Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.35 no.1 Texcoco ene. 2017
https://doi.org/10.18781/r.mex.fit.1607-3
Scientific articles
Severity of a Phytophthora capsici isolate in chayote Sechium edule plants at growth chamber level
1Estudiante de Doctorado del Programa de Edafología del Colegio de Postgraduados, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, edo. de México. C.P. 56230. México. Correo electrónico: mauricio@colpos.mx
2Laboratorio de Interacción Molecular Planta-Microorganismo, Área de Microbiología, Programa de Postgrado en Edafología del Colegio de Postgraduados, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, edo. de México. C.P. 56230. México. Correo electrónico: despinos@colpos.mx
3Programa de Fitopatología del Colegio de Postgraduados, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, edo. de México. C.P. 56230. México. Correo electrónico: olgago@colpos.mx
4Programa de Posgrado en Innovación en el Manejo de Recursos Naturales, Colegio de Postgraduados, Campus San Luis Potosí, Iturbide No. 73, Salinas de Hidalgo, San Luis Potosí. C. P. 78600. México. Correo electrónico: jocadena@colpos.mx
5Programa de Fruticultura del Colegio de Postgraduados, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, edo. de México. C.P. 56230. México. Correo electrónico: larevalo@colpos.mx
6Laboratorio de Nutrición Vegetal, Programa de Edafología del Colegio de Postgraduados, Km 36.5 Carretera. México-Texcoco, Montecillo, Texcoco, edo. de México. C.P. 56230. México. Correo electrónico: tlibia@colpos.mx
7Área de Microbiología, Programa de Edafología del Colegio de Postgraduados, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, edo. de México. C.P. 56230. México. Correo electrónico: juliandm@colpos.mx.
The severity of Phytophthora capsici in chayote plants was determined and a scale of severity is proposed, given its recent confirmation as a causative agent of disease. Plants with wilting symptoms were sampled from two commercial chayote orchards in Huatusco, Veracruz, Mexico. The isolated oomycete, from fruits and stems, was characterized morphological and molecularly, which was identified as P. capsici. Chayote plants developed in growth chamber were inoculated with 1, 3 y 6 X 105 zoospores 15 days after sowing. Necrosis of the transition zone between the stem and roots, the percentage of wilted leaves and wilting of the plant were evaluated. Three days after inoculation (dai), 2.3 to 3.0 cm of necrosis length and 50 % of wilted leaves were observed. At five day, necrosis was 2.5 to 3.15 cm with 80 % of wilted leaves. At seven day, necrosis was 2.67 to 3.3 cm with 100 % wilted leaves (dead plant). Based on the observed symptoms, a severity scale was designed to evaluate the progression of damage P. capsici in chayote plants grown under controlled conditions.
Key words: Inoculum level; Necrosis in the transitional zone between the stem and roots; leaf wilting
Se determinó la severidad de Phytophthora capsici en plantas de chayote y se propuso una escala de severidad, dada su reciente confirmación como agente causal de enfermedad. Se muestrearon plantas con síntomas de marchitez en dos huertos comerciales de chayote en Huatusco, Veracruz, México. El oomiceto aislado, a partir de frutos y tallos, fue caracterizado morfológica y molecularmente e identificado como P. capsici. Plantas de chayote desarrolladas en cámara de crecimiento fueron inoculadas con 1, 3 y 6 x 105 zoosporas 15 días después de la siembra. Se evaluaron la necrosis de la zona de transición entre el tallo y la raíz, el porcentaje de hojas marchitas y la marchitez de la planta. A los tres días después de la inoculación (ddi) se observó de 2.3 a 3.0 cm de longitud de la necrosis y 50 % de hojas marchitas. A los cinco ddi, la necrosis fue de 2.5 a 3.15 cm y 80 % de hojas marchitas. A los siete ddi, la necrosis fue de 2.67 a 3.3 cm con 100 % de hojas marchitas (planta muerta). Con base en los síntomas observados, se diseñó una escala de severidad para evaluar el avance del daño de P. capsici en plantas de chayote bajo condiciones controladas.
Palabras clave: Nivel de inóculo; Necrosis en la zona de transición entre el tallo y las raíces; Marchitez foliar
Introduction
The genus Phytophthora is one of the causes of soil diseases with the greatest economic impact in the world (Lamour et al., 2012). This pathogen is highly dynamic and destructive, since it attacks roots, stems, leaves, and fruits of a large variety of vegetable crops (Lamour et al., 2012). It also has sexual and asexual cycles that make it difficult to control the disease it causes (Ploetz et al., 2002).
Initially, Rivera et al. (1992) and Olguín (2010) reported Pythium sp., Fusarium oxysporum and F. sambucinum as causative agents of the rotting of chayote in Costa Rica and Mexico, respectively. Later, Olguín et al. (2013) identified Phytophthora capsici as the causative agent of rotting in the transition area between the stem and the roots of the chayote plants. This oomycete is present in the soils of the production areas of the state of Veracruz, Mexico, where humidity levels are high.
The area of Huatusco, Veracruz, Mexico, in particular, is one of the most important areas in the production of chayote (Sechium edule (Jacq.) Sw.) worldwide. Recently in plants in orchards there have been stains on leaves with symptoms of wilting due to the rotting of the transition área between the stem and the root, which affects the absorption of water, leading to the death of the plant (Cadena-Iñiguez et al., 2006 y 2011; Cadena-Iñiguez and Arévalo-Galarza, 2008; Lira, 1992).
The plant yield for the chayote plant is of approximately 130 ton of fruit ha-1 in commercial plantations with densities of 100 to 128 plants ha-1. Production per plant is of approximately 47 to 120 kg in a period of six months; therefore the death of several plants per hectare brings significant economic losses (Cadena-Iñiguez et al., 2008). On a national scale, from 2010 to 2014 the average chayote production was 162,600 tons (SIAP, 2016).
However, little is known on the severity induced by P. capsici, given its recent dada etiological confirmation. In this sense, taking the measurements and quantitative and qualitative characteristics of the intensity of a disease (incidence or severity), is a crucial requirement in basic epidemiological studies. Using this information, loss estimation models could be produced in the future, along with the economic importance of the damage (Campbell and Madden, 1990; Kranz, 1988).
Due to the above, the aims of this work were to determine the degree of severity of P. capsici in S. edule plants at growth chamber level, with a controlled environment, and to create a visual scale of severity for its use and evaluation in production systems. The intention of this is to contribute to laying the foundations for a disease control system.
Materials and methods
Sampling
The sampling area corresponded to two commercial orchards in the municipal area of Huatusco, Veracruz, Mexico, located in the coordinates 19° 7´23.1” N, 97° 0´ 5.2” W, and 19° 7´ 21.6” N, 97° 0´ 4.9” W, at an altitude of 1,333 masl. The types of soils present in the sampling area were Luvisols and Cambisols. The climate is semiwarm-humid with an average temperature of 18.8ºC; average annual rainfall is 1,763 mm and the characteristic vegetation is of the evergreen subtropics. In December 2014, samples were gathered of chayote plants, var. Ventlali with symptoms of characteristic rotting (loss of turgidity, softening of plant tissue, presence of mycelia, and strong smell) in fruits and the transition area between stem and root. The samples were transported to the lab in plastic bags, which were labeled and placed in a cooler.
Isolation and identification of microorganisms related to the disease
Ten samples were taken of both fruit and of the transition area between stem and root, from each of the two areas of study. In this case, the number of samples taken from each location was standardized, unlike reports by Olguín et al. (2013). The infected tissue of chayote plants and fruits was washed with distilled water and cut into 6 sections of approximately 5 mm in length. The result was 60 sections (30 of fruit and 30 of the stem-root transition area). Likewise, 5 asymptomatic samples taken from fruits and the stem-root transition area were analyzed. The sections were disinfected with a commercial sodium hypochlorite solution 1 % for 1 min; they were rinsed several times with sterile distilled water, and dried with sterile paper towels. Later, the sections were cultivated in water-agar plates (acidified with lactic acid at 25 %) and incubated at 28 °C. Once myceliar growth was observed, using a hole puncher, discs of 0.5 cm in diameter were taken from the developed cultures, and there were replanted in a V8 juice-agar (V8) and potato dextrose agar (PDA), and incubated at 28°C for 48 h (French and Hebert, 1980; French-Monar et al., 2006). The oomycete developed was purified in V8-agar medium plate using monosporangia. The qualitative and quantitative characteristics of the asexual structures (sporangiophores and sporangia) of the oomycete were analyzed from semipermanent preparations with the use of a compound microscope (OLYMPUS. Mod: CX31RBSFA). The sporangia were measured after 10 days of incubation in V8 medium. With the result of 100 measurements, we identified the oomycete using the descriptions by Newhook et al. (1978), Stamps (1985), O’Donnell (1992), and Gallegly and Hong (2008). The rest of the fungal isolations present in the samples were purified by transferring cultures, and were identified using the taxonomic keys of Barnett and Hunter (1998).
Pathogenicity test of the isolated chayote oomycete
The oomycete with the highest frequency (76 %) was used to carry out the pathogenicity test. The chayote fruits were washed with soap and water, then disinfested with alcohol at 70 %. Using a blade, two cuts were made, each 1 cm deep, inside of which we placed a culture medium disc, of 0.5 cm in diameter, which contained the oomycete. Eighteen chayote fruits were inoculated. The control fruits were only added growth medium without oomycete growth. Afterwards, the inoculated fruit were placed in a wet paper towel on top of a base of styrofoam. The fruits were kept inside sealed plastic bags. The paper towel was added sterile distilled water to maintain moisture saturation conditions in the system.To stimulate the growth of the oomycete, the incubation temperature was kept between 24 and 27 °C, with a photoperoid of 12-12 h. Once the fruits were invaded by the mycelium of the oomycete, the latter was reisolated in V8 medium plates. The morphology of the cultures obtained was compared with that of the original isolation in commercial orchards. The pathogenicity test was performed twice.
DNA amplification and sequencing of the oomycete
After four days’ growth of the oomycete in V8 medium plates, a portion of approximately 5 mm of mycelium was tekan and placed in a 200 μL test tube with30 μL of the lysis solution Lyse N Go (Pierce, USA). The tube was then heated at 95 °C for 5 min and centrifuged for 10 min at 5,000 x g. Finally, 5 μL of supernatant were taken to carry out the amplifications of the ITS by PCR.
For the amplification of the ITS area, we used the universal primers ITS5 (5’-GGAAGTAAAAGTCGTAACAAGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC) (White et al., 1990), which amplify a fragment of 580 pairs of bases, approximately (pb). Another pair of primers used was ITS5 and NL4 (5’-GGTCCGTGTTTCAAGACGG- 3’) (O’Donnell, 1992), to amplify a fragment of 1,100 pb, which included the region of the ITS and ~600 pb of the gene 28S rRNA. The reaction mixture for PCR was prepared in a final volume of 25 μL, containing the enzyme 1X Taq DNA polymerase, 0.8 mM deoxynucleotide triphosphates (0.2 mM each), 100 ng DNA, 20 pmol of each primer, and 2 units of GoTaq DNA (Promega, USA). The amplifications were carried out with an initial denaturation cycle at 95 °C for 2 min; 35 denaturation cycles at 95 °C for 1 min, alignment at 57 °C for 1 min, and a final extension at 72 °C for 2 min; finally, one amplification cycle at 72 °C for 10 min (Silva-Rojas et al., 2009).
All the PCR reactions were carried out in a thermocycler (Model: TECHNER TC-512, brand: Bibby Scientific). The amplifications were verified by electrophoresis in an agarose gel at 1.2 % prepared with the buffer 1X TAE (Tris-AcetatoEDTA) and run at 87 V cm-3 for 1 h. The gel became stained with ethidium bromide (3 mg L-1), and the bands were viewed in a UV transilluminator (Model: GL-3120, brand: Scientific). The amplified products were cleaned using the purification kit (Model: QIAquick PCR, brand : Qiagen), following instructions by the manufacturer. These products were sequenced in both directions using an automized system DNA sequencing system (Model 3730XL, brand: Applied BioSystems), to ensure no incorrect nucleotide readings.
Comparing the ITS and LSU sequences
The sequences form both regions were assembled and edited using BioEdit version 7.0.5 (Hall, 1999), used to create a consensual sequence. This sequence was compared with sequences deposited in the GenBank of the National Center for Biotechnology Information (NCBI), with the option BLASTN 2.2.19 (Brown et al., 2014; Zhang et al., 2000).
Evaluation of the severity of the disease Planting the plant material
For the pregermination of the chayote fruits, we used a 40 x 60 cm black, plastic bag, in which 20 fruits were placed for 20 days. The fruits had previously been washed with soap and rinsed with a 0.6 % chlorine solution. Once the root and stem sprouted, each fruit was placed in a black plastic bag (25 x 25 cm) containing 2,500 cm3 of substrate composed of sand-agrolite-vemicompost (40:40:20), treated twice with steam under 120 pounds of pressure for three hours. The fruits were kept in a growth chamber (Model CEL 37-14, brand: SHERER,) at a temperature of 26±1 °C, with a photoperiod of 14 light hours and 10 of darkness, and a light intensity of 6, 768 lux of fluorescent light. The plants were initially watered at field capacity, and later, irrigated every three days with 300 mL of water per plant until the end of the experiment.
Preparation of the inoculant
The oomycete was cultivated in V8 medium plates for 7 days at 28 °C. Each Petri dish was added an isotonic solution of sodium chloride at 0.9 % (AbbottMR) for 10 min. The isotonic solution was poured out, and using a dissection needle, the dish was divided in four parts. Each part of the medium containing the oomycete was placed in a sterile Petri dish. Sterile distilled water was then added, until it covered the medium, and the dishes were placed under a cold white light at 26 °C for 48 h. The release of the zoospores was then induced, exposing the Petri dishes to two temperatures (4 °C and room temperature) for 30 min. Finally, the number of zoospores was quantified using a cytometer (Marienfeld®). Inoculation was performed when the plants had an average of 16 leaves (15 days after planting) applying a suspension of 1, 3 and 6 x 105 zoospores to each plant in the transition zone between stem and root. Each treatment (level of inoculant) consisted of four plants and the control. To determine the level of inoculant, the minimum level of of P. capsici used by Trujillo-Viramontes et al. (2005) in JEP chili plants, was taken into consideration.
The progress of the damage induced by P. capsici was measured in cm from the transition area between stem and root. Apart from the length of the necrosis, we determined the number of wilted leaves and guides, as well as the presence of mycelia in the seed 3, 5 and 7 days after inoculation (ddi). Based on the symptoms and measurements observed, as in the case of other crops (Navarrete and Acosta, 1999), we designed a scale of severity to evaluate the damage of P. capsici in chayote plants in growth chambers under controlled conditions. The pathogenicity evaluation test was set up on three occasions with similar results.
Statistical analysis
The data of each variable underwent an analysis of variance and comparison of average, using the Tukey test (p≤ 0.05) (SAS Institute, 2002-2003).
Results and Discussion
Detecting Phythopthora in diseased chayote plants
Out of the 60 sections (30 fruit and 30 stem sections) planted in V8 agar, in 46 (76 %) Phythopthora sp grew, in 9 (15 %) Fusarium, and in 3 (4 %), Alternaria. The rest of the sections corresponded to saprophytic fungi and bacteria. Babadoost and Pavon (2013) indicate that the high frequency of P. capsici, as well as its virulence, determine its role as a causative agent of the disease. However, pathogenicity tests are required for each host plant. Rivera et al. (1992) and Olguín (2010) initially reported Pythium sp., Fusarium oxysporum and F. sambucinum as causative agents of rotting of chayote in Costa Rica and Mexico, respectively. However, the low frequency of Fusarium found in this investigation could indicate that the wilting of the chayote plants is not induced by this fungus.
Morphological characterization of the rotcausing oomycete
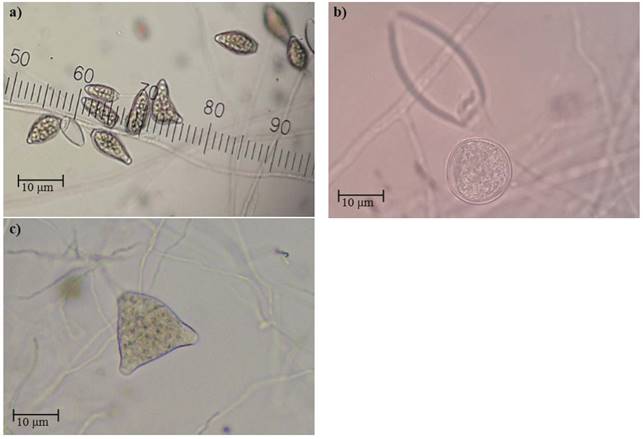
The cultures developed in V8 medium plates presented an abundance of aerial hyphae, of considerable length, cotton-like, little radial, and compact. The mycelium was cenocytic, hyaline, branched, and with an irregular diameter. The sporangia produced in V8 and sterile water medium plates (Figure 1a) presented different sizes and shapes: ovoid, starchy (on average, 55 x 32 µm), with a prominent papilla, expired, mainly with only one papilla (seldom were there any with two found) (Figure 1b), long and persistent peduncle, sometimes symmetrical and other times, assymetrical and with clamidospores (Figure 1c). The morphometric data of the sporangia indicate the presence of Phytophthora capsici in the transition area between stem and roots and the fruits of the chayote, which agrees with reports by Olguín et al. (2013), who indicated that this oomycete is the causative agent of rotting in chayote plants.
Pathogenicity test for Phytophthora sp. in plantlets in growth chambers with a controlled environment
The oomycete inoculated in the chayote fruits indicates the same characteristic rotting of the fruits it was isolated from: loss of turgidity, rotting and the presence of white mycelia 5 days after inoculation (Figure 2a). Under humid conditions (80 to 100 %) the growth of the pathogen was conspicuous (Figure 2b). In pieces of fruits with mycelia placed in sterile distilled water and under continuous white light (Figure 2c), we observed abundant sporangia, irregularly spread out (Figure 2d).

Figure 2. a) Chayote fruits with white mycelia, loss of turgency, and symptoms of rotting; b) Fruits under conditions of humidity, between 80 and 100 %, with a conspicuous growth of the pathogen; c) pieces of fruit with mycelia, placed in sterile distilled water under continuous white light; and d) sporangia irregularly spread out.
Analyses of DNA sequences
The four sequences obtained in this study were placed in the GenBank-NCBI under the accession numbers KM087089, KM087090.1, KM087091, and KM087092. DNA sequencing confirmed that the isolated strain of the necrotic chayote tissue samples gathered in the area of study belonged to Phytophthora capsici, with an identity of 99 and 100 % with respect to the sequences of different isolations of the GenBank. It is worth mentioning that the strain with accession number KM087090.1, which showed an identity of 100 % (Table 1), was used in the severity test.
Severity of P. capsici in chayote plants
No significant differences (α=0.05) were observed between the three levels of P. capsici inoculant for the variebles number of wilted leaves, necrosis in the transition area between stem and roots, and wilting of chayote plants (Figure 3a, b, and c). When the plants were inoculated with P. capcisi, differences were observed with the control plants (not inoculated), like in reports by Uribe-Lorio et al. (2014) in their work with chili pepper plants. The control plants remained healthy throughout the experiment. As in the present study, other authors (Trujillo-Viramontes et al., 2005) report that inoculation of 3 x 105 zoospores of the oomycete is enough to induce a response of the chili plant. Although no significant differences were found in the three levels of P. capsisci inoculant used, there were numerical differences. Probably, 3 x 105 zoospores per plant is the top threshold, above which damage in chayote plants may not be statistically significant.
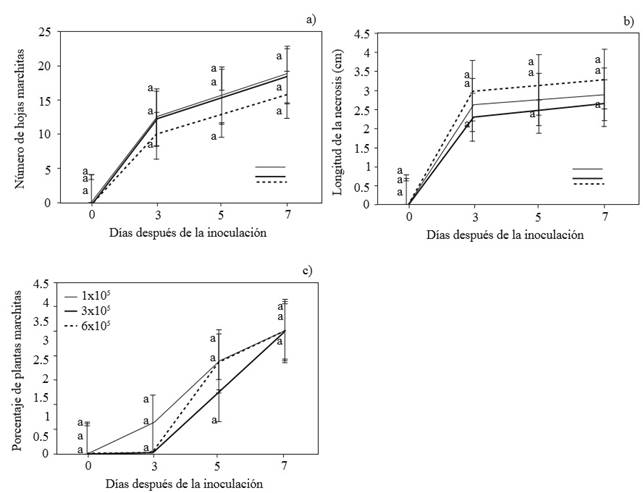
Figure 3. Number of wilted leaves (a), length of the necrosis in the transition area between the stem and the roots (b) and percentage of wilted chayote plants (c) inoculated with 1, 2, and 6 x 105 P. capsici zoospores. Each dot represents the average of 4 repetitions, Tukey (p≤ 0.05).
Using the data obtained, we built the disease progress curve. Regardless of the level of zoospores inoculated, at three ddi, we observed 50 % of wilted leaves, and 2.3 to 3.0 cm of necrosis in the transition area between stem and roots (Figure 4). At five ddi, wilting of leaves was 80% with the progress of necrosis at 2.5 to 3.15 cm and the presence of mycelia on the seed (the fruit). At seven ddi leaves presented 100 % wilting, necrosis from 2.67 to 3.3 cm, and the presence of mycelia in guides, seed, and roots. Therefore, the three levels of P. capsici inoculant were equally effective for the induction of wilting of chayote plants. Comparatively, the time of appearance of symptoms of the disease was lower than that reported by Olguín et al. (2013). The level of inoculation was crucial, while Olguín et al. (2013) used 11,500 sporangia mL-1, this investigation used 1, 3 and 6 x 105 zoospores mL-1.

Figure 4. Progress of the necrosis in the transition area between the stem and the roots of chayote plantlets three days after inoculation with 300,000 P. capsici zoospores.
The necrosis in the transition area between stem and roots was observed starting on day 3 after inoculation, regardless of the level of inoculant. Early necrosis may have accelerated plant wilting
At five ddi, plant wilting was observed to be above 50 %, also regardless of the level of inoculant, which shows the pathogenicity of the isolation studied, according to reports by Olguín et al. (2013). At seven ddi, necrosis development was, on average, 3.0 cm, which contributed to the wilting of plants at 100 %. Probably, the degradation of cell walls and middle lamella of the parenchyma tissue of the vascular system, typically caused by P. capsici, appeared in the transition area between stem and roots, which prevented the flow of water and nutrients for the adequate development of the plant (Li, et al., 2011; Wang et al., 2011).
The substrate used was crucial in the experiment, since it provided high humidity conditions which favored the development and spreading of P. capsici. High humidity levels favored the mobility of zoospores, ensuring the infection by the oomycete. Silva-Rojas et al. (2009) indicate that, indeed, this environmental factor is crucial for the spreading of P. capsici.
The progress curve of the disease (Figure 4) was the base from which to create a Severity Scale (Table 2), which can be used to evaluate damages produced by P. capsici in chayote under controlled conditions. The scale was validated on three occasions. The variables used to create the scale were: percentage of wilted leaves, length of necrosis in the transition area between stem and roots, percentage of wilted plant, and the presence of mycelia. This attempts to become a contribution for the basis of a system for the control of the disease induced by P. capsici in chayote plants.
Conclusions
The strain PCSE2, with the accession number KM087090.1 identified as P. capsici, isolated from diseased chayote plants in Huatusco, Veracruz, Mexico, induced symptoms from an inoculant level of 1 x 105 zoospores, resulting in the wilting and death of plants at 5 and 7 ddi. The inoculation of 3 x 105 zoospores promoted the presence of plants with symptoms of wilting after 3 ddi, probably because the necrosis in the transition area between stem and roots did not allow the flow of water and nutrients for the adequate development of the plant.
Although no significant differences (α=0.05) were observed between the P. capsici inoculant for the variables number of wilted leaves, necrosis in the transition area between stem and roots, and wilting of chayote plants, with the disease progress curve, we designed a scale of severity, as an initial tool to evaluate the damage produced by P. capsici in chayote plants grown under controlled conditions.
Acknowledgements
To the National Science and Technology Council (CONACYT)-National Quality Graduate Program (PNPC), for funding this work.
REFERENCES
Babadoost M and Pavon C. 2013. Survival of oospores of Phytophthora capsici in soil. Plant Dis. 97:1478-1483. http://dx.doi.org/10.1094/PDIS-12-12-1123-RE. [ Links ]
Barnett LH and Hunter BB. 1998. Illustrated Genera of Imperfect Fungi. Fourth Edition. The American Phytopathological Society. St. Paul. Minnesota, U.S.A. 218 p. Disponible en línea: http://www.apsnet.org/apsstore/shopapspress/pages/41922.aspx [ Links ]
Browm SP, Rigdon-Huss AR and Jumpponen A. 2014. Analyses of ITS and LSU gene regions provide congruent result on fungal community responses. Fungal Ecology. 9:65-68. doi:10.1016/j.funeco.2014.02.002 [ Links ]
Cadena-Iñiguez J, Ruiz PLM, Avendaño ACH, Cisneros VM, Soto HMR y Aguirre MJF. 2006. Origen y Biodiversidad de Sechium edule en el Estado de Veracruz México. Chapingo, Huatusco Veracruz, México. Revista Centro Regional Universitario Oriente pp. 1-16. [ Links ]
Cadena-Iñiguez J y Arévalo-Galarza ML. 2008. Rescatando y Aprovechando los Recursos Fitogenéticos de Mesoamérica. Montecillo, Texcoco, Estado de México. 16 p. Disponible en línea: http://gobernanzabiodiversidad.mx/bibliotecadigital/component/content/article/50-casos-de-estudio/989-gisem-rescatando-y-aprovechando-los-recursos-fitogeneticos-de-mesoamerica-volumen-1-chayote [ Links ]
Cadena-Iñiguez J, Arrazate ACH, Soto HMR, Ruiz PLM and Aguirre MJF. 2008. Intraspecific variation of Sechium edule in the state of Veracruz, Mexico. Genetic Resources and Crop Evolution. 55:835-847. DOI: 10.1007/s10722-007-9288-4 [ Links ]
Cadena-Iñiguez J, Soto HMR, Arévalo GML, Avendaño ACH, Aguirre MJF y Ruiz PLM. 2011. Caracterización bioquímica de variedades domesticadas de chayote Sechium edule (Jacq.) Sw. comparadas con parientes silvestres. Revista Chapingo Serie Horticultura, Vol. XVII, Edición Especial 2:45-55. Doi: dx.doi.org/10.5154/r.rchsh.2011.17.044 [ Links ]
Campbell CL and Madden LV. 1990. Introduction to Plant Disease Epidemiology. John Wiley and Sons. New York, USA. 532 p [ Links ]
French ER y Hebert TT. 1980. Métodos de investigación fitopatológica. 148 p. Instituto Interamericano de Ciencias Agrícolas (IICA), San José, Costa Rica. [ Links ]
French-Monar RD, Jones JB and Roberts PD. 2006. Characterization of Phytophthora capsici associated with roots of weeds on Florida vegetables farms. Plants Disease. 90:345-350. http://dx.doi.org/10.1094/PD-90-0345 [ Links ]
Gallegly ME and Hong C. 2008. Identifying species by morphology and DNA fingerprints. The American Phytopathological Society. St. Paul. Minnesota, U.S.A. 158 p. [ Links ]
Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41:95-98. Disponible en línea: https://www.google.com.mx/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwic3YOUlO3NAhVGqh4KHeokCuEQFggaMAA&url=http%3A%2F%2Fbrownlab.mbio.ncsu.edu%2FJWB%2Fpapers%2F1999Hall1.pdf&usg=AFQjCNEUNhUvahTBCOXPfD1PdvNFuRrQDA&sig2=OGI0P2DpO8w48wA7_ubq5A&bvm=bv.126130881,d.dmo&cad=rja [ Links ]
Kranz J. 1988. Measuring Plant Disease. In: Kranz J and Rotem J (eds.). Experimental Techniques in Plant Disease Epidemiology. Springer-Verlag Berlin, Heidelberg. pp. 35-50. DOI: 10.1007/978-3-642-95534-1_4 [ Links ]
Lamour KH, Stam R, Jupe J and Huitema E. 2012. The oomycete broad-host-range pathogen Phytophthora capsici. Molecular Plant Pathology 13(4):329-337. DOI: 10.1111/j.1364-3703.2011.00754.x [ Links ]
Li P, Feng B, Wang H, Tooley PW and Zhang, X. 2011. Isolation of nine Phytophthora capsici pectin methylesterase genes which are differentially expressed in various plant species. Microbiology 51: 61-70. DOI: 10.1002/jobm.201000317 [ Links ]
Lira R. 1992. Chayote (Sechium edule). In: Hernández Bermejo JE y León J. (Eds.). Cultivos marginados. FAO. Roma. pp. 77-82. [ Links ]
Navarrete NR y Acosta GJ. 1999. Reacción de variedades de frijol común a Fusarium spp. y Rhizoctonia solani en el Altiplano de México. Agronomía Mesoamericana 10(1): 37-46. [ Links ]
Newhook FJ, Waterhouse GM and Stamps DJ. 1978. Tabular key to the species of Phytophthora de Bary. Mycol. Pap. 143. 21 p. Commonwealth Mycological Institute, Kew, Surrey. England. [ Links ]
O’Donnell K. 1992. Ribosomal DNA internal transcribed spacer are highly divergent in the phytopathogenic ascomycete Fusarium sambucinum (Gibberella pulicaries). Current Genetics 22:213-220. DOI: 10.1007/BF00351728 [ Links ]
Olguín, H. G. 2010. Identificación y caracterización morfológica, cultural y molecular de hongos asociados a Sechium edule (Jacq.) Sw. en México. Tesis de licenciatura. Universidad Autónoma Chapingo. Texcoco, Edo. de México. 118p. [ Links ]
Olguín FG, Valdovinos PG, Cadena-Iñiguez J y Arévalo GML. 2013. Etiología de la marchitez de plantas de chayote (Sechium edule) en el Estado de Veracruz. Revista Mexicana de Fitopatología. 2:161-169. Disponible en línea: http://www.redalyc.org/articulo.oa?id=61231509007 [ Links ]
Ploetz R, Heine G, Haynes J and Watson M. 2002. An investigation of biological attributes that may contribute to the importance of Phytophthora capsici as a vegetable pathogen in Florida. Ann Appl Biol. 140:61-67. DOI: 10.1111/j.1744-7348.2002.tb00157.x [ Links ]
Rivera G, Brenes F y Gamboa W. 1992. Hoja divulgativa sobre las principales enfermedades del chayote (Sechium edule (Jacq.) Swartz). El Cocoro, COOPECHAYOTE R.L. 2p. [ Links ]
Stamps DJ. 1985. Phytophthora capsici. Description of pathogenic fungi and bacteria. No. 836. 2 p. Commonwealth Mycological Institute, Kew, Surrey. England. [ Links ]
SAS-Institute. 2002-2003. Statistical Analysis System Institute. Software Version 9.1, SAS Institute, Vary, NC, USA. DOI: 10.2307/2234420 [ Links ]
SIAP. 2016. Servicio de Información Agroalimentaria y Pesquera. Sistema de Información Agroalimentaria de Consulta (SIACON) 1980-2014. SAGARPA. Consultado en agosto de 2016. [ Links ]
Silva-Rojas, HV, Fernández-Pavía SP, Góngora-Canul C, Macías-López BC y Ávila-Quesada GD. 2009. Distribución espacio temporal de la marchitez del chile (Capsicum annuum L.) en Chihuahua e identificación del agente causal Phytophthora capsici Leo. Revista Mexicana de Fitopatología. 27(2): 134-147. Disponible en línea: https://www.google.com.mx/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwjL4Petmu3NAhXKsB4KHazCBnQQFggaMAA&url=http%3A%2F%2Fwww.scielo.org.mx%2Fscielo.php%3Fscript%3Dsci_arttext%26pid%3DS0185-33092009000200006&usg=AFQjCNEJeKA09eSU3tD-H3MlYQ2IR-WxaoA&sig2=p4zS4arDdXNL5nhV52Cplw&bvm=bv.126130881,d.dmo [ Links ]
Trujillo-Viramontes, F, Zavaleta-Mejía E, Rojas-Martínez RI, y Lara J. 2005. Tiempo de inoculación y nivel de inóculo, factores determinantes para el rompimiento de resistencia a Phytophthora capsici inducido por Nacobbus aberrans en chile (Capsicum annuum). Nematropica 35:37-44. https://www.google.com.mx/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwjL9bb98_nOAhUBxoMKHdVzDQAQFggbMAA&url=http%3A%2F%2Fjournals.fcla.edu%2Fnematropica%2Farticle%2Fdownload%2F69712%2F67372&usg=AFQjCNHLz2Z4ZkeOshK7YiIX8SSHFPmUXw&sig2=L_lNCZNgDJQhUiYt0542oQ [ Links ]
Uribe-Lorio L, Castro BL, Arauz CF, Henríquez HC y Blanco MM. 2014. Pudrición basal causada por Phytophthora capsici en plantas de chile tratadas con vermicompost. Agron. Mesoam. 25(2):243-253. DOI: http://dx.doi.org/10.15517/am.v25i2.15427 [ Links ]
Wang H, Li F and Zhang X. 2011. Comparison of expression, purification and characterization of a new pectatelyase from Phytophthora capsici using two different methods. BMC Biotechnology 11:32. DOI: 10.1186/1472-6750-1132 [ Links ]
White TJ, Bruns T, Lee S and Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Pp. 315-322 In: PCR Protocols: A Guide to Methods and Applications, Eds. Innis MA, Gelfand DH, Sninsky JJ and White TJ. Academic Press, Inc., New York. doi:10.1016/B978-0-12-372180-8.50042-1 [ Links ]
Zhang; Z., Schwartz S, Wagner L and Miller W. 2000. A greedy algorithm aligning DNA sequences. Journal of Computational Biology. 7:203:214. doi: 10.1089/10665270050081478 [ Links ]
Received: June 12, 2016; Accepted: September 14, 2016











 texto en
texto en