Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.35 n.2 Texcoco May. 2017
https://doi.org/10.18781/r.mex.fit.1606-7
Phytopathological notes
Distribution, life cycle and histological changes by Heterodera sp. in carrot in Puebla
1Departamento de Parasitología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional. Plan de Ayala y Carpio s/n, Colonia Casco de Santo Tomás, Delegación Miguel Hidalgo, CDMX, C.P. 11340.
In 2009, in the Tepeaca Valley, Puebla, it was found a cyst-forming nematode of the genus Heterodera associated with carrot. At this time, it is unknown the distribution in some producing municipalities, its life cycle and the histological changes on infected plant roots. Between 2008 and 2016, 28 carrot fields were sampled in six municipalities of the Tepeaca Valley. The roots were separated and white females were searched, the cysts were extracted from soil. Carrot cv. Mexicana were grown in naturally infested soil in the greenhouse, the roots were stained with fuchsine lacto glycerol acid to know the development time of each cycle phase. To describe the histological changes, the infected roots were imbibed in paraffin and sections stained with fast green-fuchsine for analysis. Heterodera sp. was found in 93 % of the plots and completed its cycle in 73 days at 20-25 °C. The histological alterations show the presence of feeding sites (syncytia) with dense and granular cytoplasm, with hypertrophied nuclei, dissolution and thickening of cell walls located mainly in the cortex and vascular cylinder. There was also the presence of lignificate areas with hyperplasia and hypertrophy.
Key words: Histopathology; Daucus carota cv mexicana; cyst-forming nematode; sedentary endoparasite
En el 2009 se encontró en el Valle de Tepeaca, Puebla, un nematodo formador de quistes del género Heterodera asociado a zanahoria. A la fecha se desconoce la distribución en algunos municipios productores, su ciclo de vida y las alteraciones histológicas en las raíces de plantas infectadas. Durante 2008 a 2016 se muestrearon 28 parcelas con zanahoria de seis municipios del Valle de Tepeaca. Se separaron las raíces y en ellas se buscaron hembras blancas adheridas, del suelo se extrajeron los quistes. En invernadero se sembró zanahoria cv. Mexicana en suelo naturalmente infestado, las raíces se tiñeron con fucsina ácida-lactoglicerol para conocer el tiempo de desarrollo de cada fase del ciclo. Para describir las alteraciones en raíces infectadas, éstas se incluyeron en parafina con lo que se hicieron cortes, se tiñeron con fucsina-verde rápido para su análisis. Heterodera sp. se encontró en el 93 % de las parcelas y completó su ciclo en 73 días a 20-25 oC. Las alteraciones histológicas mostraron la presencia de sitios de alimentación (sincitios) con citoplasma denso y granuloso, con núcleos hipertrofiados, disolución y engrosamiento de paredes celulares, ubicados principalmente en la corteza y cilindro vascular. Además hubo presencia de zonas lignificadas con hiperplasia e hipertrofia.
Palabras clave: Histopatología; Daucus carota cv mexicana; nematodo formador de quistes; endoparásito sedentario
The genus Heterodera belongs to the group of cyst-forming nematodes (CFN) and are distinguished for having lemon shaped females, which retain the eggs inside their bodies. After their death, the cuticle changes and thickens to form the cyst, which protects the eggs from hostile environmental conditions. It can remain viable in the soil for up to 30 years (Siddiqi, 2000; Subbotin et al., 2010; Manzanilla-López and Marbán-Mendoza, 2012). This nematode is economically important for agriculture due to the losses it produces in crops, caused by the induction of feeding sites known as syncytia, which alter the radicle systems of plants, limiting the absorption of water and nutrients (Sharma, 1998; Subbotin et al., 2010; Subbotin and Franco, 2012). Tovar-Soto et al. (2009, 2010) found a CFN of the genus Heterodera associated to carrot (Daucus carota) fields in the agricultural area of the Valley of Tepeaca, Puebla, composed of several farming municipalities (Lugo-Morín et al., 2010). In 2014 Puebla was the second largest carrot producer in Mexico, with harvests of 27, 109 ton (SAGARPA, 2016). Although this nematode has been found in the area, its distribution in some carrot-producing municipalities is unknown, as is its life cycle and the histological alterations it induces in the roots; these data are crucial to establish control tactics. The aim was to estimate the distribution of the CFN Heterodera sp. in some carrotproducing municipalities in the Valley of Tepeaca, as well as to determine the time of development of the different phases of its life cycle and describe the histological alterations it induces in the roots of this vegetable. Between 2008 and 2016, 28 carrot fields were sampled in the municipalities of Acatzingo, Los Reyes de Juárez, Quecholac, San Nicolás Buenos Aires, San Salvador el Seco, and Tepeaca. In each field chosen, one sample was taken at random, composed of 10 soil subsamples (200-250 g) and plants (3-6) in different phenological stages; this made up the representative sample (2-2.5 kg of soil and 30-60 plants) (De la Jara-Alcocer et al., 1994). For each sample, the plants were separated from the soil, their roots were washed with water, 5 g were weighed and then placed in a Petri dish, where they were observed under a Motic stereoscopic microscope for the search of white females attached to them. The cysts were obtained from 200 cm3 of soil previously dried using the Fenwick flotation technique (De la Jara-Alcocer et al., 1994). The cysts separated in each sample were separated to observe shape, color and presence of vulvar cone (n=20). Then, cuts were made to recognize the type of fenestration (Subbotin et al., 2010). In order to determine the time of development of each of the phases of the nematode cycle in the root, we set up an experiment in a greenhouse with 36 threekilo bags with naturally infested soil from a carrot field in the municipality of Acatzingo. In each bag, we planted 10 seeds of carrot cv. Mexicana, which were kept at 20-25 °C. Ten days after germination (DAG), plants were removed from three bags, their roots were washed with water and stained with fuchsine lacto glycerol acid. Later, they were placed in a Petri dish to observe the phases of the nematode under a Motic stereoscopic microscope. Every seven days for 12 weeks, the same procedure was followed with the remaining bags (Byrd et al., 1983). For the histopathological study, six styrofoam pots were set up with 1 kg of tindalized soil, and ten seeds of carrot cv. Mexicana were planted. Three of the pots were inoculated with eggs and juveniles from stage 2 (J2) of the nematode, and the other three were left without inoculating (controls). The pots were left for 60 days in the greenhouse at 20-25 °C; after this time, the plants were removed from their pots, their roots were washed, and they were fixated with a mixture of ethanolformaldehyde-acetic acid (FAA), where they were kept for 48 h. Straight afterwards, they were cut into 1-3 mm pieces, dehydrated in ethanol at different concentrations (70, 80, 90, 96 and 100 %), where they were kept for 15 min for each concentration. They were then made transparent using absolute ethanol-xylol and xylol 15 min in each. Later, they were included in paraffins with different melting points (52-54, 54-56 and 56-58ºC) (Carvajal-Sandoval, 1996). Once the material was included, serial cuts 10 µm thick were made longitudinally and transversally with a Reichert rotary microtome. The cuts were mounted onto a microscope slide with a flotation bath in a mixture of water and gelatin. Next, the cuts were dewaxed with xylol and absolute ethanol-xylol for 5 minutes in each and then passed through ethanol at different concentrations (100, 96 and 70 %) for 15 min each. Finally, they were rinsed for 10 minutes in distilled water, stained with fast green-fuchsine, dehydrated with 2 changes of absolute ethanol-xylol for 15 minutes each, and mounted with synthetic resin for their analysis (Carvajal-Sandoval, 1996).
The carrot roots from 17 fields sampled (61 %) showed white, pearly, lemon-shaped females attached, with vulvar cone, and a prominent gelatinous matrix in which they hold part of the eggs (egg sac), indicating that carrot is a good host in which they complete their life cycles (Figure 1A and D). Young cysts were also found in roots; a high percentage displayed the gelatinous matrix mentioned above in females; these were also lemon-shaped and with a vulvar cone. Cysts were also found in soil in 93 % of the fields studied, the population density of which ranged from 3 to 1391 cysts in 200 cm3 of soil (Table1) (Figure 1A, B and D). When cutting at the level of the vulvar cone, an ambifenestra appears (Figure 1C); with these criteria we confirmed that the population found in the fields sampled with carrots in the area studied belong to the genus Heterodera (Sharma, 1998; Subbotin et al., 2010).
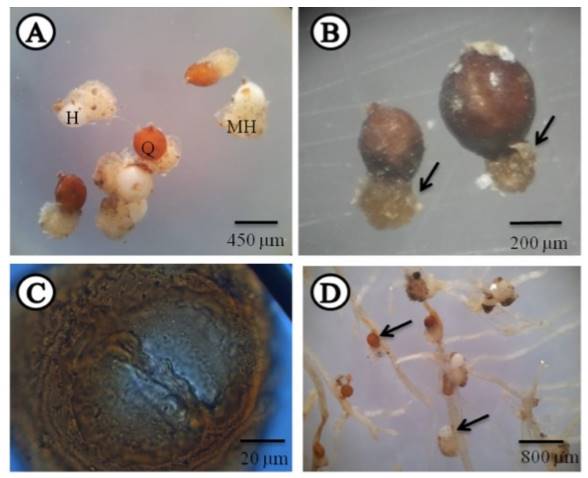
Figure 1. Cysts and white females of Heterodera sp. A) Females and cysts with egg sacs. B) Different sized cysts. Arrows indicate the egg sac. C) Cut of a cyst at the level of the vulvar cone showing the ambifenestra and the bridge. D) Carrot roots with females and cysts attached with egg sacs. H= Female, Q= Cyst, MH= Egg Sacs, P= Bridge.
Table 1. Number of Heterodera sp. cysts per 200 cm3 of field soil in the area under study and presence or absence of white females attached to carrot roots.
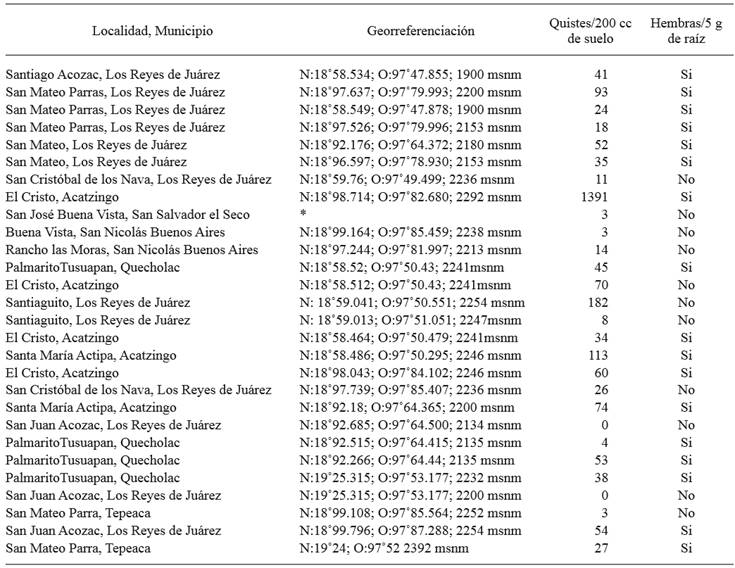
*Georeferentiation data were not taken.
In the life cycle, the J2 appeared inside the lateral roots 10 DAG; likewise, at 24 and 38 DAG, stages J3 y J4 appeared, respectively (Figure 2). On the other hand, the males were observed on the roots inside an exuvia at 52 and up to 59 DAG; female adults were observed attached to the roots starting on day 66. The first cysts attached to the roots were identified by their light brown color, at 73 and up to 94 DAG (Figure 2).
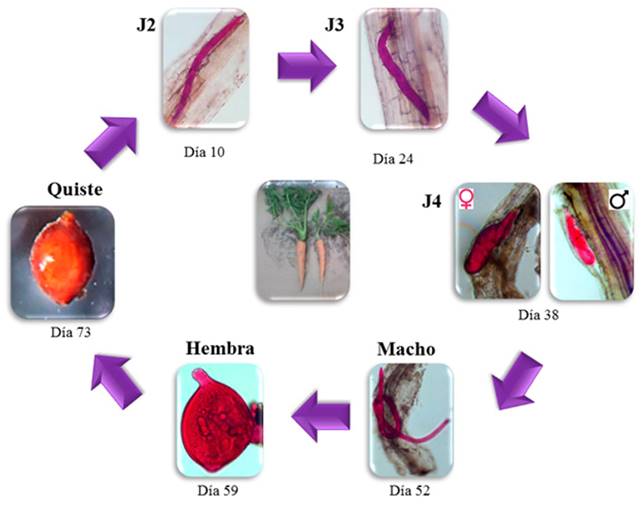
Figure 2. Life cycle phases of development of the cyst forming nematode Heterodera sp. in carrot cv. Mexicana roots under greenhouse conditions. Notice the different phases and the times that appeared in the root.
The histopathological study showed, in the transversal cuts of infected roots, feeding sites known as syncytia, located mainly in the vascular cylinder, causing the displacement of the xylem and phloem vessels (Figure 3C, D and F), where thickening and dissolution of cell walls were observed, with dense, granular and vacuolated cytoplasm (Figure 3C, D and F). There was also hyperplasia, and the presence of hypertrophied nuclei (Figure 3E). Also notorious was the presence of lignified areas (Figure 3C, E and F). These cellular changes are induced by the nematode to be able to feed (Endo, 1971). All these changes have been documented in different species of the genus Heterodera in different crops (Endo, 1971; Sasanelli and Vovlas, 2013; Barrot, 2016). This contrasts with the cuts in roots with healthy tissue (control) in the longitudinal and transversal plane, where no alterations were found (Figure3A and B).
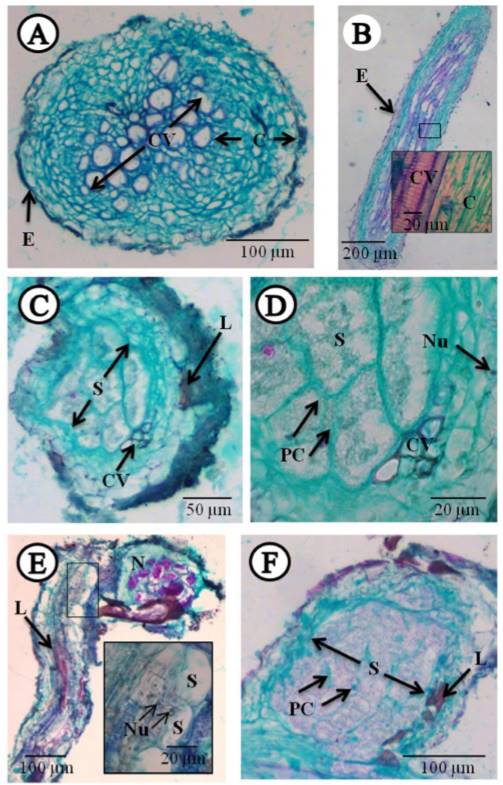
Figure 3. Longitudinal and transversal cuts to the microtome in healthy and infected carrot roots with the cyst-forming nematode Heterodera sp., stained with fast green-fuchsine. A) Transversal cut of healthy root (control). B) Longitudinal cut of healthy root (control). C-D) Transversal cuts of root infected by the nematode. E- F) Longitudinal cut of root with nematode parasite. C= Cortex, CV= Vascular cylinder, E= epidermis, L= Lignification, N= Nematode, Nu= Nucleus, PC= Cell wall, S= Syncytium.
Conclusions
Heterodera sp. was found in 93 % of the fields sampled and completed its life cycle in the greenhouse in 73 days at 20-25 °C. Histological alterations showed the presence of feeding sites (syncytia) located mainly in the cortex and vascular cylinder, with dense and granular cytoplasm, and hypertrophied nuclei, dissolution and thickening of cell walls. There were also lignified areas with hyperplasia and hypertrophy.
Acknowledgements
This paper shows the results of the Master’s degree thesis by the first author, who would like to thank CONACYT for the scholarship granted to carry out his Graduate studies, as well as the BEIFI-IPN system for the economic support provided.
REFERENCES
Byrd DW, Kirkpatrick T and Barker KR. 1983. An improved technique for clearing and staining plant tissues for detection of nematodes. Journal of Nematology 15: 142-143. Disponible en línea: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2618249/pdf/142.pdf [ Links ]
Barrot L. Resistance to Heterodera carotae and methods for use. FR., WO2016166262 A1, 20 de Octubre de 2016. PCT/EP2016/058304, 15 Abr 2016. Disponible en línea: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016166262&recNum=1&maxRec=&office=&prevFilt er=&sortOption=&queryString=&tab=PCT+Biblio [ Links ]
Carvajal-Sandoval A. 1996. Manual de Histología Vegetal. Instituto Politécnico Nacional. México, D. F. 42 p. [ Links ]
De la Jara-Alcocer F, Zeron-Bravo F, Torres-Coronel R y Tovar-Soto A. 1994. Manual de Prácticas de Nematología Agrícola. 2a Edición. Escuela Nacional de Ciencias Biológicas-IPN. México, D. F., México. 107 p. [ Links ]
Endo B.Y. 1971. Nematode-induced syncytia (Giant cells). Host-parasite relationships of Heteroderidae. pp. 91-117. In: Zuckerman BM, Mai WF and Rohde RA (eds.). Plant Parasitic Nematodes. II. Cytogenetics, Host-parasite Interactions, and Physiology. Academic Press. New York, USA. 347 p. [ Links ]
Lugo-Morín DR, Ramírez-Juárez J, Méndez-Espinoza JA y Peña-Olvera B. 2010. Redes sociales asimétricas en el sistema hortícola del Valle de Tepeaca, México. Economía, Sociedad y Territorio 10: 207-230. Disponible en línea: http://www.redalyc.org/articulo.oa?id=11112509008 [ Links ]
Manzanilla-López RH and Marbán-Mendoza N. 2012. Practical Plant Nematology. Biblioteca básica de Agricultura. México. 881 p. [ Links ]
SAGARPA. Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. 2016. Servicio de Información Agroalimentaria y Pesquera. www.siap.gob.mx/cierre-de-la-produccion-agricola-por-cultivo/ (consulta, marzo 2016). [ Links ]
Sassaneli N and Vovlas N. 2013. Pathogenicity and Host Parasite Relationships of Heterodera cruciferae in Cabbage. Plant Disease. 97(3):333-338. http://dx.doi.org/10.1094/PDIS-07-12-0699-RE [ Links ]
Sharma SB. 1998. The Cyst Nematodes. Springer Science & Business Media. Great Britain, UK. 452 p. [ Links ]
Siddiqi MR. 2000. Tylenchida: Parasites of Plants and Insects. Second Edition. CAB International, Wallingford, U.K. 835 p. [ Links ]
Subbotin SA, Mundo-Ocampo M, and Baldwin JG. 2010. Systematics of cyst nematodes (Nematoda: Heteroderinae). 8A. Nematology monographs and perspectives. Netherlands. 351 p. [ Links ]
Subbotin SA and Franco J. 2012. Cyst nematodes. pp. 299-357. In: Manzanilla-López R.H., and Marbán-Mendoza N. (eds.). Practical Plant Nematology. Colegio de Postgraduados, México. Biblioteca básica de Agricultura. México. 881 p. [ Links ]
Tovar-Soto A, Hernández-López JM y Torres-Coronel R. 2009. Nematodos Formadores de Quistes en la Zona Hortícola del Estado de Puebla. XI Congreso Internacional/XXXVI Nacional de la Sociedad Mexicana de Fitopatología, A.C. Sociedad Mexicana de Fitopatología. Acapulco, Gro., México. 4 p. [ Links ]
Tovar-Soto A, Medina-Canales MG y Torres-Coronel R. 2010. Nematodos Fitoparásitos Asociados a Hortalizas en el Valle de Tepeaca, Puebla, México. XII Congreso Internacional/XXXVII Nacional de la Sociedad Mexicana de Fitopatología, A.C. Sociedad Mexicana de Fitopatología. Mérida, Yuc. México. 4 p. [ Links ]
Received: June 30, 2016; Accepted: December 06, 2016











 text in
text in 


