Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.35 n.2 Texcoco May. 2017
https://doi.org/10.18781/r.mex.fit.1701-2
Phytopathological notes
Etiology of a black spot symptom in Agave angustifolia: An in vitro approach towards its biological control
1Universidad de la Sierra Sur, Guillermo Rojas Mijangos S/N, Ciudad Universitaria CP 70800, Miahuatlán de Porfirio Díaz, Oaxaca, México.
2Centro de Investigación en Biotecnología Aplicada-IPN, Ex-Hacienda San Juan Molino Carretera Estatal Tecuexcomac-Tepetitla Km 1.5, CP 90700, Tlaxcala, México.
3Universidad de la Sierra Sur, Guillermo Rojas Mijangos S/N, Ciudad Universitaria CP 70800, Miahuatlán de Porfirio Díaz, Oaxaca, México.
We present the molecular identification of a Cladosporium species isolated from necrotic spots of diseased stalks of Agave angustifolia, which were collected in San Luis Amatlán, Oaxaca, Mexico. Analyses of the internal transcribed spacer (ITS) from the 18S ribosomal RNA gene and the partial coding region (cDNA) from an allergenic conserved heat shock protein 70 (HSP70), revealed its similarity with Cladosporium herbarum (Pers.: Fr.) Link. The in vitro assessment of the ethanolic extracts from Zaluzania montagnifolia roots and its main ent-kaurenoids resulted in the growth inhibition of the fungus. Minimum inhibitory concentration (MIC) values were obtained by the agar dilution and broth microdilution methods. The ethanolic extract, ent-kaurenoic acid (ent-16-kauren-19-oic acid) and grandiflorenic acid (ent-kaur-9(11),16-dien-19-oic acid) showed MIC values of 150.4±3.5, 100.3±2.1, and 80.7±1.4 μg mL-1, respectively.
Key words: Cladosporium herbarum; ITS; maguey mezcalero; ent-kaurene derivatives; growth inhibition
Presentamos la identificación molecular de una especie de Cladosporium aislada de las manchas necróticas de los tallos enfermos de Agave angustifolia, los cuales se colectaron en San Luis Amatlán Oaxaca, México. Los análisis de los espaciadores transcritos internos (ITS) del gen de RNA ribosomal 18S y de la secuencia parcial codificante (cDNA) de una proteína alergénica conservada de choque térmico 70 (HSP70), revelaron su similitud con Cladosporium herbarum (Pers.: Fr.) Link. La evaluación in vitro de los extractos etanólicos de las raíces Zaluzania montagnifolia y de sus principales ent-kaurenoides resultó en la inhibición del crecimiento normal del hongo. La concentración mínima inhibitoria (MIC) se obtuvo por el método de dilución en agar y microdilución en caldo. El extracto etanólico, el ácido ent-kaurenoico (ent-16-kauren-19-oic acid) y el ácido grandiflorenico (ent-kaur-9(11),16-dien-19-oic acid) mostraron valores MIC de 150.4±3.5, 100.3±2.1 y 80.7±1.4 μg mL-1, respectivamente.
Palabras clave: Cladosporium herbarum; ITS; maguey mezcalero; derivados del ent-kaureno; inhibición del crecimiento
The Cladosporium genus is comprised by around 500 members with 40 officially named species and 180 unnamed strains reported worlwide (Alonso, 2012). These polyphyletic species are characterized by its morphophysiological heterogeneity and by its extraordinary capacity to colonize non-living and living hosts including humans and many plant species (Bensch et al., 2012). Despite many of these fungi are known to be common endophytes, some species can act as opportunistic microorganisms associated to leaf spots and other lesions in fruits from plants with agronomic importance (Schubert, 2005). Cladosporium herbarum (Pers.: Fr.) Link, is one of the three major species complexes of the genus Cladosporium which usually colonizes falling or dead leaves or even herbaceous and woody plants as secondary invader on necrotic leaf spots (Bensch et al., 2015). C. herbarum produces rot diseases in a number of agronomic fruit crops such as pear, grape, cherry and passion fruit (Barbosa et al., 2001). Its teleomorph, Mycosphaerella tassiana (De Not.) Johans is associated to the brown leaf spot disease of date palm (Barbosa et al., 2001). C. herbarum has been documented as an opportunistic phytopathogen isolated from Agave americana cultivated in Italy (Bensch et al., 2012). However, the incidence of cladosporiosis in Agave plants cultivated in Latin America has been poorly described. Some regions of Mexico such as Jalisco and Oaxaca economically depend on Agave crops to commercialize traditional distillates. Thus, the identification of its main phytopathogens and the formulation of alternatives for their biological control should be very valuable. Considering that some Cladosporium species could aggravate the necrosis of many vegetables including Agave plants and also contain some allergens for humans (Achatz et al., 1995), its alternative biological control should be investigated.
The Agave crops from the district of Miahuatlán de Porfirio Díaz Oaxaca are usually surrounded by xeric scrublands. In this ecosystem, there are several wild plants that have not been tested for its capacity as antimicrobials (Meave et al., 2012). Thus, the evaluation of crude extracts and its majoritarian compounds on selected phytopathogenic species, could originate less expensive alternatives to achieve its biological control. In this context, some plant diseases could reasonably be treated with native plant sources. One of the most abundant plants associated to this geographical location is Zaluzania montagnifolia, a native Asteraceae commonly known as “yegachin” or “vara ceniza” (Villa-Ruano et al., 2013). The plant is considered as a source of kaurane type diterpenes with antimicrobial activity on several animal- and plant pathogenic species (Villa-Ruano et al., 2016). Considering the availability of Z. montagnifolia in this region and the known antimicrobial activity of its main chemical components, the objectives of this wok were to determine the identity of the main phytopathogen involved in the generation of necrotic spots in diseased Agave plants, as well as to evaluate the ethanolic extracts and pure ent-kaurenoids of Z. montagnifolia on the in vitro growth of that phytopathogen.
Isolation of Cladosporium sp. from diseased A. angustifolia
Ten stalks from Agave angustifolia showing necrotic spots were collected in open-field crops from San Luis Amatlán Oaxaca, México (16° 39’ 02’’ N, 96° 49’ 95’’ W, 1,500 masl) in July 2016. The stalks were carefully washed three times with a solution of 2 % sodium hypoclorite. After this step, the necrotic spots were scratched out with a sterile surgical blade and then immersed in a solution of absolute ethanol (J.T. baker, USA) for 1 min. The tissue was dried under N2 stream and cut into small pieces in a sterile cabinet for posteriorly being incubated in PDA medium (Difco®, USA) at 30 °C in darkness for 5 days. The microorganisms were purified by cross streaking method and maintained in fresh petri dishes containing the same culture media. The most dominant microorganism (Cladosporium sp.) was subjected to molecular identification and it was subsequently tested for its possible involvement in the generation of necrotic spots.
Molecular determination of Cladosporium sp. isolated from diseased A. angustifolia
100 mg of a mixture of hyphal cells and conidiophores were taken from PDA containing the filamentous fungus. The cells were disrupted with acid-washed glass beads (G-9268, 425-600 μm 0.5 mm diameter, Sigma-Aldrich Co., USA.) in the presence of 300 µL of DNAzol reagent (Thermofisher TM, USA). The following steps were carried out in accordance with the manufacturer’s recommendations. The isolated DNA was washed twice with a mix of phenol:chloroform:isoamyl alcohol (25:24:1, v/v: Sigma-Aldrich Co., USA). PCR reactions were carried out using 1 µg of DNA dissolved in a final volume of 50 µL. The identity of the microorganism was determined by the amplification of internal transcribed spacers (ITS) using the primers ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) and performing the PCR conditions described by White et al. (1990). The amplicon was cloned into TOPO TA cloning vector (Thermofisher TM, USA) and sequenced in triplicate in an ABI PRISM 3700 instrument sequencer (ABI, Foster City, CA). The allergenic protein of C. herbarum (heat shock protein 70, accession S83210) was amplified by reverser transcription from total RNA, using TRIZOL (Thermofisher TM, USA) and SuperScritp II enzyme (Thermofisher TM, USA). PCR assays were carried out with Platinum® DNA polymerase high fidelity (Thermofisher TM, USA) and the primers GAGATCCTTCTTCTCGACGTCG and CCTTCTAATCGTTAACGCCATG. The PCR protocol to amplify this cDNA was 94 °C for 5 min of initial denaturation, followed by 35 cycles at 94 °C for 30 s, 57 °C for 30 s, 72 °C for 1 min and a final extension of 72 °C for 4 min. The amplicon was visualized in an agarose 1 % gel electrophoresis after staining with ethidium bromide and it was simultaneously cloned and sequenced as previously described. The sequences were compared with those deposited in the nucleotide data base of the NCBI (by nucleotide BLAST) and MycoBank.
Pathogenicity tests of Cladosporium sp. on A. angustifolia stalks
Healthy young stalks of A. angustifolia were excised from the plant and were immersed in water to avoid dehydration along the experimental tests and finally maintained at 25 °C in normal photoperiod (12 hours light). Conidiophores from the fungus were directly harvested with a sterile needle and were immediately inoculated in the stalks by mechanic penetration in order to examine Koch’s postulates. The process was carried in a sterile cabinet. The appearance of the spots was observed during 47 days.
Isolation of ent-kaurenoids and its in vitro evaluation on Cladosporium sp.
500 g of dried roots from Zaluzania montagnifolia were extracted for 10 days at room temperature with 2 L of absolute ethanol (J.T. Baker, USA). The crude ethanolic extract was filtered with Whatman filter paper grade 1 and subsequently reduced until dryness in a rotary evaporator (Buchi R200). The resulting gum was recovered and then resuspended in the same solvent in order to obtain stock solutions of 100 mg mL-1. These solutions were directly used for broth microdilution and agar dilution methods or were fractioned by HPLC for the obtainment of kaurenoic acid (ent-16-kauren-19-oic acid) and grandiflorenic acid (ent-kaur-9(11),16-dien-19-oic acid). Semipreparative purification was carried out in a Hewlett Packard 1050 system coupled to a HP G1306A diode array detector equipped with a Varian C18 column (250×4.5 mm I.D.; 5 μm particle size). The metabolites were collected during continuous runs (100 μL injection volume) using an isocratic mobile phase consisting of 70 % acetonitrile (J.T. Baker, USA) with 0.05 % acetic acid (v/v) at 1 mL min−1 flow rate as previously described by Villa-Ruano et al. (2009). The retention time was compared with that of authentic standards available in our laboratory. The diterpenoids were resuspended in methanol (J.T. Baker, USA) for further antifungal assessment. Agar diffusion method was carried out by dissolving distinct concentrations (dose-response curves of 10-300 µg mL-1) of ethanolic extracts and/or pure diterpenoids in 10 mL of PDA medium (Valgas et al., 2007). The phytopathogenic fungus was inoculated in the medium by massive streaking and these experiments were performed in quintuplicate. The plates were visualized in a white light transilluminator. The obtainment of MIC values was achieved by the broth microdilution method for filamentous fungi proposed by Pfaller et al. (2000) using resazurin (Sigma-Aldrich Co., USA) as an indicator of cell viability. The dose-response curves (10-300 µg mL-1) to obtain MIC values were performed in quintuplicate. Colorimetric reactions were measured at 545 nm. The MIC values were additionally validated by ANOVA-Tukey Test (p<0.01) using the software GraphPad 6.05.
As a result of the incubation of the necrotic tissue in PDA medium, it was isolated a brown-green filamentous fungi (between others isolates) with small terminal conidia (Figure 1 A-B). This fungus was observed as the most dominant species in all the incubations. Macroscopy and microscopy of the microorganism suggested its similarity with Cladosporium species (Bensch et al., 2012). PCR reactions with genomic DNA and cDNAs from reverse transcription produced fragments of ~500 and ~800 bp (Figure 1 C). The analyses of the ITS sequence with NCBI and MycoBank data bases demonstrated its homology (94 %) with the 18S ribosomal RNA gene from the isolate “olrim85” (ID: AY354234.1) of C. herbarum (Figure 2A). Whereas, the coding sequence of the allergenic protein had 99 % homology with the sequence ID:X81860.1 from C. herbarum deposited in the NCBI (Figure 2B). These molecular data strongly suggested the identity of the fungus as C. herbarum.
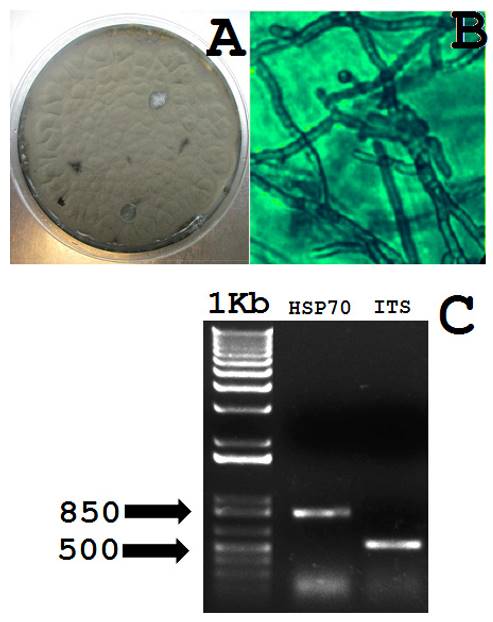
Figure 1. Macroscopy and optical microscopy of C. herbarum isolated from diseased Agave stalks. A, C. herbarum grown in PDA medium. B, hyphal organization and conodiophores of C. herbarum, the image was recorded at 40X. C, Molecular identification of C. herbarum by the amplification of a partial cDNA from the allergenic HSP70 (~800 bp) and the ITS from 18S ribosomal RNA gene (~500bp). The approximated molecular weights are shown.

Figure 2. Results of the BLAST alignment for the original DNA sequences (query) from C. herbarum and its homology with those deposited in the nucleotide data base from the NCBI (subject).
The inoculation of the fungus in asymptomatic Agave stalks exhibited necrotic symptoms since day 4 (Figure 3B). Despite the penetration with the sterile needle produced a damage in the stalks (Figure 3A), the inoculation of the fungus caused an evident systemic response and a darkening of the vasculature (Figure 3B). The systemic response was also perceived by emerging yellow spots around the necrotic cells which were visible since day 15 (Figure 3B). Interestingly, the lesions generated by the sole mechanical damage were almost imperceptible at day 47 (Figure 3A). Contrarily, those infected with the fungus were clearly aggravated at the same period of time (Figure 3B). These data strongly suggested the susceptibility of those Agave stalks to the C. herbarum infection. Despite this evidence supports the fact that C. herbarum is associated to the generation of necrotic spots, the involvement of another opportunistic microorganisms in the infection of open-air crops, cannot be discarded.

Figure 3. Effect of mechanical damage (A) and mechanical damage with inoculation by C. herbarum (B) in healthy young stalks from A. angustifolia.
The in vitro antifungal activity of the ethanolic extracts from the roots of Z. montagnifolia against C. herbarum showed a clear inhibitory effect (Figure 4). Agar dilution method using pure ent-kaurenoic and grandiflorenic acids (usually the main constituents of the root extracts of the plant) exhibited their involvement in the observed antifungal effect (Figure 4). As shown in Figure 4, the fungus perfectly grew in sole PDA medium (0 µg mL-1), whereas an evident growth inhibition was observed as the concentration of each component assessed was increased (20-120 µg mL-1). These semi-quantitative experiments showed that the ethanolic extract containing both diterpenes was able to inhibit the normal grow of the fungus at less than 0.5 mg mL-1. The assays performed with the broth microdilution method allowed to the obtainment of MIC values. These values reflected the concentration of the antimicrobial agent in which there are no signs of growth. The values were 150.4±3.5, 100.3±2.1, and 80.7±1.4 µg mL-1, for ethanolic extract, kaurenoic acid and grandiflorenic acid, respectively (Figure 5). Significant differences (p<0.01) were observed among those treatments (Figure 5). Thus, the use of the ethanolic extract should be considered as a less expensive alternative for further in vivo trials in order to determine its possible use in the biological control of C. herbarum. The antimicrobial activity of these assayed diterpenes has been already proposed and confirmed in diverse bacterial and fungal species (Villa-Ruano et al., 2016). This work contributes to the investigation of novel antimicrobial activities from plant extracts containing ent-kaurenoids. Considering that Z. montagnifolia can be found in the vegetation of those regions, its viability as a source for further antifungal in vivo test should be contemplated.
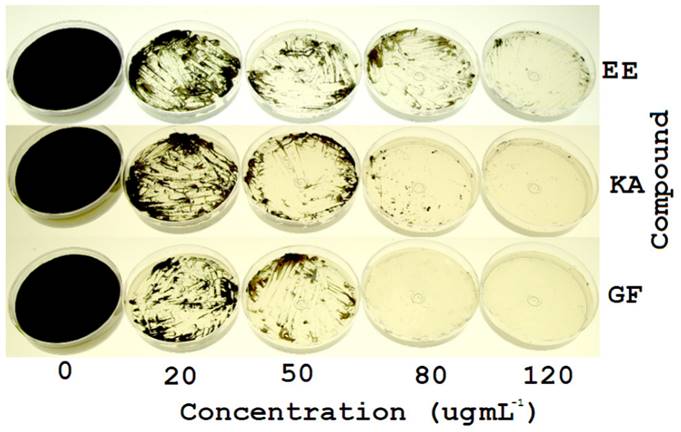
Figure 4. Effect of different concentrations of the ethanolic extract (EE) of Z. montagnifolia, ent-kaurenoic acid (KA) and grandiflorenic acid (GF) in the in vitro growth of C. herbarum.
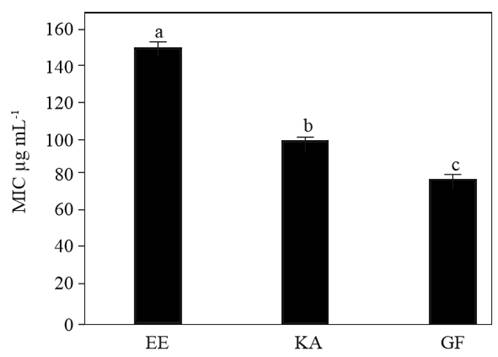
Figure 5. Comparison of MIC values of the ethanolic extract from Z. montagnifolia (EE), ent-kaurenoic acid (KA) and grandiflorenic acid (GF) in the in vitro growth of Cladosporium herbarum. Bars indicate the standard deviation of five repetitions (n=5) and diverse letters indicate significant differences between treatments by ANOVA-Tukey test (p<0.01).
Conclusions
A phytopathogenic fungus was isolated from the necrotic spots of diseased A. angustifolia plants collected in San Luis Amatlán Oaxaca, Mexico. Based upon molecular analyses and molecular characters the fungus was identified as C. herbarum. The ethanolic extracts and pure kaurenoids from Z. montagnifolia showed in vitro antifungal effect at <0.5 mg mL-1 on this fungus.
Literatura citada
Achatz G., Oberkofler H., Lechenauer E., Simon B., Unger A., Kandler D., Ebner C., Prillinger H., Kraft D. and Breitenbach M. 1995. Molecular cloning of major and minor allergens of Alternaria alternata and Cladosporium herbarum. Molecular Inmunology 32:213-227. http://dx.doi.org/10.1016/0161-5890(94)00108-D [ Links ]
Alonso S. F. B. 2012. Cladosporium: género fúngico que deteriora soportes documentales y afecta a la salud del hombre. Boletin del Archivo Nacional 20:104-118. http://www.arnac.cu/wp-content/uploads/2013/03/Cladosporium.pdf [ Links ]
Barbosa M.A.G., Rehn K.G., Menezes M. and Mariano R.LR. 2001. Antagonism of Trichoderma species on Cladosporium herbarum and their enzimatic characterization. Brazilian Journal of Microbiology 32:98-104. http://dx.doi.org/10.1590/S1517-83822001000200005 [ Links ]
Bensch K., Braun U., Groenewald J.Z. and Crous P.W. 2012. The genus Cladosporium. Studies in Mycology 72:1-401. http://dx.doi.org/10.3114/sim0003 [ Links ]
Bensch K., Groenewald J.Z., Braun U., Dijksterhuis J., Yáñez-Morales M.J. and Crous P.W. 2015. Common but different: the expanding realm of Cladosporium. Studies in Mycology 82:23-74. http://dx.doi.org/10.1016/j.simyco.2015.10.001 [ Links ]
Meave J.A., Romero-Romero M.A., Salas-Morales SH, Pérez-García EA, Gallardo-Cruz JA. 2012. Diversidad, amenazas y oportunidades para la conservación del bosque tropical caducifolio en el estado de Oaxaca, México. Ecosistemas 21:85-100. http://www.revistaecosistemas.net/index.php/ecosistemas/article/viewFile/29/25 [ Links ]
Pfaller M.A., Messer S.A., Mills K. and Bolmström A. 2000. In vitro susceptibility testing of filamentous fungi: comparison of etest and reference microdilution methods for determining itraconazole MICs. Journal of Clinical Microbiology 38:3359-3361. http://jcm.asm.org/content/38/9/3359.full.pdf+html [ Links ]
Schubert K. 2005.Taxonomic revision of the genus Cladosporium s. lat. 3. A revision of Cladosporium species described by J.J. Davis and H.C. Greene (WIS). Mycotaxon 92:55-76. http://www.mycotaxon.com/vol/abstracts/92/92-55.html [ Links ]
Valgas C., de Sousa S.M., Smania E.F.A., Smania A. (2007) Screening methods to determine antibacterial activity of natural products. Brazilian Journal of Microbiology 38:369-380. http://www.scielo.br/pdf/bjm/v38n2/v38n2a34.pdf [ Links ]
Villa-Ruano N., Betancourt-Jiménez M.G. and Lozoya-Gloria E. 2009. Biosynthesis of uterotonic diterpenes from Montanoa tomentosa (zoapatle). Journal of Plant Physiology 166:1961-1967. http://dx.doi.org/10.1016/j.jplph.2009.06.004 [ Links ]
Villa-Ruano N., Lozoya-Gloria E. and Pacheco Hernández Y. 2016. Kaurenoic acid: a diterpene with a wide range of biological activities. In: Studies in Natural Products Chemistry Vol.51, Atta-ur-Rahman (ed.) Elsevier. 151-174p. http://dx.doi.org/10.1016/B978-0-444-63932-5.00003-6 [ Links ]
White T.J., Bruns T., Lee S., and Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications. Innis MA, Gelfand DH, Sninsky JJ, and White TJ (eds). Academic Press, Inc., San Diego, Calif. 315-322 p. http://dx.doi.org/10.1016/B978-0-12-372180-8.50042-1 [ Links ]
Received: January 12, 2017; Accepted: February 15, 2017











 text in
text in 


