Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.35 n.3 Texcoco Sep. 2017
https://doi.org/10.18781/r.mex.fit.1703-3
Scientific articles
Larrea tridentata extracts as an ecological strategy against Fusarium oxysporum radicis-lycopersici in tomato plants under greenhouse conditions
1Instituto Tecnológico de Sonora, 5 de Febrero 818 sur, Ciudad Obregón, Sonora, CP. 85000, México.
2Laboratorio de Ecología Química, Centro de Investigación en Alimentación y Desarrollo A.C., km 0.6 Carretera Hermosillo-La Victoria, Hermosillo, Sonora, CP. 83000 México.
The antifungal ability of extracts of Larrea tridentata in the inhibition of Fusarium oxysporum radicis-lycopersici in vitro and in vivo was evaluated. Once the Fusarium strain was characterized, the methodology of poisoned culture medium was used to determine the inhibition of mycelial growth at various concentrations (0-2000 ppm) and solvents (dichloromethane, methanol, ethanol and water). In the in vivo assay, ten treatments were evaluated: 2000 and 3000 ppm for DCM and EtOH, 3000 and 6000 ppm for MeOH and H2O and two controls; one plant as experimental unit and ten replicates. Plant height, leaf number, chlorophyll, severity, as well as incidence and survival were determined in tomato plants inoculated with the fungus and different extracts of L. tridentata. The extracts with a higher percentage of inhibition and vegetative development are dichloromethane at 3000 ppm and methanol at 4000 ppm, both in vitro and in vivo. Extracts of Larrea tridentata can be considered as part of an integrated management plan for the control of crown rot in tomatoes caused by Fusarium oxysporum radicis-lycopersici.
Key words: biofungicide; protected agriculture; integrated management; Creosote bush
Se evaluó la capacidad antifúngica de extractos de Larrea tridentata en la inhibición de Fusarium oxysporum radicis-lycopersici bajo condiciones experimentales en invernadero. Una vez que la cepa de Fusarium se caracterizó, se determinó el crecimiento micelial in vitro y su efecto inhibitorio con los extractos de L. tridentata por el método del medio envenenado a diferentes concentraciones (0-2000 ppm) y disolventes (diclorometano, metanol, etanol y agua). En el ensayo in vivo se evaluaron diez tratamientos: 2000 y 3000 ppm para DCM y EtOH, 3000 y 6000 ppm para MeOH y H2O y dos testigos, considerando una planta como unidad experimental y diez repeticiones. Se evaluó la altura de la planta, número de hojas, clorofila, severidad, incidencia y sobrevivencia en plantas de tomate inoculadas con el hongo y tratadas con los diferentes extractos de L. tridentata. Los extractos con mayor porcentaje de inhibición y desarrollo vegetativo fueron: diclorometano a 3000 ppm y metanol a 4000 ppm, tanto in vitro como in vivo. Los extractos de L. tridentata pueden considerarse dentro de un plan de manejo integrado para el control de la pudrición de la corona en tomate causada por F. oxysporum radicis-lycopersici.
Palabras clave: biofungicida; agricultura protegida; manejo integrado; gobernadora
Tomato (Solanum lycopersicum) is grown par excellence in protected agriculture in Mexico, consolidating this country as the main exporter to the United States and Canada (Atlas Agroalimentario, 2016). Among the main problems faced by this crop is the appearance of fungal diseases, such as Tomato Crown Rot (TCR), the causal agent of which is the saprophytic fungus Fusarium oxysporum f. sp. radicis-lycopersici (Forl). TCR is one of the most destructive diseases, since it causes losses in the yields of greenhouse and field production systems (Szczechura et al., 2013). Some of the most important damages caused to plants include growth delay, root rotting, large necrotic wounds in the neck and base of the stem, and therefore senescence (McGovern, 2015). The presence of Forl has been reported in the states of Morelos (Domínguez, 2012) and Sinaloa, where its presence caused losses of up to 50 % in tomato harvests (Apodaca et al., 2004). Conventional control strategies are based on the use of grafts, solarization, crop rotation, and mainly, on the use of synthetic chemical products, which produce well-known damages to human health, negative impacts on the environment and the considerable increase of production costs (Cordero-Ramírez et al., 2013). Given the negative implications of the improper use of chemical fungicides, the development of new forms of control is crucial to reduce the dependence on these products (Villa-Martínez et al., 2014). Some of the alternatives to chemical control in integrated pest and disease management, are the use of plant extracts with fungicidal properties, such as Larrea tridentata. Commonly known as “gobernadora,” it is the predominant bush of the Sonoran desert and a prominent source of natual compounds with about 50 % of its leaves (in dry weight) as extractable matter (Arteaga et al., 2005). Out of the most widely studied bioactive phenolic compounds, one worth highlighting is nordihydroguaiartic acid (NDGA) (Martins et al., 2013), an antioxidant found in the resin of cells near the epidemic layers of leaves and stems. This biocompound is present in all the species of the genus; also, interpopulational studies on L. tridentata carried out in the Sonora desert revealed that the NDGA concentrations found in the resin of their leaves varied depending on the latitude and time of year. The flowers, leaves, green and some woody stems (<5 mm diameter) contain NDGA; the highest concentration was observed in leaves with 38.3 mg g-1 and green stems with 32.5 mg g-1 (Hyder, et al., 2002; Lira-Saldivar, 2003). Therefore, knowing the content of NDGA in the L. tridentata extracts will provide information on its effect in the control of pathogenic fungi in plants. The antifungal properties of L. tridentata have been confirmed in different investigations for about 40 years using in vitro tests with about 17 important plant pathogenic fungi in the agricultural area (López et al., 2005; Lira-Saldivar et al., 2006); however, there are very few studies in vitro against Forl, and carrying out a test under these conditions would set the standards to test the standards in vivo, since, despite the promising results that have been published, the evidence of tests carried out in greenhouses or fields is insufficient. The aim of this study was to quantify the content of NDGA in de L. tridentata extracts to evaluate its effectiveness in vitro and in greenhouse conditions to establish its potential as a biofungicide in the control of Forl.
MATERIALS AND METHODS
This investigation was carried out in the year 2014 in the Plant Biotechnology laboratory and in the Experimental Transfer and Technology Center 910 of the Sonora Institute of Technology (ITSON) in Ciudad Obregón, Sonora, as well as the Ecological Chemistry laboratory in the Food and Development Research Center (CIAD) A.C. Hermosillo, Sonora.
Obtaining the Forl strain
The Forl strain was previously isolated from the rhizosphere of tomato plants grown in a commercial greenhouse in the Yaqui Valley, Sonora. The fungus was distinguished by comparing its colonial and morphological characteristics using specialized literature (Leslie and Summerell, 2016; Nelson et al., 1983), which was identified molecularly with specific primers according to Hirano and Arie (2006) (data not published). A copy of the isolation is kept as Forlmx-19 in the microorganism bank of the Ecological Chemistry Lab in the microorganism bank of the Ecological Chemistry Lab in the Research Center for Food and Development, Civil Association (CIAD A.C.), Hermosillo, Sonora.
Obtaining L. tridentata extracts and content of nordihydroguaiaretic acid (NDGA)
Preparation of the extracts. Extracts were produced using four solvents: dichloromethane (DCM), methanol (MeOH), ethanol (EtOH), and water (H2O) using young L. tridentata leaf and stem samples from the Sonora desert (Peñuelas et al., 2015). For the DCM and MeOH extracts, 90 g of dry and crushed L. tridentata leaves were used to for separate extractions, with each solvent in a Soxhlet system at 39 and 64.7 °C, respectively, for 15 h of reflux, followed by rotatory evaporation. For the EtOH and aqueous extracts, 1 kg of fresh material was softened in 10 L of an ethanol:water solution 7:3 (v/v) for 8 days at room temperature (25 °C) and in the dark. After time it was filtered using gauze and then paper (Whatman #5). The filtrate underwent rotatory evaporation; the solid phase is considered as EtOH extract, and the supernatant was frozen at -45 °C and freeze-dried to be considered as the aqueous extract. The NDGA content was quantified in the four extracts obtained using reverse phase high performance liquid chromatography (RP-HPLC), in a HPLC system (Hewlett Packard series 1100, Germany) connected to a diode arrangement detector (DAD) in the Ecological Chemistry Laboratory in CIAD A.C. Hermosillo, Sonora. We used the methodology proposed by Hyder et al. (2002) with an ODS C-18 hypersil column and as a mobile phase: A: H3PO4 1 % and B: 85 % acetonitrile-14 % H2O-1 % H3PO4 in a 60/40 proportion, with Isocratic elusion, and a flow of 1 mL min-1. The detection was carried out at a wavelength of 283 nm. For the quantification, a calibration curve was created with the NDGA standard (purity≥90 %, Sigma Aldrich, United States). The volume of injection of the NDGA standard and the samples was 25 μL.; the data were analyzed using the software Chemstation (Hewlett Packard, Germany).
Determination of the percentage of inhibition of Forl by L. tridentata extracts
We used the poisoned medium method (Guerrero-Rodríguez et al., 2007), which consists in pouring the extracts in the agar dextrose potato (ADP) culture medium in sterile, 5 cm petri dishes; after the medium has solidified, a sample of the vigorous growth margin of the 12-day old strain was planted by poking the center. The period of incubation lasted 7 days at 28 °C.
Variables evaluated
The mycelial growth was recorded every day starting on day 3 of incubation, measuring in mm the two crossed diameters; monitoring ceased when the control reached 100% of the Petri dish. The inhibition was determined using the formula of the percentage of inhibition = (dc-dt/dt)x100, where dc is the average diameter of the mycelial growth of the control, and dt is the diameter of the mycelial growth with the different extracts (Lira-Saldivar et al. 2003).
Experimental design
A 4x5 factorial analysis was used, where A were the extracts, and B, the concentrations (Table 1). The experimental design was completely random, and an ANOVA was used, along with a Tukey average comparison test (p<0.05) in the software Statgraphics 5.1.
Biotest in vivo
This was carried out under greenhouse conditions in the Experimental Transfer and Technology Center 910 of the Sonora Institute of Technology (ITSON) in the Yaqui Valley, Sonora. One hundred commercial cocktail tomato seedlings var. F1 Prolyco2 with 35 days of growth were used. The agronomic management of the crop (fertilization, irrigation, and cultural handling) was carried out according to the Sonora Technical Agricultural Agenda (SAGARPA, 2015).
Inoculation of Forl. The streak plate method was previously used to culture the Forl strain in Roux bottles with 250 ml of ADP with 3.5 ml of tartaric acid at 10 %, previously sterilized and gellified according to NOM-111-SSA1-1994 and Camacho et al. (2009). They were incubated at 28 °C for 7 days until the mycelium developed. Glass and 20 al 0.02 % tween beads were placed on the agar; using soft movements, the conidia were moved to a sterilized beaker. They we re counted in a Neubauer chamber, and adjusted to a concentration of 107 conidia mL-1 with distilled water. The roots of the plantlets were submerged for 30 min in this inoculant solution after having cut an incision into their secondary roots with a scalpel. They were then transplanted into 9 L pots containing clay loam soil from the Yaqui Valley, Sonora.
Application of treatments. To achieve the adhesion of the different concentrations of the extracts evaluated, the agricultural adherent® (Técnica Mineral S.A. de C.V) was used, at 1 L ha-1. The extracts were reactivated in acetone at 100 ml ml-1 (extract DCM and EtOH) and acetone:water (extract MeOH and H2O), the agricultural adherent was added and mixed until a suspension was obtained. Every 7 days after transplanting, uncovering the top section of the root, the pertinent extract was applied to it.
Variables evaluated
The following measurements were taken, 49 days after inoculation (dai): chlorophyll index with Minolta SPAD 502 (between 11:00 and 14:00 h) in chlorophyll units (CU), plant height with measuring tape (from the base of the stem to the apex) and number of leaves. After the emergence of the 5th true leaf, each plant was evaluated on a scale of 1 to 5 (Table 2), depending on the intensity of the disease (Marlatt et al. 1996). Once classified, the severity index was calculated using the formula by Towsend and Heuberguer (1943): Severity= (∑nv/ 5N) x 100, where n= plants per each category; v= value of each category; N= plants in experimental units. The data were normalized with a natural logarithm before. The incidence was calculated by the percentage of plants that showed some symptom of Forl infection. Plants were considered survivors if they remained alive after the completion of the experiment.
Experimental design
A totally random design was applied with 10 treatments and 10 repetitions (Table 3). The concentrations of the treatments were defined according to the results of the CMI performed previously. An ANOVA was carried out, as well as a multiple range test using the software Statgraphics plus version 5.1.
RESULTS AND DISCUSSION
Determination of the percentage of Forl inhibition by the effect of applying L. tridentata extracts
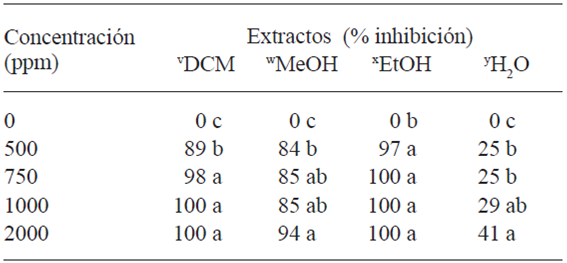
Table 4 shows the activity of the extracts on the mycelial growth of Forl. The L. tridentata extract with DCM displayed an inhibition of 98 % at 750 ppm; 94 % for MeOH at 2000 ppm , and 97 % for EtOH at 500 ppm, which indicates that the compounds extracted with EtOH had a greater fungicidal power over Forl in vitro. These results were used as a basis to determine the doses for the treatments in vivo, considering the concentration of each extract (p≤0.05) the completely inhibited the mycelial growth of the fungus. The concentration was increased to 3000 y 6000ppm of the extract H2O, since it presented little effect on the growth inhibition of Forl.
The highest inhibition of the hydroalcoholic extract of L. tridentata has been reported at 7000 ppm for Aspergillus flavus and 5000 ppm for Penicillium sp. (Moreno-Limón et al., 2011). The antifungal effect of L. tridentata is attributed to phenolic compounds, because polyphenolic extracts inhibited to the maximum with 0.7 ppm in Pythium sp., Colletotrichum truncatum, C. coccodes, Alternaria alternata, F. verticillioides, F. solani, F. Sambucinum and Rhizoctonia solani (Osorio et al., 2010).
Among the phenolic compounds of L. tridentata, NDGA stands out since it is extracted from the resin in leaves and stems (Lira et al., 2003). The amount of this compound extracted is directly related to the degree of polarity of the solvent (Moreno-Limón et al., 2011). In this way, this study quantified 68, 64, 6.07, and 2.8 mg g-1 of NDGA in the extracts of DCM, EtOH, MeOH, and H2O, respectively, correlating the concentrations quantified in the extracts with the effect on the mycelial growth shown in Table 4.
Table 4 Effect of the L. tridentata extracts on the mycelial growth of Forl
v dichloromethane, w methanol, x ethanol, y water.
Different letters represent significant differences between treatments (Tukey p≤0.05).
Giving a standard recommendation for the use of extracts in the control of phytopathogenic microorganisms, since there is not only the influence of the basic triangle host-pathogen-weather, but also of factors such as solvents and conditions of extraction, latitude and altitude of the area of sampling of L. tridentata (Lira-Saldivar et al., 2003), variability in breeds and pathogenicity among the special forms of F. oxysporum Fol and Forl; furthermore, in vivo one must consider the effect of the biodiversity of organisms in the soil and the surroundings.
Biotest in vivo
Chorophyll index. The content of chlorophyll in the leaves is shown in Table 5. All the extracts displayed higher levels (p<0.05) in comparison with the absolute control and the commercial fungicide. The treatments worth pointing out are those with H2O 6000, DCM 3000, and MeOH 4000 ppm with 49, 48, and 45 units of chlorophyll, respectively, which are values higher than those reported for healthy tomato plants (35 and 37 UC) (Mercado-Luna et al., 2010). The remaining treatments presented values within this range, except for the control and the positive control, which showed the damage caused by Forl.
The UCs provide an indirect and quantitative measure of the amount of N in the plant, as well as the effect of weather variables (Rincón-Castillo and Ligarreto, 2010; Vázquez et al., 2012). The plants treated with L. tridentata extracts maintained an overall state of well-being, even after having been infected with Forl.
Plant height. Table 5 shows the values for plant height in each treatment; all the extracts were statistically higher (p≤0.05) to the absolute control and commercial fungicide, from the first week to the end of the experiment. Treatments H2O 4000, MeOH 4000, and DCM 3000 ppm, presented plants 49, 48, 42 % taller than plants that received the commercial fungicide. Increases in plant height were reported for tomato plants (144 and 148 %) by Díaz-Díaz et al. (2013) with L. tridentata extracts in concentrations of 3000 and 2000 ppm with the use of MeOH and acetone as solvents. The absolute control (0 ppm) showed general growth delay caused by the typical symptomatology of Forl: invasion of the root, premature loss of lower leaves and rot of the radicle. The Neem (Azardiachta indica) and L. tridentata extracts favor height (up to 35 % in comparison to the control) in tomato plants when they have been infected with F. oxysporum (Hadian et al., 2011).
Leaf number. All the treatments presented a higher number of leaves (p≤0.05) than the absolute control, particularly the treatments MeOH 4000, DCM 3000, H2O 4000 ppm, with 52, 50, and 45 % more leaves than the commercial fungicide, respectively (Table 5). Díaz-Díaz et al. (2013) recorded a 25 % increase in dry weight when applying extracts of L. tridentata by 3000 and 2000 ppm in tomato plants inoculated with P. capsici, which accounts for a greater vegetative growth, as in the results presented in this investigation; the use of L. tridentata as an infusion is also reported as a stimulant tomato plant growth in combination with lanoline and cocoa butter, since it increases dry (77 %) and fresh weights (100 %) (Recinos, 2010). No signs of toxicity were observed in the doses used, as was the case in the aqueous extracts of L. tridentata at 30 % and after 5 days of application (Rojob, 2008). The infestation by Forl implies the wilting of the lower leaves towards the top (Fernández-Herrera et al., 2013), and therefore the lower levels of infestation helped the plants generate and preserve a higher number of leaves than the absolute control and the commercial fungicide.
Table 5 Vegetative development of tomato plants inoculated with Forl and treated with L. tridentata extracts at 49 dai

u commercial fungicide, v dichloromethane, w ethanol, x methanol, y water. Different letters represent significant differences between treatments (p≤0.05).
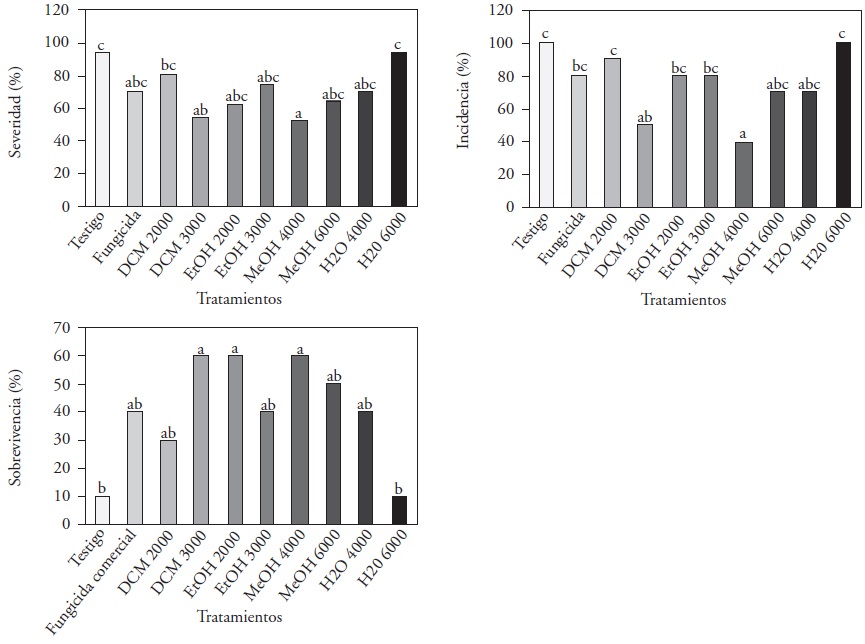
Severity. Significant differences (p≤0.05) were found between the treatments with MeOH at 4000 and DCM at 3000 ppm, which displayed severity indices of 55 and 57 % (respectively) in comparison with the 94% of the absolute control and H2O 6000 ppm. Díaz-Díaz et al. (2013) reported reductions of up to 83 % in severity when evaluating tomato plants infected with P. capsici and treated with L. tridentata extracts and acetone at concentrations of 2000 ppm. The results shown (Figure 1-A) indicate a reduction of 45% in the severity in MeOH 4000ppm, and a relation is established between the variables of vegetative growth and. There is a similar behavior between treatments DCM 3000, MeOH 4000, and H2O 4000 ppm, which gave lower severity indices and higher levels of chlorophyll, height, and number of leaves than the commercial fungicide, taking into account the first report on the severity of Forl on tomato plants that have been treated with L. tridentata extracts.
The extract DCM 3000 ppm presented the lowest severity index and a higher vegetative development than the commercial fungicide, as well as an inhibition of 100% at 1000 ppm in the Forl in vitro biotest and a higher NDGA content. In regard to this, Martins et al. (2012) stated that the antifungal activity of L. tridentata can be attributed to three compounds: NDGA, kaempferol, and quercetin, which are highly compatible with the solvent dichloromethante, which is more available due to the polarity of the three compounds. Hanaa et al. (2011) and Villa-Martínez (2015) used aqueous extracts of neem and willow (Salix babylonica) in tomato plantlets, which reduced the incidence of the disease of wilting by F. oxysporum in tomato plantlets by up to 30%, by the increase of the defensive antioxidant enzymes secreted by the tomato plants, such as peroxidase, catalase and the dismutase superoxidase.
Incidence. Statistically significant differences (p≤0.05) were obtained; 100 % of the plants treated with the control and H2O 6000 ppm treatments registered symptoms of Forl infection (Figure 1-B); however, when applying the extracts DCM 3000 and MeOH 4000 ppm, the incidence reached only 40 and 50 %, respectively. No reports were found on in vivo tests for the control of Forl with L. Tridentata extracts; however, in carrot plants, there were reports of a 5 % incidence of blight caused by A. dauci when applying a hydrosoluble extract of L. tridentata mixed with chitosan (Hernández-Castillo et al. 2006).
Survival. At 49 dai, one out of every 10 plants treated with the control and H2O at 6000 ppm survived, whereas with extracts DCM 3000 and MeOH 4000 ppm favored the survival of 60 % of the plants. With treatments EtOH 2000 and MeOH 6000 ppm, 50 % and the commercial fungicide, 40 % (Figura 1-C). These reports show a reduction in the aggressiveness of Forl due to the effect of the L. tridentata plant extracts, which may be due to the phenolic substances present in the extracts react chemically with the sensitive systems of the enzymes, making them catalytically inactive with this pathogen, therefore favoring the survival of the plants (Tappel and Marr, 1954).
CONCLUSIONS
The results obtained lead to conclude that the L. tridentata extracts can be used as a part of an integrated management of the rotting of the tomato crown caused by F. oxysporum radicis-lycopersici under in vivo conditions. The consistency of the extracts of DCM at 3000 ppm and MeOH at 4000 ppm on the incidence and severity of Forl and the survival in tomato plants, stand out. It is important to mention that this is the first report in vivo which evaluates the antifungal capability of L. tridentata extracts on Forl in tomato plants in the Yaqui Valley, although it is recommendable to take the investigation to a commercial greenhouse level with more environmentally friendly solvents.
LITERATURA CITADA
Apodaca M, Zavaleta E, García R, Osada S y Valenzuela J. 2002. Frecuencia de campos infestados con Fusarium oxysporum f. sp. radicis-lycopersici en Sinaloa México y su control. Revista Mexicana de Fitopatología 20(1):1-7. http://www.redalyc.org/articulo.oa?id=61220101 [ Links ]
Apodaca M, Zavaleta E, Osada S, García R y Valenzuela J. 2004. Hospedantes asintomáticos de Fusarium oxysporum Schlechtend. f. sp. radicis lycopersici W.R. Jarvis y Shoemaker en Sinaloa, México. Revista Mexicana de Fitopatología 22(1):7-13. http://www.redalyc.org/articulo.oa?id=61222102 [ Links ]
Arteaga, S, Andrade-Cetto A and Cárdenas R. 2005. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. Journal of Ethnopharmacology 98(3):231-239. DOI: 10.1016/j.jep.2005.02.002 [ Links ]
Atlas Agroalimentario SIAP. 2016. Atlas Agroalimentario del Servicio de Información Agroalimentaria y Pesquera. México, D.F. http://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2016/Atlas-Agroalimentario-2016 [ Links ]
Camacho A, Giles M, Ortegón A, Palao M, Serrano B y Velázquez O. 2009. Técnicas para el análisis microbiológico de alimentos. Segunda Edición. Facultad de Química, UNAM. México. http://depa.fquim.unam.mx/amyd/archivero/TecnicBasic-Diluciones_6526.pdf [ Links ]
Cordero-Ramírez JD, López-Rivera R, Figueroa Lopez AM, Mancera-López ME, Martínez-Álvarez JC, Apodaca-Sánchez MA and Maldonado Mendoza IE. 2013. Native soil bacteria isolates in Mexico exhibit a promising antagonist effect against Fusarium oxysporum f. sp. radicis-lycopersici. Journal of Basic Microbiology 53:838-847. DOI: 101.1002/jobm.201200128 [ Links ]
Díaz-Díaz A, Hernández-Castillo FD, Belmares-Cerda RE, Gallegos-Morales G, Rodríguez-Herrera R y Aguilar-González CN. 2013. Efecto de extractos de Larrea tridentata y Flourensia cernua en el desarrollo de plantas de tomate inoculadas con Phytophthora capsici. Agraria 10(2):49-58. http://www.redinnovagro.in/docs/Revista_Agraria_vol(10)_No(2).pdf [ Links ]
Domínguez AG. 2012. Aislamiento, identificación y distribución de Fusarium spp. en jitomate cultivado en suelo bajo invernadero. Tesis de Maestría. Instituto Politécnico Nacional. Centro de Desarrollo de Productos Bióticos (CEPROBI). Yautepec, Morelos, México. http://tesis.ipn.mx:8080/xmlui/bitstream/handle/123456789/12423/Grisel%20Dom%C3%ADnguez%20Arizmendi%20Noviembre%202012.pdf?sequence=1 [ Links ]
Fernández-Herrera E, Guerrero J, Rueda E y Acosta M. 2013. Patógenos y síntomas asociados a la marchitez del tomate (Solanmum lycopersicum L.) en Texcoco México. Revista Ciencias biológicas y de la salud. Universidad de Sonora 15(3):46-50. http://www.biotecnia.uson.mx/revistas/articulos/24-Articulo%207.pdf [ Links ]
González HA. 2014. Principios de bioquímica clínica y patología molecular. Segunda Edición. Elsevier. España. 488p. [ Links ]
González S y Carrasco J. 2002. Pasos para eliminar el bromuro de metilo en chile, Tierra adentro, Mayo- Junio, 46-49. http://www2.inia.cl/medios/tierraadentro/pdf/N68-p46_49.pdf [ Links ]
Guerrero-Rodríguez E, Solís-Gaona S, Hernández-Castillo F, Flores-Olivas A, Sandoval-López V y Jasso-Cantú D. 2007. Actividad biológica in vitro de extractos de Flourencia cernua D.C. en patógenos de postcosecha; Alternaria alternata (Fr.:Fr) Keissl., Colletrotrichum gloeosporioides (Penz.) Penz. y Sacc. y Penicillium digitatum (Pers.:Fr) Sacc. Revista Mexicana de Fitopatología 25(1):48-53. http://www.redalyc.org/pdf/612/61225107.pdf [ Links ]
Hadian S, Rahnama K, Jamali S and Eskandari A. 2011. Comparing Neem extract with chemical control on Fusarium oxysporum and Meloidogyne incognita complex of tomato. Advances in Environmental Biology 5(8):2052-2057. http://www.aensiweb.com/old/aeb/2011/2052-2057.pdf [ Links ]
Hanna F, Abdou Z, Salama D, Ibrahim M and Sror H. 2011. Effect of neem and willow aqueous extract fusarium wilt disease in tomato seedlings: Induction of antioxidant defensive enzymes. Annals of agricultural Science 56: 1-7. DOI: http://doi.org/10.1016/j.aoas.2011.05.007 [ Links ]
Hernández-Castillo F, Aguirre-Aguirre A, Lira-Saldivar R, Guerrero E y Gallegos-Morales G. 2006. Bioeficacia de productos orgánicos, biológicos y químicos contra Alternaria dauci Kühn y su efecto en el cultivo de zanahoria. PHYTON-International Journal of Experimental Botany 75:91-101. http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1851-56572006000100009 [ Links ]
Hirano Y and Arie T. 2006. PCR-based differentiation of Fusarium oxysporum f. sp. lycopersici and radicis-lycopersici and races of F. oxysporum f. sp. lycopersici. Journal of General Plant Pathology 72:273-283. DOI: 10.1007/s10327-006-0287-7 [ Links ]
SAGARPA-SENASICA-INIFAP. 2015. Agenda Técnica Agrícola de Sonora. Segunda Edición. SAGARPA. México, D.F. 383p. [ Links ]
Lira-Saldivar R. 2003. Estado actual del conocimiento sobre las propiedades biocidas de la gobernadora (Larrea tridentata (D.C.) Coville). Revista Mexicana de Fitopatología 21(2):214-222. http://www.redalyc.org/pdf/612/61221217.pdf [ Links ]
Lira-Saldivar R, Gamboa-Alvarado R, Villareal-Cárdenas L, López-Campos R and Jiménez-Díaz F. 2002. Hydrosoluble extracts of Larrea tridentata from two desertic zones in the north of Mexico and their inhibitory effect on Fusarium oxysporum. PHYTON-International Journal of Experimental Botany 167-172. http://cat.inist.fr/?aModele=afficheN&cpsidt=15426010 [ Links ]
Lira-Saldivar R, Hernández M and Hernández F. 2006. Activity of Larrea tridentata (D.C) Coville L. Extracts and chitosan against fungi that effect horticultural crops. Chapingo Serie Horticultura 12(2):211-216. DOI: dx.doi.org/10.5154/r.rchsh.2006.05.023 [ Links ]
Leslie J and Summerell B. 2006. The Fusarium laboratory manual. Blackwell Publishing. USA. 388 p. DOI: 10.1002/9780470278376 [ Links ]
López A, López S, Vázquez M, Rodríguez S, Mendoza M y Padrón E. 2005. Inhibición del crecimiento micelial de Fusarium oxysporum Schlechtend f. sp. lycopersici (Sacc.) Snyder y Hansen. Rhizotocnia solani Kühn and Verticillium dahliae Kleb. Mediante extractos vegetales acuosos. Revista Mexicana de Fitopatología 23(2):183-190. http://www.redalyc.org/pdf/612/61223212.pdf [ Links ]
Lira-Saldivar R, Balvantín G, Hernández R, Gamboa D, Jasso R and Jiménez F. 2003. Evaluation of resin content and the antifungal effect of Larrea tridentata (Sesse and Moc. Ex D.C.) Coville extracts from two mexican deserts against Pythium sp. Pringsh. Revista Mexicana de Fitopatología 21(2):97-101. http://www.redalyc.org/pdf/612/61221201.pdf [ Links ]
Marlatt M, Corell J and Kauffman P. 1996. Two genetically distinct populations of Fusarium oxysporum f. sp. lycopersici race 3 in the United States. Plant Disease 80:1336-1342. DOI: 10.1094/PD-80-1336. [ Links ]
Martins S, Aguilar CN, Teixeira JA and Mussato SI. 2012. Bioactive compounds (phytoestrogens) recovery from Larrea tridentata leaves by solvents extraction. Separation and Purification Technology 88:163-167. DOI: http://doi.org/10.1016/j.seppur.2011.12.020 [ Links ]
Martins S, Amorim E, Sobrinho T, Saraiva AM, Pisciottano M, Aguilar C, Teixeira JA and Mussatto SI. 2013. Antibacterial activity of crude methanolic extract and fractions obtained from Larrea tridentata leaves. Industrial Crops and Products 41:306-311. DOI: http://doi.org/10.1016/j.indcrop.2012.04.037 [ Links ]
McGovern RJ. 2015. Management of tomato disease caused by Fusarium oxysporum. Crop Protection 73:78-92. DOI: http://doi.org/10.1016/j.cropro.2015.02.021 [ Links ]
Méndez M, Rodríguez R, Ruiz J, Morales-Adame D, Castillo F, Hernández-Castillo FD and Aguilar CN. 2012. Antibacterial activity of plant extracts obtained with alternative organics solvents against food-borne pathogen bacteria. Industrial Crops and Products 37:445-450. DOI: http://doi.org/10.1016/j.indcrop.2011.07.017 [ Links ]
Mercado-Luna A, Rico-García E, Lara-Herrera A, Soto-Zarazúa G, Ocampo-Velázquez R, Guevara-González R, Herrera-Ruiz G and Torres-Pacheco I. 2010. Nitrogen determination on tomato (Lycopersicon esculentum Mill.) seedlings by color image analysis (RGB). African Journal of Biotechnology 9(33):5326-5332. DOI: 10.5897/AJB10.130 [ Links ]
Moreno-Limón S, González-Solís LN, Salcedo-Martínez SM, Cárdenas-Ávila MI y Perales-Ramírez A. 2011. Efecto antifúngico de extractos de gobernadora (Larrea tridentata) sobre la inhibición in vitro de Aspergillus flavus y Penicillium sp. Polibotánica 32:193-205. http://www.scielo.org.mx/pdf/polib/n32/n32a12.pdf [ Links ]
Nelson PE, Tousson TA and Marasas WFO. 1983. Fusarium species: an ilustrated manual for identification. Pennsylvania State University Press. [ Links ]
NOM-111-SSA1-1994. Norma Oficial Mexicana, bienes y servicios. Método para la cuenta de mohos y levaduras en alimentos. http://www.economia-noms.gob.mx (consulta, enero 2017). [ Links ]
Osorio E, Flores M, Hernández D, Ventura D, Rodríguez R and Aguilar C. 2010. Biological efficiency of polyphenolic extracts from pecan nuts shell (Carya Illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Industrial Crops and Products 31:153-157. http://www.sciencedirect.com/science/article/pii/S0926669009001861 [ Links ]
Peñuelas- Rubio O, Arellano-Gil M, Vargas-Arispuro I, Lares-Villa F, Cantú-Soto E, Hernández-Rodríguez SE, Gutiérrez-Coronado MA y Mungarro-Ibarra C. 2015. Bioactividad in vitro de extractos de gobernadora (Larrea tridentata) sobre la inhibición de hongos poscosecha: Alternaria tenuissima, Aspergillus niger, Penicillium polonicum y Rhizopus oryzae. Revista Polibotánica 40(12):183-198. DOI: 10.18387/polibotanica.40.12 [ Links ]
Quilambaqui H. 2005. Aislamiento e identificación de especies de Fusarium spp. asociadas al declinamiento del espárrago (Asparagus officinalis L.) en cinco municipios de Guanajuato, México. Revista Tecnológica ESPOL 18(1):135-140. http://www.rte.espol.edu.ec/index.php/tecnologica/article/viewFile/238/180 [ Links ]
Recinos DAL. 2010. Efecto del uso de extractos de gobernadora (Larrea tridentata) en plántula de tomate (Solanum lycopersicum L.) bajo condiciones de invernadero. Tesis de Licenciatura. Universidad Autónoma Agraria Antonio Narro. Saltillo, Coah., México. pp. 28-30. http://repositorio.uaaan.mx:8080/xmlui/bitstream/handle/123456789/4422/T17687%20RECINOS%20DIAZ,%20ANA%20LILIAN%20%2061068.pdf?sequence=1 [ Links ]
Rincón-Castillo A y Ligarreto G. 2010. Relación entre nitrógeno foliar y el contenido de clorofila, en maíz asociado con pastos en el Piedemonte Llanero colombiano. Ciencia y Tecnología Agropecuaria 11(2): 122-128. http://www.redalyc.org/pdf/4499/449945029003.pdf [ Links ]
Rojob A. 2008. Control in vitro con extractos vegetales de patógenos que afectan el cultivo de jitomate (Lycopersicon esculentum). Tesis de licenciatura de la Universidad Autónoma de Querétaro. http://ri.uaq.mx/bitstream/123456789/2802/1/RI002644.pdf [ Links ]
Szczechura W, Staniaszek M and Habdas H. 2013. Fusarium oxysporum f. sp. radicis-lycopersici-the cause of Fusarium crown and root rot in tomato cultivation. Journal of Plant Protection Research 53(2): 172-176. DOI: 10.2478/jppr-2013-0026 [ Links ]
Tappel A and Marr G. 1954. Effect of alpha-tocophenol, propyl gallate and nordihydroguaiaretic acid on enzymatic reaction. Agricultural and Food Chemistry 2(11):554-558. DOI: http://pubs.acs.org/doi/pdf/10.1021/jf60031a003 [ Links ]
Towsend GR and Heuberger JW. 1943. Methods for estimating losses caused by diseases in fungicide experiments. Plant Disease Reporter 27:340-343. [ Links ]
Ulacio D, Salas J, Querales P y Sanabria M. 2002. Microbiota del suelo de zonas productoras de papa del estado Mérida y su relación con Rizhoctonia solani. Bioagro (Venezuela) 14(1):11-16. http://www.ucla.edu.ve/bioagro/Rev14(1)/2.%20Micobiota%20del%20suelo.pdf [ Links ]
Vázquez M, Jiménez S, Torres I, Anaya I, Mendoza H y Guevara R. 2012. Comportamiento de plantas de tomate (Solanum lycopersicum) asperjadas con ácido salicílico cultivadas bajo diferentes condiciones climáticas en invernadero. Ciencia@UAQ 5(1):1-9. http://www.uaq.mx/investigacion/revista_ciencia@uaq/ArchivosPDF/v5-n1/articulo6.pdf [ Links ]
Villa-Martínez A, Pérez-Leal R, Morales-Morales H, Basurto Sotelo M, Soto-Parra J y Martínez-Escudero E. 2014. Situación actual en el control de Fusarium spp. y evaluación de la actividad antifúngica de extractos vegetales. Acta Agronómica 64(2):194-205. DOI: http://dx.doi.org/10.15446/acag.v64n2.43358 [ Links ]
Vogt V, Cifuente D, Tonn C, Sabini L and Rosas S. 2013. Antifungal activity in vitro and in vivo of extracts and lignans isolated from Larrea divaricata Cav. against phytopathogenic fungus. Industrial Crops and Products 42:583-586. DOI: 10.1016/j.indcrop.2012.06.009 [ Links ]
Received: March 10, 2017; Accepted: May 02, 2017











 text in
text in