens and microbial population in conventional and organic apple orchards. Plant and Soil 256: 315-324. http://doi.org/10.1023/A:1026103001592 [ Links ]
Manici LM, Kelderer M, Franke-Whittle IH, Rühmer T, Baab G, Nicoletti F y Neaf A. 2013. Relationship between root-endophytic microbial communities and replant diseases in specialized apple growing areas in Europe. Applied Soil Ecology 72: 207-214. http://doi.org/10.1016/j.apsoil.2013.07.011 [ Links ]
Pérez-Corral DA, García-González NY, Gallegos-Morales G, Ruiz-Cisneros MF, Berlanga-Reyes DI y Rios-Velasco C. 2015. Aislamiento de actinomicetos asociados a rizosfera de árboles de manzano antagónicos a Fusarium equiseti. Revista Mexicana de Ciencias Agrícolas 6(7): 1629-1638. Disponible en línea: http://www.scielo.org.mx/pdf/remexca/v6n7/v6n7a16.pdf [ Links ]
Raeder U y Broda P. 1985. Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology 1: 17-20. http://dx.doi.org/10.1111/j.1472-765X.1985.tb01479.x [ Links ]
Ramírez-Legarreta MR, Jacobo-Cuéllar JL, Marioni-Ávila MR y Parra-Quezada RA. 2004. Eficiencia del uso de plaguicidas en huertos de manzano [Malus sylvestris (L.) Mill. var. domestica (Borkh.) Mansf.] en Chihuahua, México. Revista Mexicana de Fitopatología 22: 403-413. Disponible en línea: http://www.redalyc.org/pdf/612/61222314.pdf [ Links ]
Ramírez-Legarreta MR, Ruiz-Corral JA, Medina-García G, Jacobo-Cuéllar JL, Parra-Quezada RA, Ávila-Marioni MR y Armando-Álvarez JP. 2011. Perspectivas del sistema de producción de manzano en Chihuahua, ante el cambio climático. Revista Mexicana de Ciencias Agrícolas 2: 265-279. Disponible en línea: http://www.scielo.org.mx/pdf/remexca/v2nspe2/v2spe2a8.pdf [ Links ]
Rios-Velasco C, Caro-Cisneros JM, Berlanga-Reyes DI, Ruiz-Cisneros MF, Ornelas-Paz JJ, Salas-Marina MA, Villalobos-Pérez E y Guerrero-Prieto VM. 2016. Identification and antagonistic activity in vitro of Bacillus spp. and Trichoderma spp. isolates against common phytopathogenic fungi. Revista Mexicana de Fitopatología 34(1): 84-99. http://dx.doi.org/10.18781/R.MEX.FIT.1507-1 [ Links ]
Roiger DJ, y Jeffers SN. 1991. Evaluation of Trichoderma spp, for biological control of Phytophthora crown and root rot of apple seedlings. Phytopathology 81: 910-917. http://doi.org/10.1094/Phyto-81-910 [ Links ]
Rumberger, A., Merwin, I.A., y Thies, J.E. 2007. Microbial community development in the rhizosphere of apple trees at a replant disease site. Soil Biology and Biochemistry. 39: 1645-1654. https://doi.org/10.1016/j.soilbio.2007.01.023 [ Links ]
Samaniego-Gaxiola JA. 2007. Research perspectives on Phymatotrichopsis omnivora and the disease it causes. Agricultura Técnica en México 33: 309-318. Disponible en línea: http://www.scielo.org.mx/pdf/agritm/v33n3/v33n3a10.pdf [ Links ]
SAS Institute. 2002. SAS User’s Guide. Version 9.0. SAS Institute, Cary, NC. [ Links ]
Scherm B, Balmas V, Spanu F, Pani G, Delogu G, Pasquali M y Migheli Q. 2013. Fusarium culmorum: causal agent of foot and root rot and head blight on wheat. Molecular Plant Pathology 14(4): 323-341. https://doi.org/10.1111/mpp.12011 [ Links ]
Schoenborn L, Yates PS, Grinton BE, Hugenholtz P, y Janssen PH. 2004. Liquid serial dilution is inferior to solid media for isolation of cultures representative of the phylum-level diversity of soil bacteria. Applied and Environmental Microbiology 70(7): 4363-4366. https://doi.org/10.1128/AEM.70.7.4363-4366.2004 [ Links ]
Serdani M, Kang JC, Andersen B y Crous PW. 2002. Characterisation of Alternaria species-groups associated with core rot of apples in South Africa. Mycological Research 106: 561-569. https://doi.org/10.1017/S0953756202005993 [ Links ]
Silo-Suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J y Handelsman J. 1994. Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Applied and Enviromental Microbiology 60: 2023-2030. Disponible en línea: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC201597/pdf/aem00023-0329.pdf [ Links ]
Souto GI, Correa OS, Montecchia MS, Kerber NL, Pucheu NL, Bachur M y García AF. 2004. Genetic and functional characterization of a Bacillus sp. strain excreting surfactin and antifungal metabolites partially identified as iturin-like compounds. Journal of Applied Microbiology 97: 1247-1256. http://dx.doi.org/10.1111/j.1365-2672.2004.02408.x [ Links ]
Tewoldemedhin YT, Mazzola M, Botha WJ, Spies CFJ y McLeod A. 2011a. Characterization of fungi (Fusarium and Rhizoctonia) and oomycetes (Phytophthora and Pythium) associated with apple orchards in South Africa. European Journal of Plant Pathology 130: 215-229. http://dx.doi.org/10.1007/s10658-011-9747-9 [ Links ]
Tewoldemedhin YT, Mazzola M, Labuschagne I y McLeod A. 2011b. A multi-phasic approach reveals that apple replant disease is caused by multiple biological agents, with some agents acting synergistically. Soil Biology and Biochemistry 43: 1917-1927. http://doi.org/10.1016/j.soilbio.2011.05.014 [ Links ]
Uthkede RS y Smith EM. 1991. Phytophthora and Pythium species associated with root rot of young apple trees and their control. Soil Biology and Biochemistry 23: 1059-1063. https://doi.org/10.1016/0038-0717(91)90044-K [ Links ]
Watanabe T. 2010. Pictorial atlas of soil and seed fungi: Morphologies of cultured fungi and key to species (3rd ed.): CRC Press. [ Links ]
Wheatley RE. 2002. The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek 81: 357-364. http://dx.doi.org/10.1023/A:1020592802234 [ Links ]
White TJ, Bruns T, Lee S y Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In Press, A. (Ed.), PCR protocols: A guide to methods and applications. pp. 315-322. http://dx.doi.org/10.1016/B978-0-12-372180-8.50042-1 [ Links ]
Wilcox WF. 1993. Incidence and severity of crown and root rots on four apple rootstocks following exposure to Phytophthora species and waterlogging. Journal of American Society of Horticultural Science 118(1): 63-67. Disponible en línea: http://journal.ashspublications.org/content/118/1/63.full.pdf+html [ Links ]
Yao S, Merwin I A, Abawi GS y Thies JE. 2006. Soil fumigation and compost amendment alter oil microbial community composition but do not improve tree growth or yield in an apple replant site. Soil Biology and Biochemistry 38: 587-599. http://doi.org/10.1016/j.soilbio.2005.06.026 [ Links ]
Acknowledgments
Received: April 20, 2017; Accepted: July 09, 2017











 text in
text in 


