Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.35 no.2 Texcoco may. 2017
https://doi.org/10.18781/r.mex.fit.1612-4
Scientific articles
Sensitivity of Colletotrichum acutatum isolates obtained from strawberry to tiophanate-methyl and azoxystrobin fungicides
1Posgrado en Protección Vegetal, Departamento de Parasitología Agrícola, Universidad Autónoma Chapingo. Km 38.5 carretera México-Texcoco, Texcoco, Estado de México, México. CP 56235 Tel. 5959521500.
2Producción de Semillas, Colegio de Postgraduados, Campus Montecillo. Km 36.5 carretera México-Texcoco, Texcoco, Estado de México, México. CP 56230 Tel. 5959520265.
3Departamento de Parasitología Agrícola, Universidad Autónoma Chapingo. Km 38.5 carretera México-Texcoco, Texcoco, Estado de México, México. CP 56235 Tel. 5959521608.
4Centro Regional Morelia, y Posgrado en Protección Vegetal, Universidad Autónoma Chapingo. Periférico Paseo de la República No. 1000, Morelia, Michoacán, México. CP 58170 Tel. 4433161489.
The objective of this study was to determine the sensitivity of Colletotrichum acutatum to the fungicides thiophanate-methyl and azoxystrobin, based on mycelial growth and conidia germination. Sixty monoconidial isolates from two strawberry-producing regions of Michoacan state were evaluated in culture medium amended with fungicides at 0.001, 0.01, 0.1, 1 and 10, 100 and 2000 μg mL-1. The effective dose that reduced mycelial growth by 50 % (ED50) of thiophanate-methyl varied from 0.28 to 9.72 μg mL-1 in the Maravatío Valley and from 1.39 to 2.99 μg mL-1 in the Zamora Valley. In conidia, the ED50 ranged from 0.9 to 396.4 μg mL-1 in the Maravatío Valley and from 0.43 to 63.32 μg mL-1 in the Zamora Valley. For azoxystrobin the ED50 in mycelium ranged from 0.04 to 0.36 μg mL-1 in Maravatío and from 0.07 to 0.99 μg mL-1 in Zamora. In the conidia the ED50 varied from 0.01 to 0.56 μg mL-1 for Maravatío and 0.006 to 0.15 μg mL-1 in Zamora. The ED50 distributions indicated that C. acutatum isolates were sensitive to azoxystrobin and moderately resistant to methyl thiophanate.
Key words: Benzimidazole; QoI; fungicide resistance
El objetivo de este estudio fue determinar la sensibilidad de Colletotrichum acutatum a los fungicidas metil tiofanato y azoxystrobin, basada en el crecimiento de micelio y germinación de conidios. Se evaluaron 60 aislados monoconidiales de dos regiones productoras de fresa del estado de Michoacán, en medio de cultivo enmendado con 0.001, 0.01, 0.1, 1 y 10, 100 y 2000 μg mL-1 de fungicidas. La dosis efectiva que redujo en un 50% el crecimiento micelial (DE50) del metil tiofanato varió de 0.28 a 9.72 μg mL-1 en el valle de Maravatío y de 1.39 a 2.99 μg mL-1 en el valle de Zamora. En conidios la DE50 varió entre 0.9 a 396.4 μg mL-1 en el valle de Maravatío y de 0.43 a 63.32 μg mL-1 en el valle de Zamora. Para el azoxystrobin las DE50 en micelio variaron de 0.04 a 0.36 μg mL-1 en Maravatío y de 0.07 a 0.99 μg mL-1 en Zamora. En conidios las DE50 variaron de 0.01 a 0.56 μg mL-1 para Maravatío y 0.006 a 0.15 μg mL-1 en Zamora. Las distribuciones de las DE50 indicaron que los aislados de C. acutatum fueron sensibles al azoxystrobin y moderadamente resistentes al metil tiofanato.
Palabras clave: Benzimidazoles; QoI; Resistencia a fungicidas
In Mexico, the area planted to strawberry (Fragaria × ananassa) accounts for more than 10,000 hectares, nearly 60 % of which is located in the state of Michoacán (SIAP, 2016). Among the phytopathological factors that mostly affect strawberry production is anthracnose. This disease has been associated mainly with the species Colletotrichum acutatum (Turecheck et al., 2006), especially in farm systems where the crop is grown in open fields with a low to medium level of technology, or in protected systems where the crop is exposed to rain. This condition favors the spread of conidia by splashing from primary infection sources and from plant to plant (Smith, 2008).
The disease management is based mainly on establishing pathogen-free plants (Smith, 2008; Freeman, 2008), elimination of symptomatic plants and fruits and removing crop residues and alternate hosts (Freeman, 2008; Parikka and Lemmetty, 2009). Resistant varieties and biological control have also been used (Wharton and Diéguez-Uribeondo, 2004; Hammerschlag et al., 2006). Although preventive fungicides, such as Captan, Thiram, and different copper-based formulations, are the most frequently used, and other fungicides with specific mode of action, such as cyprodinil+fludioxonil, pyraclostrobin, azoxystrobin and thiophanate methyl, are often used as well (Turechek et al., 2006; Wedge et al., 2007). The specific mode of action of these fungicides and their frequent use pose a risk because they cause the pathogen to develop resistance. Therefore, they must be utilized following certain guidelines for effectively managing resistance (the correct dose, number of applications, mixing fungicides that have a different mode of action, alternation and application timing) (van den Bosh et al., 2014). It is also essential to frequently monitor the sensitivity of fungal and oomycete populations in different areas at the regional or national level so as to detect changes in the distribution of sensitivity caused by different mechanisms. If necessary, implement appropriate measures to delay, postpone or prevent the development of resistance, which could cause significant crop losses due to the reduction of the fungicides’ effective life (van den Bosh et al., 2014; Corio-Costet, 2015). This approach must be included in all integrated disease management strategies (Hollomon, 2015).
Given that C. acutatum is one of the causal agents of anthracnose in several crops, and especially in citrus and strawberry, knowing their sensitivity to the most frequently used fungicides would allow us to make better decisions when formulating integrated disease management strategies. In the case of citrus, Mondal et al. (2005) reported base lines (before fungicide use) with DE50 between 0.09-0.77 µg mL-1, and 0.01 a 0.034 μg mL-1 for azoxystrobin and pyraclostrobin, respectively. On the other hand, Forcelini et al. (2016) evaluated the resistance of C. acutatum isolates to strobilurin fungicides or QoI (quinone outside inhibitors) on strawberry crops in Florida, based on mycelial growth and germ tube length. DE50 values for azoxystrobin were 0.22 µg mL-1 in isolates collected from 1994 to 2011; for pyraclostrobin, the average DE50 was 0.012 µg mL-1. Isolates obtained later from sites that had a history of use of these fungicides showed no sensitivity with values higher than 10µg mL-1 and 100µg mL-1 for pyraclostrobin and azoxystrobin, respectively. Sequencing of the cytochrome b gene confirmed that the resistant isolates had the G143A and F129L mutations.
Benzimidazole fungicides (benomyl, thiabendazole, carbendazim and thiophanate methyl, among others) have been widely used on strawberry crops in Mexico for over three decades. Furthermore, QoI (strobilurin) fungicides have been on the market since the mid-90s; in Mexico, their use on strawberry has been approved. However, the frequent use of these active ingredients may result in the lack of effectiveness in the field. The objective of this study was to determine the sensitivity of C. acutatum isolates obtained from strawberries collected in the Maravatío and Zamora valleys, state of Michoacán, to thiophanate methyl and azoxystrobin, based on mycelial growth and conidia germination. By knowing the sensitivity status of C. acutatum, which causes strawberry anthracnose in the two main strawberry producing areas of Mexico, we will be able to adjust resistance management programs to postpone, delay or prevent this phenomenon from developing and thus contribute to more effective disease management.
Materials and methods
Collecting and obtaining isolates
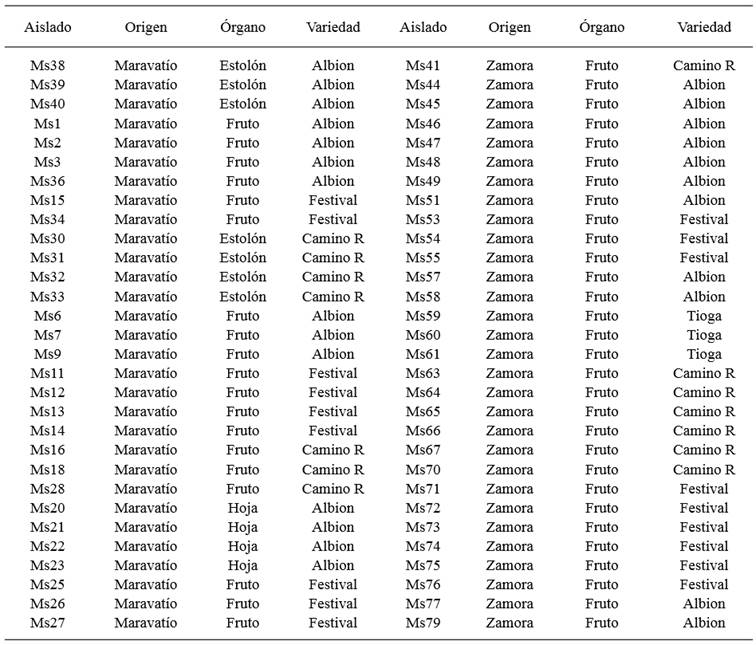
In 2009-2011, leaves, stolons and strawberries showing typical anthracnose symptoms were collected in commercial plots of Festival, Albión and Camino Real strawberry varieties in the Maravatío and Zamora valleys. To capture greater variability in the samples, they were taken from plots separated by a distance of 1 to 3 km, approximately.
The sensitivity test was conducted at the Phytopathology Laboratory of the Centro Regional Universitario Centro-Occidente (CRUCO), Universidad Autónoma de Chapingo, in Morelia, Michoacán, and the pathogen’s molecular identification was performed at the Laboratory of Biotechnology and Seed Pathology for Postgraduate Students in Genetic Resources and Productivity (PREGEP), Colegio de Postgraduados, in Montecillo, State of Mexico.
Obtaining monoconidial isolates
From strawberries, leaves and stolons showing symptoms, portions of tissue approximately 2 cm were selected from the area showing symptoms, washed with water, disinfected with a 1 % sodium hypochlorite solution for 2-3 minutes, and rinsed with sterile distilled water. They were then dried in a laminar flow cabinet and grown on potato-dextrose-agar (PDA) (Difco, Becton, Dickinson and Company, Sparks, MD 21152, USA). From the stock cultures, monoconidial cultures were prepared using serial dilutions; the conidia were placed on 2 % water agar (WA) and incubated for 10-18 hours at room temperature. Later, using a dissection needle, the germinated conidia were extracted one by one and placed in Petri dishes containing PDA. The monosporic isolates (Table 1) were stored at 4o C and later used in fungicide sensitivity tests.
Molecular identification
Monoconidial isolates from the Maravatío (8 isolates) and Zamora (11 isolates) valleys were selected. DNA extraction was carried out following the protocol of Doyle and Doyle (1990) with minor modifications. Later, through PCR, the ITS1, 5.8S and ITS2 regions of the ribosomal DNA were amplified using ITS5/ITS4 primers (White et al., 1990) in a C1000 TouchTM Thermal Cycler (Bio-Rad, USA) as follows: initial denaturation at 95 °C for 4 min, followed by 35 cycles at 95° for 2 min, 55 °C for 1 min, 72 °C for 2 min, and a final extension at 72 °C for 2 min. Later, the PCR products were cleaned using a QIAquick purification kit (Qiagen Inc., Chatsworth, CA, USA), according to the manufacturer’s instructions. Sequencing was performed in Macrogen, Maryland, USA; then the sequences were edited in Bioedit Sequence Alignment Editor v.7.0.5 (Hall, 1999), and consensus sequences were generated. The sequences were then compared with the sequences stored in the Genbank database of the National Center for Biotechnology Information (NCBI) using the BLASTNucleotide (Zhang et al., 2000) program; we took into consideration the highest value resulting from the comparisons.
Fungicide application
Commercial formulations of thiophanate methyl from the benzimidazole group (Cercobin M®, BASF Mexicana, Zapopan, Jalisco) at 70 % concentration were used in the tests, as well as azoxystrobin, which belongs to the group of quinone outside inhibitors (QoI) (Amistar®), at 50 % concentration of the active ingredient (Syngenta Agro S.A de C.V. México, D.F.).
Stock solutions and amended culture medium preparation
Stock solutions of each fungicide were prepared by mixing the active ingredient with sterile distilled water. Using the concentrated solution, 1:10 serial dilutions were prepared to logarithmically obtain the desired concentrations. The solution containing the corresponding fungicide concentration was added to the medium when it cooled to 50-60 °C, constantly stirring to ensure uniform homogenization, and immediately placed in 15 mL Petri dishes.
Mycelial growth test
Six doses of each fungicide were evaluated: 100, 10, 1, 0.1, 0.01 and 0.001 µg mL-1 of azoxystrobin, and 2000, 1000, 100, 10, 1 and 0.1 µg mL-1 of methyl thiophanate, plus an absolute tester to which no fungicide was added. From the margins of 6-day old active colonies of each of the monoconidial isolates, we obtained disks 0.5 cm in diameter, which were placed in the middle of 90 x 15 mm Petri dishes containing culture medium to which the fungicide had been added. Three replications (Petri dishes) of each dose were prepared. The dishes were incubated in darkness at room temperature (20±4 °C) for five days. After this period, the diameters of the colonies were measured perpendicularly in two directions to obtain the average.
Conidia germination test
The same six doses of each fungicide were evaluated, plus a tester to which no fungicide was added. Three replications of each dose (50x15 mm Petri dishes) were used. Monoconidial suspensions were prepared at concentrations of 103 to 104 conidia mL-1. The suspensions were grown in 2 % WA culture medium to which fungicide had been added, only as a germination surface, prepared in the same way as in the mycelial growth inhibition stage. Six hours after being sown, the number of germinated and non-germinated conidia in each replication of a 100-conidia random sample were counted. A conidium was considered germinated when the length of its germ tube was 2 times greater than the diameter of the conidium.
Estimation of effective doses 50 (ED50) and data analysis
Based on the two measurements of the diameter of the colony during the mycelial growth tests, we obtained the average, and after the three replications (Petri dishes) —which were used to calculate growth or germination compared to the control per each fungicide dose and isolate, we adjusted the model and estimated the ED50 using the log- Logistic model with upper asymptote using the NLMIXED procedure of SAS (Statistical Analysis System version 9.3). The model is expressed as follows:
Where y denotes relative mycelial growth or relative conidial germination, α is the upper asymptote (maximum y value possible), β the slope at the inflection point, and ED50 the dose at which growth/germination is reduced by 50% (Schabenberguer and Pierce, 2001; Rebollar-Alviter et al., 2007). Given that not all the initial values resulted in convergence for all the isolates, different sets of values were explored at the beginning of the iterations in order to achieve convergence. The adjustment to the model was confirmed considering the residuals pattern for each isolate (Rebollar-Alviter et al., 2007). Once ED50 was estimated, an exploratory data analysis was conducted, and the sensitivity distributions were illustrated with frequency histograms.
Before estimating the statistical moments, normality tests were conducted on the original data, and the ED50 values were converted to 10-base logarithms. The estimated moment, the dispersion of basic statistics, the bias or misalignment parameters and the normality tests were conducted using the Proc univariate included in SAS software (version 9.3). Once the general sensitivity distribution of the two strawberry producing areas was explored, the data from each area were individually analyzed to determine significant differences between the distributions in each strawberry producing valley in terms of the sensitivity of C. acutatum isolates. This was done because the two production areas may exert different levels of selection pressure due to their contrasting technology levels. The comparison was made using the Kolmogorov-Smirnov test and the NPAR1WAY (SAS version 9.3) procedure to determine whether the distributions came from a single population or different populations.
Results and Discussion
Morphological and molecular identification of isolates
All the pathogen isolates associated with strawberries showing anthracnose symptoms and damage collected in the two strawberry producing areas of Michoacán proved to be Colletotrichum acutatum. The isolates were characterized by forming white colonies with dark gray centers and concentric orange circles both at the center and at the edges. The conidia were smooth, unicellular, aseptate, spindle-shaped and measured 10-14 x 4-6µm, and similar to those reported by Damm et al. (2012). The sequences obtained by PCR amplification of the region included in the Internal Transcribed Spacer (ITS) of the rDNA of the 20 isolates were 99 % identical to the AM991131 and AY266405 accessions placed in the NCBI’s GenBank.
ED50 estimation
The log-Logistic model properly described the data of the dose-response to the fungicides used in this experiment for each of the isolates used. An example of ED50 estimation in the Ms12 isolate in response to mycelial growth using azoxystrobin is shown in Figure 1. This model has proved to be very useful for estimating ED50 when studying fungicide resistance and for detecting the hormesis phenomenon in these compounds (Schabenberger and Pierce, 2001; Rebollar-Alviter et al., 2007; Flores and Garzon, 2013).
Distribution of the sensitivity of C. acutatum isolates to thiophanate methyl
The sensitivity of C. acutatum isolates to thiophanate methyl at the mycelial stage ranged from one 0.28 to 9.72 µg mL-1 dose of ED50 with an average dose of 2.39 µg mL-1 for the Maravatío valley, but it fit a normal distribution when the data were converted to the base-10 logarithm. The distribution of sensitivity in Zamora had a normal distribution and thus no data transformation of any type was required (Figure 2); its values ranged from 1.39 to 2.99 µg mL-1, and were similar to the ones obtained for Maravatío. In the case of conidia, their ED50 doses showed a wide distribution ranging from 0.9 to 396.4 µg mL-1, with a mean of 20.9 µg mL-1 for the Maravatío valley; the values for the Zamora valley were lower and ranged from 0.43 to 63.32 µg mL-1, with an average of 10.9 and a median of 5.0 µg mL-1 (Table 2). In this case, there were no significant differences between the sensitivity distribution in both valleys for mycelium (Pr>Ksa=0.13), but there were significant differences for conidia (Pr>Ksa=0.0004). However, considering the mode of action of thiophanate methyl, mycelial growth is the process that most precisely reflects the isolates’ sensitivity, since benzimidazoles act by joining the subunits of the β-tubulin (TUB2) protein, which affects the microtubule assembly, and therefore interferes with processes such as mitosis and cytoskeletal formation. This is directly manifested in cell division (Ishii, 2012); point mutations at this site result in a lack of effectiveness in the field (Ma and Michailidis, 2005).

Figure 2. Sensitivity distribution of Colletotrichum acutatum to azoxystrobin fungicide for conidia germination test. A= Maravatío valley; B= Zamora valley. Sensitivity distribution to thiophanate methyl (mycelium), C= Maravatío valley; D= Zamora valley.
Table 2. Basic statistics and moments of ED50 (µg mL-1) values for thiophanate methyl in Colletotrichum acutatum mycelium and conidia obtained from strawberries collected in Maravatío and Zamora, Michoacán.

xStandard deviation.
In studies conducted by Peres et al. (2004) using C. gloesporioides and C. acutatum obtained from citrus, the authors found that in the case of benomyl (benzimidazole), C. acutamum isolates were moderately resistant, since their growth was inhibited by approximately 55 % at DE50= 0.1 µg mL-1 and 80 % at DE50=1 µg mL-1. The latter isolates showed point mutations in the TUB2 gene. Chung et al. (2006) studied different Colletotrichum species and reported that in C. acutatum isolates they estimated ED50 between 1 and 10 µg mL-1 of thiophanate methyl, indicating that they were more resistant than C. gloeosporioides. Kim et al. (2007) also reported using ED50 doses between 3.8 and 8.8 µg mL-1 in C. acutatum isolates obtained from chili in concentrations higher than 100 µg mL-1, but found no point mutations in the TUB2 gene. The results of our study show that, based on mycelial growth, the isolates from both strawberry producing areas showed ED50 no higher than 3 µg mL-1. For this reason, it may be considered that C. acutatum populations have moderate resistance to benzimidazole fungicides such as thiophanate methyl, and more likely to benomyl, because of they share the same mode action, as previously shown (Peres et al., 2004; Valero et al., 2010). These results suggest the need to implement benzimidazole resistance management strategies in the study areas given the stability found in some fungi such as Botrytis cinerea (Hollomon, 2015); among these strategies are the use of fungicide mixtures or alternating fungicides of different mode of action and not using them for eradication purposes (after symptom development) (Brent and Derek, 2007).
Distribution of the sensitivity of C. acutatum isolates to azoxystrobin
The distribution of the sensitivity to azoxystrobin of C. acutatum isolates in mycelium ranged between 0.04 to 0.36 µg mL-1 ED50, with a median of 0.12 µg mL-1 in the Maravatío valley, while in the Zamora valley, the ED50 values ranged from 0.07 to 0.99 µg mL-1, with the same median value. For conidia, the estimated ED50 ranged from 0.01 to 0.56 µg mL-1 and 0.006 a 0.15, with a median of 0.05 and 0.04 µg mL-1, for Maravatío and Zamora, respectively (Table 3; Figure 2). Converting the ED50 values to base-10 logarithms satisfactorily normalized the distribution curve, except for the isolates from Zamora in the mycelium sensitivity test. The Kolmogorov-Smirnov test used to compare the two sensitivity distributions (for conidia and mycelium) did not show significant differences between the two study areas (Pr>Ks=0.58 for conidia; Pr>Ks=0.79 for mycelium).
Table 3. Basic statistics and moments of ED50 (µg mL-1) values for azoxystrobin in Colletotrichum acutatum mycelium and conidia obtained from strawberries collected in Maravatío and Zamora valleys.

xStandard deviation.
Mondal et al. (2005) reported ED50 base values of 0.09 to 0.99 with a median of 0.4 µg mL-1 of azoxistrobin for C. acutatum mycelia obtained from citrus in Florida. Based on these results, they selected discriminatory doses of 1 ug mL-1. Later, Forcelini et al. (2016) determined that azoxystrobin sensitivity in C. acutatum isolates obtained from strawberry in Florida was 0.30 µg mL-1. According to the authors, isolates showing inhibition up to ED50=3 µg mL-1 were considered sensitive; those with up to 100 µg mL-1 were considered moderately resistant, and those with sensitivity higher than 100 µg mL-1 were considered completely resistant. Moderately resistant isolates were associated with point mutations at codon 129 of the cytochrome bc1 gene, where the aminoacid phenylalanine changed to leucine (F129L), while in the highly resistant isolates, the mutation was associated with the change from the aminoacid glycine to alanine (G143A). Based on these studies, we may conclude that all the isolates tested in this study are sensitive to azoxystrobin, since the data show no evidence of resistance in the two strawberry producing areas studied based on ED50 distribution, although this active ingredient and other molecules of the same group have been in the Mexican market for over 10 years. However, given that this group is considered to be high risk because it induces resistance in fungi, it is necessary to implement resistance management strategies (Hollomon, 2015) in order to avoid changes in sensitivity distribution in the short and long term. The implementation of resistance management strategies must take into account general principles that determine the development of resistant isolates (van den Bosch et al., 2014).
Conclusions
The sensitivity of C. acutatum isolates to thiophanate metyl based on mycelium ranged from ED50 0.28 to 9.72 µg mL-1 in the Maravatío valley, and from 1.39 to 2.99 µg mL-1 in the Zamora valley; for conidia ED50 ranged from 0.9 to 396.4 µg mL-1 in the Maravatío valley, and from 0.43 to 63.32 µg mL-1 in the Zamora valley.
As for azoxystrobin in mycelium, the ED50 ranged from 0.04 to 0.36 µg mL-1 in the Maravatío valley, and from 0.07 to 0.99 µg mL-1 in the Zamora valley. In conidia, ED50 ranged from 0.01 to 0.56 µg mL-1 for the Maravatío valley, and from 0.006 to 0.15 µg mL-1 for the Zamora valley.
The data obtained based on sensitivity distribution (ED50) show that the C. acutatum isolates from Maravatío and Zamora, Michoacán, showed moderate resistance to thiophanate methyl, and complete sensitivity to azoxystrobin. In both cases, it is necessary to implement strategies for managing resistance to benzimidazole and strobirulin fungicides.
Acknowledgements
The authors wish to thank the Consejo Nacional de Ciencia y Tecnología, Mexico, for funding the first author’s postgraduate studies in Plant Protection at the Universidad Autónoma Chapingo.
REFERENCES
Brent KJ and Hollomon DW. 2007. Fungicide resistance in crop pathogens: how can it be managed?. Fungicide Resistance Action Committee. Frac Monograph no. 1. http://www.frac.info/docs/default-source/publications/monographs/monograph-1.pdf [ Links ]
Chung WH, Ishii H, Nishimura K, Fukaya M, Yano K and Kajitani Y. 2006. Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan. Plant Disease 90:506-512. http://dx.doi.org/10.1094/PD-90-0506 [ Links ]
Costet-Costet M. 2015. Monitoring resistance in obligate pathogens by bioassays relating to field use: grapevine powdery and downy mildews. Pp: 251-280 in: Ishii H, and Hollomon WWF (eds). Fungicide resistance in plant pathogens. Springer, Japan. 485 pp. http://dx.doi.org/10.1007/978-4-431-55642-8 [ Links ]
Damm U, Cannon PF, Wouldenberg JHC and Crous PW. 2012. The Colletotrichum species complex. Studies in Mycology 73:37-113. http://dx.doi.org/10.3114/sim0010 [ Links ]
Doyle JJ and Doyle JL. 1990. Isolation of plant DNA from fresh tissue. Focus 12:13-15. http://ci.nii.ac.jp/naid/10005384640 [ Links ]
Flores FJ and Garzon C. 2013. Detection and assessment of chemical hormesis on the radial growth in vitro of Oomycetes and fungal pathogens. Dose-Response 11:361-373. http://dx.doi.org/10.2203/dose-response.12-026.Garzon [ Links ]
Forcelini BB, Seijo TE, Amiri A and Peres NA. 2016. Resistance in strawberry isolates of Colletotrichum acutatum from Florida to quinone- outside inhibitor fungicides. Plant Disease 100:2050-2056. http://dx.doi.org/10.1094/PDIS-01-16-0118-RE [ Links ]
Freeman S. 2008. Management, survival strategies, and host range of Colletotrichum acutatum on strawberry. Hort Science 43:66-68. http://hortsci.ashspublications.org/content/43/1.toc [ Links ]
Hammerschlag F, Garcés S and Koch-Dean M. 2006. In vitro response of strawberry cultivars and regenerants to Colletotrichum acutatum. Plant Cell, Tissue and Organ Culture 84:255. http://dx.doi.org/10.1007/s11240-005-9027-5 [ Links ]
Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41:95-98. http://brownlab.mbio.ncsu.edu/JWB/papers/1999Hall1.pdf. [ Links ]
Hollomon DW. 2015. Fungicide resistance: facing the challenge. Plant Protection Science 51:170-176. http://dx.doi.org/10.17221/42/2015-PPS [ Links ]
Ishii H. 2012. Resistance in Venturia nashicola to benzimidazoles fungicides and sterol demetylation Inhibitors. Pp: 21-31. In: Thind, T. (ed). Fungicide resistance in crop protection. CAB International. UK. 297 pp. http://dx.doi.org/10.1079/9781845939052.0157 [ Links ]
Kim Y, Min J, Kang B, Bach N, Choi W, Park E and Kim H. 2007. Analyses of the less benzimidazole-sensitivity of the isolates of Colletotrichum spp. causing the anthracnose in pepper and strawberry. The Plant Pathology Journal 23:187-192. http://dx.doi.org/10.5423/PPJ.2007.23.3.187. [ Links ]
Ma Z and Michailides TJ. 2005. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Protection 24:853-863. http://dx.doi.org/10.1016/j.cropro.2005.01.011. [ Links ]
Mondal SN, Bhatia A, Shilts T and Timmer LW. 2005. Baseline sensitivities of fungal pathogens of fruit and foliage of citrus to azoxystrobin, pyraclostrobin and fenbuconazole. Plant Disease 89:1186-1194. http://dx.doi.org/10.1094/PD-89-1186 [ Links ]
Parikka P and Lemetty A. 2009. Colletotrichum acutatum: survival in plant debries and infection of some weeds and cultivated plants. Acta Horticulturae 842:307-310. http://dx.doi.org/10.17660/ActaHortic.2009.842.55 [ Links ]
Peres NAR, Souza NL, Peever TL and Timmer LW. 2004. Benomyl sensitivity of isolates of Colletotrichum acutatum and C. gloeosporioides from citrus. Plant Disease 88:125-130. http://dx.doi.org/10.1094/PDIS.2004.88.2.125 [ Links ]
Rebollar-Alviter A, Madden LV, Jeffers SN and Ellis MA. 2007. Baseline and differential sensitivity to two QoI fungicides among isolates of Phytophthora cactorum that cause leather rot and crown rot on strawberry. Plant Disease 91:1625-1637. http://dx.doi.org/10.1094/PDIS-91-12-1625 [ Links ]
Schabenberger O and Pierce FJ. 2001. Contemporary Statistical Model for the Plant and Soil Science. CRC Press, Boca Raton, FL. USA. 738 p. [ Links ]
SIAP. 2016. Sistema de Información Agroalimentaria y Pesquera. Producción agrícola por Cultivo. http://www.siap.gob.mx (consulta en Marzo del 2016). [ Links ]
Smith BJ. 2008. Epidemiology and pathology of strawberry anthracnose: A North American perspective. Hort Science 43:69-73. http://hortsci.ashspublications.org/content/43/1.toc [ Links ]
Turechek WW, Peres NAR and Werner NA. 2006. Pre- and post-infection activity of pyraclostrobin for control of anthracnose fruit rot of strawberry caused by Colletotrichum acutatum. Plant Disease 90:862-868. http://dx.doi.org/10.1094/PD-90-0862 [ Links ]
Valero M, García-Martínez S, Giner MJ, Aranzazu A and Ruiz JJ. 2010. Benomyl sensitivity assays and species-specific PCR reactions highlight association of two Colletotrichum gloeosporioides types and C. acutatum with rumple disease on Primofiori lemons. European Journal of Plant Pathology 127:399-405. http://dx.doi.org/10.1007/s10658-010-9606-0 [ Links ]
van den Bosh F, Oliver R, van den Berg F and Paveley N. 2014. Governing principles can guide fungicide-resistance management tactics. Annual Review of Phytopathology 52:175-195. https://dx.doi.org/10.1146/annurevphyto-102313-050158 [ Links ]
Wedge D, Smith JN, Quebedeaux JP and Constantin RJ. 2007. Fungicide management strategies for control of strawberry fruit rot diseases in Louisiana and Mississippi. Crop Protection 26:1449-1558. http://dx.doi.org/10.1016/j.cropro.2006.12.007 [ Links ]
Wharton SP and Diéguez-Uribeondo J. 2004. The biology of Colletotrichum acutatum. Anales del jardín botánico de Madrid 61:3:22. http://dx.doi.org/10.3989/ajbm.2004.v61.i1.61 [ Links ]
White TJ, Bruns T, Lee S, and Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. A guide to methods and applications. Pp. 315-322. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds. PCR Protocols, Academic Press, Inc. San Diego, CA, USA. https://nature.berkeley.edu/brunslab/papers/white1990.pdf [ Links ]
Zhang Z, Schwartz S, Wagner L and Miller W. 2000. A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7:203-214. http://dx.doi.org/10.1089/10665270050081478 [ Links ]
Received: December 11, 2016; Accepted: January 17, 2017











 texto en
texto en