Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.35 n.2 Texcoco May. 2017
https://doi.org/10.18781/r.mex.fit.1701-5
Phytopathological notes
Pathogenic characteristics of the Asian soybean rust (Phakopsora pachyrhizi) in Mexico
1Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias (INIFAP), Campo Experimental Las Huastecas, Carretera Tampico-Mante Kilómetro 55, Villa Cuauhtémoc, Altamira, Tamaulipas, C.P. 89610, México.
2Japan International Research Center for Agricultural Sciences (JIRCAS), 1-1 Ohwashi, Tsukuba, Ibaraki 305-8686, Japan.
In Mexico, the Asian soybean rust caused by Phakopsora pachyrhizi, reduces soybean yield by 25 % to 80 %. The use of resistant cultivars is the most cost-effective strategy for management of this disease. The objective of this study was to determine the pathogenic characteristics of P. pachyrhizi populations in Mexico in order to look for effective resistance in soybean germplasm. Using 12 soybean differentials carrying 0-3 resistance genes, the pathogenic profiles of four P. pachyrhizi populations sampled in northeastern Mexico in 2015, were evaluated. Three of the populations had similar pathogenic characteristics. Cluster analysis supported the distinction by the location of two clusters of Mexican P. pachyrhizi populations apart, in the dendrogram of South American and Japanese rust populations. This indicates a large variation in the soybean rust populations in Mexico. In addition, no soybean differential carrying a single resistance gene showed resistance to all the four Mexican populations; however, the differential carrying three genes was resistant to all the P. pachyrhizi populations. Therefore, introducing multiple resistance genes in soybean breeding programs will be useful to develop rust-resistant cultivars in Mexico.
Key words: Glycine max; Phakopsora pachyrhizi; differentials; gene pyramiding; pathogenicity
En México, la roya asiática de la soya causada por Phakopsora pachyrhizi reduce el rendimiento del cultivo desde un 25 hasta un 80 %. El uso de variedades resistentes es la estrategia más rentable para el manejo de esta enfermedad. El objetivo del presente estudio fue determinar las características patogénicas de poblaciones mexicanas de P. pachyrhizi, con la finalidad de encontrar resistencia eficiente a dicho patógeno en germoplasma de soya. Para evaluar los perfiles patogénicos de cuatro poblaciones de P. pachyrhizi, colectadas en el noreste de México durante el 2015, se utilizaron 12 diferenciales de soya portando 0 - 3 genes de resistencia. Tres poblaciones mostraron características patogénicas similares. El análisis clúster diferenció dos grupos en las poblaciones de P. pachyrhizi de México, los cuales se asociaron con la localidad de muestreo. El dendrograma no incluyó poblaciones de roya de Sudamérica y Japón en dichos grupos. Lo cual indicó una gran variación en las poblaciones de roya asiática de México. Por otro lado, ningún diferencial de soya portando un solo gen de resistencia fue resistente a las cuatro poblaciones mexicanas de roya; pero el diferencial con tres genes mostró resistencia a todas las muestras de P. pachyrhizi. Por lo tanto, el uso de germoplasma con múltiples genes de resistencia será útil para desarrollar variedades de soya resistentes a la roya asiática en México.
Palabras clave: Glycine max; Phakopsora pachyrhizi; diferenciales de soya; piramidación de genes; patogenicidad
Asian soybean rust (ASR) caused by the pathogen Phakopsora pachyrhizi, is a major threat to soybean in the tropics and subtropics. In Mexico, yield losses caused by ASR range from 25 % to 80 % (Terán-Vargas et al., 2007) making chemical control necessary (Yañez-López et al., 2015). Tamaulipas in northeastern Mexico, has the highest soybean production in the country, but it also has the longest period with favorable weather conditions for ASR infection (Yañez-López et al., 2015). Since all soybean commercial cultivars currently used in Mexico are susceptible to ASR, the cost of chemical control is high.
The use of resistant soybean cultivars to ASR is considered to be the most cost-effective strategy for disease management (Hartman et al., 2005). Although seven ASR resistance genes have been reported, namely Rpp (resistance to P. pachyrhizi) 1 to Rpp6 and Rpp1-b, they cannot be used for breeding without knowing which gene is effective against a given ASR population in a target region. Thus, pathogenic characteristics of P. pachyrhizi populations in South American countries have been examined by the use of a differential soybean cultivar set (Akamatsu et al., 2013; 2017). From the results, a breeding scheme for developing resistant cultivars has been implemented in South America, using a highly resistant soybean line carrying multiple resistance genes (Yamanaka et al., 2013; 2015).
In Mexico, there are few reports on resistance to ASR in soybean germplasm (Peña-del-Río et al., 2014). Because little is known about which Rpp genes confer resistance to specific P. pachyrhizi populations, the incorporation of resistance genes in soybean has not been considered in breeding programs. Thus, the aim of this study was to determine the pathogenic characteristics of P. pachyrhizi populations in Mexico.
During the 2015 soybean growing season, four P. pachyrhizi populations were sampled in fungicide untreated soybean fields in northeastern Mexico (Figure 1). Three were sampled in the municipalities of Reynosa, Matamoros, and Altamira in the State of Tamaulipas. In the State of San Luis Potosi, one population was sampled in the municipality of Tamuin. They were coded as MRP (Mexican Asian Soybean Rust Population)-4, MRP-13, MRP-16, and MRP-19, respectively. At each site, several ASR-infected leaves were collected and placed in a plastic bag. Then, they were placed on Petri dishes and the leafstalks were covered with sterilized cotton moistened with distilled water. These samples were exported to Japan International Research Center for Agricultural Sciences (JIRCAS), Japan, under import permit 27Y935. After arrival, urediniospores on leaflets were collected and suspended in 2 or 3 mL of 0.04% Tween 20 solution. The urediniospore suspensions (10-25×104 spores/mL) were inoculated on susceptible cultivar BRS184 for multiplication according to Yamanaka et al. (2016). The multiplied urediniospores were used for the following experimental inoculations.
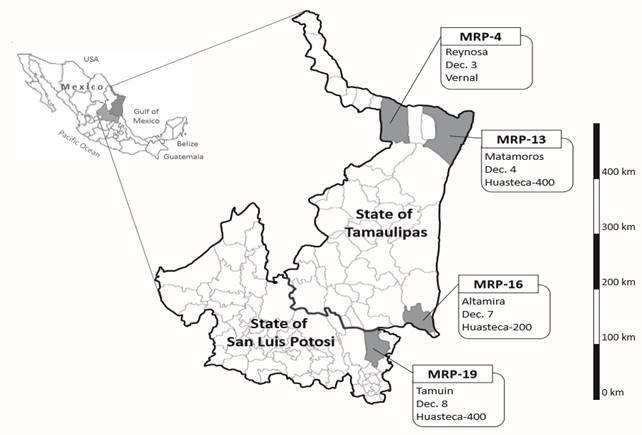
Figure 1. Locations where Phakopsora pachyrhizi populations were collected in Mexico in 2015. Labels indicate municipality, sampling date, and host cultivar.
In this study, 12 soybean differentials were used (Yamanaka et al., 2016) (Table 1); ten carry one of the ASR-resistance genes: Rpp1, Rpp2, Rpp3, Rpp4, Rpp5, Rpp6, or Rpp1-b; No6-12-1 carries Rpp2, Rpp4, and Rpp5; and BRS154 is the susceptible control.
Table 1. Reactions of 12 soybean differentials to four Phakopsora pachyrhizi populations collected in northeastern Mexico in 2015. The frequency of the resistant reaction to Mexican, South American, and Japanese P. pachyrhizi populations in 12 soybean differentials is also included in this table.
x Data of South American populations were used from the reports by Akamatsu et al. (2013; 2017), and that of Japanese populations by Yamanaka et al. (2015) and Yamaoka et al. (2014). S = Susceptible; IM = Intermediate; R = Resistant
Plants were grown in a growth chamber at 24 °C under a 14-h light period of approximately 19,000-23,000 lux provided by fluorescent lamps. The first trifoliate leaflets were detached, inoculated with a urediniospore suspension (5×104 spores/mL), placed in Petri dishes, and incubated in a growth chamber at 21 °C under a 12-h light photoperiod of approximately 3,000 lux provided by fluorescent lamps. Fourteen days after inoculation, the rust sporulation level (SL) was visually assessed as nil (0), low (1), moderate (2), or high (3). Then, the urediniospores were removed with a paint brush, and the average number of uredinia per lesion (NoU) was determined microscopically in 30 lesions. The reaction type of each differential was classified as resistant (R), intermediate (IM), or susceptible (S), according to values of SL and NoU. This criterion and the SL standard are mentioned in Yamanaka et al. (2016).
The results were compared with those of 145 South American (Argentina, Brazil, and Paraguay), and eight Japanese ASR pathogens (Akamatsu et al., 2013, 2017; Yamaoka et al., 2014), in which the data were obtained using the same soybean differentials. The reactions were changed to numerical scores: R=0, IM=1, and S=2. Distance matrices were prepared by calculating Euclidian distances between samples in R v. 3.0.1 software (https://www.r-project.org), and input into a hierarchical clustering function, which constructed a dendrogram based on the unweighted pair group method with arithmetic mean (UPGMA).
Population MRP-19 caused a resistant reaction in differential PI 462312, PI 416764, PI 200526, and No6-12-1 (Table 1); MRP-4 had similar effect on those differentials and in addition in PI 567102B. MRP-13 caused a resistant reaction in the same differentials as MRP-4, and in addition in PI 200492, while MRP-16 caused a resistant reaction in PI 587880A and No6-12-1. Although populations MRP-13, -4, and -19 showed similar behavior on differentials, there were some differences in their pathogenic patterns. Populations MRP-4 and -13 produced different lesion types on differentials PI 200492 (Rpp1), PI 230970 (Rpp2), and PI 459025 (Rpp4).
MRP-4 produced less than 30 lesions on PI 200492 (Rpp1), PI 416764 (Rpp3), and No6-12-1 (Rpp2+Rpp4+Rpp5). It produced only five lesions on PI 200492, but these lesions had many uredinia with abundant urediniospores (NoU=4.2, SL=3), giving S phenotype. Conversely, MRP-13 produced fewer uredinia and less urediniospores (NoU=0.8, SL=1), giving R phenotype to PI 200492. S and IM phenotypes were observed on PI 230970 and PI 459025 by MRP-13 inoculation, respectively; while IM and S phenotypes were observed on these differentials by MRP-4 inoculation, respectively. MRP-19 caused the same reactions on most differentials as MRP-4 and -13, but it caused an intermediate reaction on PI 567102B (Rpp6), which was resistant to MRP-4 and -13. This population also showed a different reaction on PI 200492 from MRP-4 and -13 (Table 1). As a result, MRP-13, -4, and -19 showed rather similar pathogenic patterns on the 12 soybean differentials. Aside from the differential pathogenicity, MRP-16 was the most virulent to the differentials. It caused a susceptible reaction in nine differentials, while in PI 416764 (Rpp3) it was intermediate, and resistant in PI 587880A (Rpp1-b) and No6-12-1 (Rpp2 + Rpp4 + Rpp5). MRP-16 produced few uredinia and urediniospores on PI 587880A (NoU=1.0, SL=0.8), and did not sporulate in No6-12-1 (NoU=0.0, SL=0.0).
The UPGMA dendrogram revealed that MRP-4, -13, and -19 made a single cluster distantly from MRP-16; these two clusters of Mexican P. pachyrhizi populations did not contain any populations from South America and Japan (Figure 2). The highly virulent MRP-16 belonged to the major cluster of South American samples, while the MRP-4, -13, and -19 cluster was located distantly from the South American samples even though there were some Japanese and Paraguayan samples which made a cluster outside of them.
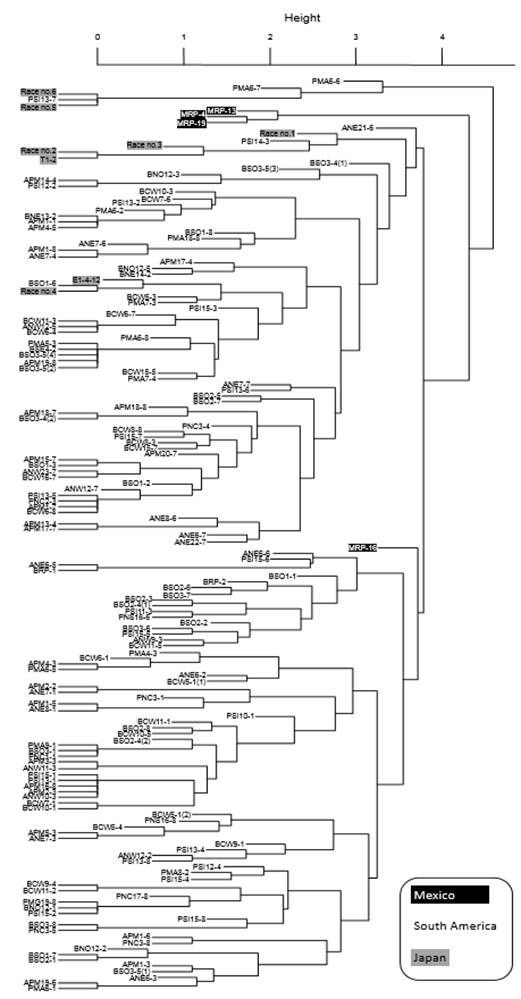
Figure 2. UPGMA dendrogram of the relationships among four Mexican, 145 South American, and eight Japanese Phakopsora pachyrhizi populations based on pathogenicity on 12 soybean differentials. Mexican and Japanese populations are colored with black and gray, respectively. South American populations are labeled as ‘A’ for Argentina, ‘B’ for Brazil, and ‘P’ for Paraguay. Data of South American populations were used from the reports by Akamatsu et al. (2013; 2017), and those from Japan by Yamanaka et al. (2015) and Yamaoka et al. (2014).
MRP-4, -13, and -19 showed not identical but similar pathogenic profiles (Table 1). Similarities in pathogenicity between MRP-4 and -13 could be due to the proximity of the sampling locations; both were collected in northern Tamaulipas about 80 km apart. MRP-19, which caused similar responses as MRP-4 and -13 in most differentials was collected in the State of San Luis Potosi (Huasteca region), approximately 450 km from the sampling sites of MRP-4 and -13, but 75 km from that of MRP-16. It was expected that the pathogenicity of MRP-19 would be similar to that of MRP-16, however, it was not the case (Table 1, Figure 2). This means that geographical differentiation in pathogenicity is rather limited in Mexican ASR pathogen within a small region in Mexico, unlike P. pachyrhizi populations collected in a small area in Japan which showed six races (Yamaoka et al., 2014). MRP-16 showed the highest virulence among the four Mexican P. pachyrhizi populations. Uredinia and urediniospores were observed except in differential No6-12-1 (Rpp2 + Rpp4 + Rpp5), which it indicates that the MRP-16 population includes at least one race which is virulent to any Rpp gene that the differentials carry. According to these observations, Mexican ASR pathogen has high pathogenic diversity and strong virulence to resistant soybean differentials.
Frequencies of resistant reactions observed in soybean differentials by the inoculations ofMexican, South American, and Japanese P. pachyrhizi populations show a clear difference between Mexican and the other two regional populations in Rpp1-b carrying differentials. Rpp1-b conferred PI 587880A and PI 594767A resistance to >80 % of ASR populations from Japan and South America. In contrast, it was less effective against Mexican P. pachyrhizi populations, since PI 587880A was susceptible to three of the four populations and PI 594767A was susceptible to all (Table 1). Another significant difference among Mexican, South American, and Japanese P. pachyrhizi population was detected in PI 459025: a resistant reaction was detected in about 50 % and 90 % of South American and Japanese P. pachyrhizi populations, respectively; however, resistant reaction was not observed by the inoculation with Mexican P. pachyrhizi populations.
The ineffectiveness of Rpp1-b detected in Mexico was previously observed in the USA (Li, 2009; Walker et al., 2014). The pathogenic similarity between the ASR populations from Mexico and the USA is also observed in Rpp3; it caused resistance reactions to most of the ASR pathogens (Walker et al., 2014; Paul et al., 2015). On the other hand, Rpp5 (PI 200526) showed a geographical difference between Mexico and USA, conferring resistance to three of the four Mexican P. pachyrhizi populations, as well as to all Japanese and most South American P. pachyrhizi populations (Table 1), but its susceptibility to 24 USA P. pachyrhizi populations was reported (Paul et al., 2015). Therefore, the virulence of Mexican pathogen to Rpp5, like that of South American and Japanese pathogens, is different from those from the USA.
Most P. pachyrhizi populations in South America are virulent to Rpp1 (PI 200492 and PI 587886). In contrast, Japanese ASR populations were avirulent to these differentials (Yamanaka et al., 2010; Yamaoka et al., 2014). In the USA, Rpp1 (PI 200492) was resistant in most cases (Li, 2009; Li et al., 2012; Twizeyimana and Hartman, 2012; Walker et al., 2014), but in Mexico, PI 200492 was resistant to MRP-13, intermediate to MRP-19, and susceptible to MRP-4 and -16. Since MRP-4 produced only few lesions, suggesting that most reactions might have been of immunity, or that a dominant race in MRP-4 might be avirulent to PI 200492. The tested Mexican ASR populations, therefore, are moderately virulent to Rpp1 (PI 200492) and the characteristics are different from those in South America, the USA, and Japan. Overall, Mexican P. pachyrhizi populations may have different characteristics from the pathogens from those regions.
Genes Rpp2 (PI 230970), Rpp1 (PI 587886), Rpp4 (PI 459025), and Rpp1-b (PI 594767A) are useless for conferring resistance to ASR in Mexican soybean cultivars, since none conferred resistance to the four MRPs. In addition, Rpp1 (PI 200492) and Rpp1-b (PI 587880A) conferred adequate resistance to only one of the four populations (Table 1), therefore, they would have a very limited used for soybean breeding in Mexico. Peña-del-Río et al. (2014) identified 23 genotypes with low susceptibility to the Mexican P. pachyrhizi population in 2007; two (PI 230970 and PI 417125) carried the Rpp2 gene. Thus, Rpp2 may have become ineffective against ASR in Mexico from 2007 to 2015.
Since Rpp3 (PI 462312 and PI 416764) and Rpp5 (PI 200526) conferred resistance to three of the populations, and Rpp6 (PI 567102B) to two (Table 1), they could be useful as sources of resistance in soybean breeding in Mexico. However, it would be risky to use these genes singly, as none of the differentials carrying single Rpp genes showed resistance to all four Mexican populations. Only No6-12-1 (Rpp2 + Rpp4 + Rpp5) was resistant to the four populations, and remained free of uredinia and urediniospore production, indicating that gene pyramiding (Yamanaka et al., 2015) may confer to soybeans not only resistance to a broad range of P. pachyrhizi races in Mexico but also a high level of resistance.
Conclusions
One of four Mexican P. pachyrhizi populations used in this study showed high virulence on resistant soybean differentials and quite different pathogenicity from the other three. Pathogenicity of Mexican populations was different from South American and Japanese ones which indicates that there should be highly virulent and diverse P. pachyrhizi races in Mexico. Gene-pyramiding such as the combination of Rpp2+Rpp4+Rpp5, is necessary to confer adequate and stable ASR resistance to soybean cultivars in Mexico.
Acknowledgments
We are grateful to the Brazilian Agricultural Research Corporation (Embrapa) for providing seeds of the singlegene differential varieties. We are deeply grateful to Dr. Héctor Cortinas-Escobar and M.C. Nicolás Maldonado-Moreno (INIFAP), and to Ing. Iván Ausencio Delgado-Robles (CESAVETAM, Altamira) for their help during collection of ASR samples in Mexico. We are also deeply grateful to Mses. Yukie Muraki, and Midori Hasegawa (JIRCAS) for their technical assistance.
REFERENCES
Akamatsu H., Yamanaka N., Soares R.M., Ivancovich A.J.G., Lavilla M.A., Bogado A.N., Morel G., Scholz R., Yamaoka Y., and Kato M. 2017. Pathogenic variation of South American Phakopsora pachyrhizi populations isolated from soybeans from 2010 to 2015. Japan Agricultural Research Quarterly (in press). [ Links ]
Akamatsu H., Yamanaka N., Yamaoka Y., Soares R.M., Morel W., Ivancovich A.J.G., Bogado A.N., Kato M., Yorinori J.T., and Suenaga K. 2013. Pathogenic diversity of soybean rust in Argentina, Brazil, and Paraguay. Journal of General Plant Pathology 79:28-40. http://dx.doi.org/10.1007/s10327-012-0421-7Hartman G.L., Miles M.R., and Frederick R.D. 2005. Breeding for resistance to soybean rust. Plant Disease 89:664-666. http://dx.doi.org/10.1094/PD-89-0664 [ Links ]
Hartman G.L., Miles M.R., and Frederick R.D. 2005. Breeding for resistance to soybean rust. Plant Disease 89:664-666. http://dx.doi.org/10.1094/PD-89-0664 [ Links ]
Li S. 2009. Reaction of soybean rust-resistant lines identified in Paraguay to Mississippi isolates of Phakopsora pachyrhizi. Crop Science 49:887-894. http://dx.doi.org/10.2135/cropsci2008.06.0305 [ Links ]
Li S., Smith J.R., Ray J.D., and Frederick R.D. 2012. Identification of a new soybean rust resistance gene in PI 567102B. Theoretical and Applied Genetics 125:133-142. http://dx.doi.org/10.1007/s00122-012-1821-y [ Links ]
Paul C., Frederick R.D., Hill C.B., Hartman G.L., and Walker D.R. 2015. Comparison of pathogenic variation among Phakopsora pachyrhizi isolates collected from the United States and international locations, and identification of soybean genotypes resistant to the US isolates. Plant Disease 99:1059-1069. http://dx.doi.org/10.1094/PDIS-09-14-0989-RE [ Links ]
Peña-del-Río M.L.Á., Maldonado-Moreno N., and Díaz-Franco A. 2014. Reaction of germplasm to Phakopsora pachyrhizi in the field. Revista Fitotecnia Mexicana 37:225-227. [ Links ]
Terán-Vargas AP, Ascencio-Luciano G, Maldonado-Moreno N, and Ávila-Valdez J. 2007. La roya asiática de la soya en México. Tamaulipas, Mexico. CIRNE-INIFAP. http://biblioteca.inifap.gob.mx:8080/jspui/handle/123456789/818 [ Links ]
Twizeyimana M., and Hartman G.L. 2012. Pathogenic variation of Phakopsora pachyrhizi isolates on soybean in the United States from 2006 to 2009. Plant Disease 96:75-81. http://dx.doi.org/10.1094/PDIS-05-11-0379 [ Links ]
Walker D.R., Harris D.K., King Z.R., Li Z., Boerma H.R., Buckley J.B., Shipe E.R., Mueller J.D., Weaver D.B., Sikora E.J., Moore S.H., Hartman G.L., Miles M.R., Harris D.K., Wright D.L., Marois J.J., and Nelson R.L. 2014. Evaluation of soybean germplasm accessions for resistance to Phakopsora pachyrhizi populations in the southeastern United States, 2009-2012. Crop Science 54:1673-1689. http://dx.doi.org/10.2135/cropsci2013.08.0513 [ Links ]
Yamanaka N., Yamaoka Y., Kato M., Lemos N.G., Passianotto A., dos Santos J.V.M., Benitez E.R., Abdelnoor R.V., Soares R.M., and Suenaga K. 2010. Development of classification criteria for resistance to soybean rust and differences in virulence among Japanese and Brazilian rust populations. Tropical Plant Pathology 35:153-162. http://dx.doi.org/10.1590/S1982-567620100003. [ Links ]
Yamanaka N., Lemos N.G., Uno M., Akamatsu H., Yamaoka Y., Abdelnoor R.V., Braccini A.L., and Suenaga K. 2013. Resistance to Asian soybean rust in soybean lines with the pyramided three Rpp genes. Crop Breeding and Applied Biotechnology 13:75-82. http://dx.doi.org/10.1590/S1984-70332013000100009 [ Links ]
Yamanaka N., Morishita M., Mori T., Lemos N.G., Hossain M.M., Akamatsu H., Kato M., and Yamaoka Y. 2015. Multiple Rpp-gene pyramiding confers resistance to Asian soybean rust isolates that are virulent on each of the pyramided genes. Tropical Plant Pathology 40:283-290. http://dx.doi.org/10.1007/s40858-015-0038-4 [ Links ]
Yamanaka N., Kato M., Akamatsu H., and Yamaoka Y. 2016. Laboratory manual for studies on soybean rust resistance. www.jircas.affrc.go.jp/english/manual/soybean_rust/soybean_rust.html. (Accessed on February, 2016). [ Links ]
Yamaoka Y., Yamanaka N., Akamatsu H., and Suenaga K. 2014. Pathogenic races of soybean rust Phakopsora pachyrhizi collected in Tsukuba and vicinity in Ibaraki, Japan. Journal of General Plant Pathology 80:184-188. http://dx.doi.org/10.1007/s10327-014-0507-5 [ Links ]
Yañez-López R., Hernández-Zul M.I., Quijano-Carranza J.A., Terán-Vargas A.P., Pérez-Moreno L., Díaz-Padilla G., and Rico-García, E. 2015. Potential distribution zones for soybean rust (Phakopsora pachyrhizi) in Mexico. Ecosistemas y Recursos Agropecuarios 2:291-302. http://www.scielo.org.mx/pdf/era/v2n6/v2n6a5.pdf [ Links ]
Received: January 30, 2017; Accepted: April 13, 2017











 text in
text in 


