Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.34 no.3 Texcoco Set. 2016
https://doi.org/10.18781/r.mex.fit.1604-3
Phytopathological notes
Histopathology of the infection process of Colletotrichum truncatum in papaya and pea leaves
1Centro de Investigación en Alimentación y Desarrollo, A. C. Coordinación Culiacán. Carretera Culiacán-Eldorado km 5.5, Campo el Diez, CP. 80110, Culiacán, Sinaloa, México.
Colletotrichum truncatum is a pathogenic fungus causing anthracnose in several hosts; the infection process of this pathogen in papaya leaf as well as studies of the same strain of C. truncatum in different hosts is unknown. The aim of this study was to describe the infection process of a Colletotrichum truncatum strain by histological techniques in papaya and pea leaves. In both hosts, direct penetration occurred through appressoria around 20 h after inoculation (hai). In papaya, intercellular hyphae grew at 24-60 hai; the necrotrophic colonization began at 60 hai; intracellular hyphae grew in mesophyll cells causing extensive cellular degradation; in contrast, in pea primary infection hyphae began at 36 hai, secondary infection hyphae (necrotrophic state) began at 72 hai. In both hosts, the acervuli were observed at 96 hai, which was associated with typical anthracnose lesions. C. truncatum behaved as intramural subcuticular pathogen in papaya and as intracellular pathogen in pea; so that, the infection strategy is dependent on the host.
Keywords: Carica papaya; Pisum sativum; intramural pathogen; intracellular pathogen
Colletotrichum truncatum es un hongo patógeno causante de antracnosis en diversos hospedantes; se desconoce el proceso de infección de este patógeno en hojas de papaya, así como el comportamiento de una misma cepa de C. truncatum en distintos hospedantes. El objetivo de este estudio fue describir, mediante técnicas histológicas, el proceso de infección de una cepa de C. truncatum en hojas de papaya y chícharo. En los dos hospedantes, la penetración del hongo ocurrió de manera directa por medio de apresorios alrededor de las 20 h después de la inoculación (hdi). En papaya, las hifas crecieron intercelularmente a las 24-60 hdi; la colonización necrotrófica inició a las 60 hdi; hifas intracelulares crecieron en células del mesófilo causando una extensiva degradación celular; en contraste, en chícharo se observaron hifas primarias de infección a partir de las 36 hdi, las hifas secundarias de infección (estado necrótrofo) se observaron a las 72 hdi. En ambos hospedantes, los acérvulos se observaron a las 96 hdi, lo cual se asoció con la producción de lesiones típicas de antracnosis. Colletotrichum truncatum se comportó como patógeno intramural subcuticular en papaya y como hemibiótrofo intracelular en chícharo; por lo que la estrategia de infección es dependiente del hospedante.
Palabras clave: Carica papaya; Pisum sativum; patógeno intramural; patógeno intracelular
Colletotrichum spp., causal agents of anthracnose, affect a wide range of hosts in both pre- and postharvest. The species of Colletotrichum use different infection strategies: intramural subcuticular or intracellular, or even both in different organs of the same host (Perfect et al., 1999; Diéguez-Uribeondo et al., 2005). Infection strategies are correlated with the specificity in the host (Pring et al., 1995), which is why it is important to know the infection process to determine the biological cycle of the disease, which could suggest the adequate use of a control strategy once we know the period in which the pathogen is developed in the host.
Anthracnose is one of the main diseases in papaya crops. In Mexico, reported losses are above 50 % (Torres-Calzada et al., 2012). Colletotrichum truncatum was reported in 2008 as the cause of anthracnose in papaya (Carica papaya) in Mexico (Tapia-Tusell et al., 2008), with an incidence of up to 40 % (Torres-Calzada et al., 2012). This species uses different infection mechanisms, since it behaves like an intracellular hemibiotrophic pathogen in pea (Pisum sativum), broad bean (Vicia faba), and lentil plants (Lens culinaris) (O´Connell et al., 1993; Latunde-Dada and Lucas, 2007), and as a subcuticular intramural pathogen in chili pepper (Capsicum annuum), chickpea (Cicer arietinum), peanut (Arachis hypogaea), and bean plants (Phaseolus vulgaris) (Pring et al., 1995; Ranathunge et al., 2012). Although there are diverse studies related to this species, the process of pathogenesis is unknown for papaya leaves; also, there is scarce information describing the infection process of a same strain of the pathogen in different hosts. The aim of this study was to describe the histological changes presented during the infection of papaya and pea leaves by C. truncatum.
We used healthy Maradol papaya (three months old) and Lincoln pea leaves (three months old). The CCM strain of C. truncatum was used as an inoculum. Beforehand, it was isolated from a papaya fruit with anthracnose symptoms, purified using monosporic cultivation, and characterized molecularly (KF147902). The conidia suspension was prepared at a concentration of 1x106 spores/mL from a culture grown for 7 days in PDA. The abaxial surfaces of 15 papaya leaves and 30 pea leaves, previously disinfected with ethanol at 70 %, were inoculated in six and three points of 1 cm2 respectively, by depositing 10 µL of inoculant. As a control treatment, 10 µL aliquots of sterile distilled water were placed on five papaya leaves and 10 pea leaves. The papaya leaves were placed in polyethylene bags with pieces of paper towels moistened with distilled water (Pandey et al., 2012), and the pea leaves were placed in glass Petri dishes with N° 2 Whatman filter paper, sterilized and moistened with distilled water to increase the relative humidity. All leaves were incubated at 25 °C ± 2 °C for six days. Sections of de 5 mm of tissue were taken from the inoculated areas at 2, 4, 6, 9, 12, 18, 20, 24, 30, 36, 48, 60, 72, 96, 120, and 148 hours after inoculation (hai), and placed in plastic casettes to include them in paraffin. The samples were submerged in an FAA fixing solution (10 % formaldehyde, 5 % acetic acid, 50 % ethanol at 96%) for at least 24 h. Later, the tissues were gradually dehydrated with ethyl alcohol (50, 70, 80, 96, and 100 %) and filtrated in (Leica) paraffin for 3 h, after having been transferred in absolute alcohol-xylene (v/v) and xylene (twice) (Rodríguez-López et al., 2013). The samples were kept in the solutions for 3 h and finally shaken twice in a centrifugal tissue processor (Thermo Scientific, STP 120, GER).
In an embedding workstation (Thermo Scientific, HistoStar, UK), the samples were placed in metallic molds with molten paraffin, they were added a in plastic casettes, molten paraffin was added, and they were left to cool. The samples were cut longitudinally and transversely to the 6 µm thick midrib in a semiautomatic microtome (Thermo Scientific, Microm HM340E, GER). The sections obtained were dyed with safranin (Sigma) at 1 % in ethyl alcohol at 50 % and fast green (Sigma) at 1 % in ethanol at 96 %, they were mounted on Entellan resin (Merck) and left to dry for 24 h (Casarrubias-Carrillo et al., 2002). The lamellae were observed under a Carl Zeiss Axiostar Imager A2 optical microscope with an integrated camera to identify tissue damage. Thirty-six observations were carried out for every sample. The appressoria were measured using the software ZEN 2012 (blue edition).
Between 2 and 18 hai only non-germinated C. truncatum conidia were observed on the surface of the leaf. Germination began after 20 hai, and on the apical end of the germination tubes, dark brown melanized appressoria were formed, balloonlike to irregularly shaped, and on average, 7.67 x 5.26 µm (Figura 1A). C. truncatum formed melanized appressoria to improve adhesion and penetration, by mechanical action and enzyme lysis (Kubo et al., 2000). The first infection hyphae emerged from the appressoria 24-48 hai (Figure 1B). In the interval of 24-60 hai, intramural hyphae were observed, which grew on cell walls (Figure 1C). At 60 hai we observed the start of the necrotrophic state, since intracellular hyphae were observed (Figure 1D); also, cavities were observed (Figure 1E), which according to Pring et al. (1995) are indicative of cell wall degradation. The acervuli (Figure 1E) were related to the dark coloration on the surface of the host in contrast with the control leaf, which showed no symptoms (Figure 1F). At 96 hai, the pathogen completed its life cycle and produced acervuli with conidia, which are the inoculum source for infections in new tissues (Ranathunge et al., 2012). The cell damage and the production of acervuli were associated with the appearance of dark, brown to black semicircular lesions, on the third day after inoculation (Figure 2A), in contrast with the control leaf (Figure 2B).
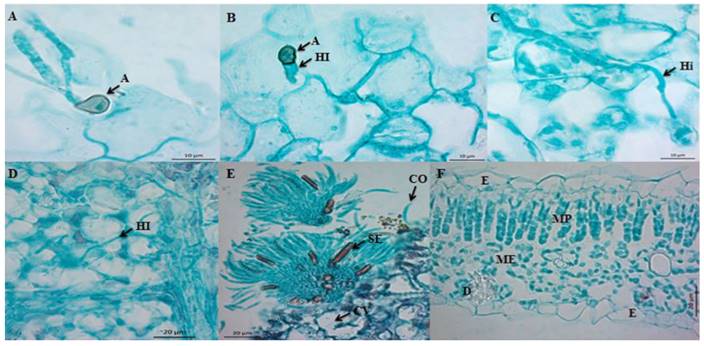
Figure 1. Microphotographs of the infection process of C. truncatum on detached Maradol papaya leaves. A) Longitudinal cut of a leaf 20 hai, formation of melanized appressoria (A) from polar germination tubes from conidia. Bar = 10 µm. B) Longitudinal cut (paradermal) of a leaf 24 hai. First infection hyphae (IH) emerging from appressoria (A). Bar = 10 µm. C) Longitudinal cut (paradermal) of a leaf 60 hai. Intramural hypha (iH). Bar = 10 µm. D) Longitudinal cut (paradermal) of a leaf 60 hai. Intracellular hypha (HI). Bar = 20 µm. E) Transversal cut of a papaya leaf infected by C. truncatum 96 hai, formation of acervulus in the host, conidiophores (CO), embedded setae (SE), and cavities (CV). Bar = 20 µm. F) Transversal cut of a healthy leaf. Epidermis (E), palisade mesophyll (MP), spongy mesophyll (ME), and druse (D). Bar = 20 µm.
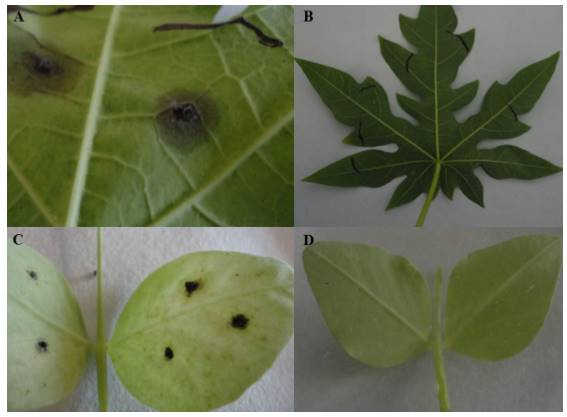
Figure 2. Anthracnose symptoms by the CCM strain of C. truncatum in different hosts. A) Maradol papaya leaf with symptoms of anthracnose 148 hai; B) Control papaya leaf; C) Lincoln pea leaf with anthracnose symptoms 148 hai; D) Control pea leaf.
The infection strategy of C. truncatum in pea leaves was different. The conidia did not germinate until 12 hai. At 18 hai, the germination tubes emerged in a polar and lateral way. From the germination tubes, dark brown melanized appressoria were formed, balloonlike to irregularly shaped, and measuring 8.24 x 5.92 µm on average. The first infection hyphae emerged from the appressoria between 18 and 20 hai, unlike what occurred in the papaya leaf (Figure 3A). We observed lobulated, intracellular branched (primary) hyphae between 36 hai and 60 hai (Figure 3B). Although the primary infection hyphae appeared in the cell lumen, no lysis was observed. The main characteristic of the intracellular invasion is the formation of primary infection hyphae, which can have different morphologies depending on the species (O´Connell et al., 2000). The primary hypha of C. truncatum is multilobulated, branched, and large (Latunde-Dada and Lucas, 2007). During the biotrophic state, the primary hypha was invaginated towards the cell lumen (Figure 3B). According to O’Connell et al. (2000), the primary hypha forms an interfacial matrix composed of glycoproteins, rich in proline and hydroxyproline that separates the plasmatic membrane from the host.
We observed thin infection hyphae (secondary hyphae), which emerged from the primary infection hyphae 72 hai (Figure 3C). According to Bhadauria et al. (2011), the transition from the biotrophic state to the necrotrophic state is associated with the growth of thin secondary hyphae, which soften the host’s tissues. The secondary hyphae colonized cells intracellularly, which caused cell damage with the formation of cell cavities (Figure 3D). According to Casarrubias-Carrillo et al. (2002) cell cavities indicate cell dissolution. The formation of acervuli began 96 hai until 148 hai and they contained conidiophores, melanized setae and conidia (Figure 3E), in contrast with the healthy leaf (Figure 3F). Cell damage was associated with the symptoms of anthracnose (Figure 2C), in contrast with the control leaf, which presented no symptoms (Figure 2D). Unlike the study carried out by O’Connell et al. (1993), the strain of C. truncatum used in this investigation produced acervuli in pea. The acervuli with conidia were produced starting at 96 hai, thus concluding the life cycle of the pathogen. The symptoms of anthracnose in pea leaves at 148 hai were limited in extension, in contrast to those observed in papaya, probably due to the strain originally being isolated from the papaya fruit.
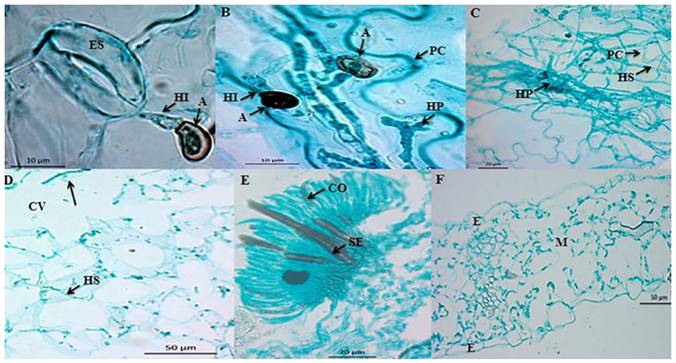
Figure 3. Microphotographs of the infection process of C. truncatum in detached Lincoln pea leaves. A) Longitudinal cut (paradermal) of a leaf 18 hai. First infection hyphae (HI) emerging from appressoria (A). Bar = 10 µm. B) Longitudinal cut (paradermal) of a leaf 36 hai. Infection hyphae (HI) emerging from appressoria (A), as well as from a lobulated primary hypha (HP) limited by the cell wall (CW) of the host. Bar = 10 µm. C) Longitudinal cut (paradermal) of a leaf 72 hai. Secondary hyphae (SH) emerging from primary hypha (HP), which penetrate the cell walls (CW) of the host. Bar = 20 µm. D) Longitudinal cut (paradermal) of a leaf 72 hai. Secondary infection hyphae (HS) invading intracellularly. Cavities (CV) are observed during the necrotrophic state. Bar = 50 µm. E) Transversal cut of a pea leaf 96 hai. Formation of acervulus on the surface of the host. Presence of conidiophores (CO) and embedded setae (SE). Bar = 20 µm. F) Transversal cut of a healthy leaf. Epidermis (E) and mesophyll (M). Bar = 50 µm.
Acknowldgements
To the project 2011-163213 “El manejo integral del cultivo de papaya en México, un acercamiento innovador” financed by SAGARPA, to CONACyT for funding the studies for I. Rojo-Báez, and to Dr. Apolinar Santamaría Miranda of the CIIDIRIPN Sinaloa for her support in performing the histopathological analysis in her laboratory.
REFERENCES
Bhadauria V, Banniza S, Vandenberg A, Selvaraj G and Wei Y. 2011. Cataloging proteins putatively secreted during the biotrophy-necrotrophy transition of the anthracnose pathogen Colletotrichum truncatum. Plant Signaling & Behavior. 6:1457-1459. Disponible en línea: http://www.tandfonline.com/doi/pdf/10.4161/psb.6.10.17700 [ Links ]
Casarrubias-Carrillo U, Cárdenas-Soriano E, Nieto-Ángel D y Gutiérrez-Alonso G. 2002. Histopatología de frutos de papaya (Carica papaya L.) infectados por Colletotrichum gloeosporioides Penz. Revista Mexicana de Fitopatología. 20: 88-93. Disponible en línea: http://www.socmexfito.org/2013-06-19-02-09-15/001-vol-20/101-revista-smf/2002/001/234-histopatologia-de-frutos-de-papaya-carica-papaya-l-infectados-por-colletotrichum-gloeosporioides-penz [ Links ]
Diéguez-Uribeondo J, Förster H, Soto-Estrada A and Adaskaveg, JE. 2005. Subcuticular-intracellular hemibiotrophic and intercellular necrotrophic development of Colletotrichum acutatum on almond. Phytopathology. 95:751-758. http://dx.doi.org/doi/pdf/10.1094/PHYTO-95-0751 [ Links ]
Kubo Y, Takano Y, Tsuji G, Horino O and Furusawa I. 2000. Regulation of melanin biosynthesis genes during appressorium formation by Colletotrichum lagenarium 99p. Prusky D, Freeman S and Dickman MB. (eds.). In: Colletotrichum Host specificity, pathology, and host-pathogen interaction. APS press, Minnesota, USA. 399 p. [ Links ]
Latunde-Dada AO and Lucas JA. 2007. Localized hemibiotrophy in Colletotrichum: cytological and molecular taxonomic similarities among C. destructivum, C. linicola and C. truncatum. Plant Pathology. 56:437-44. http://dx.doi.org/10.1111/j.1365-3059.2007.01576.x/epdf [ Links ]
O´Connell RJ, Uronu AB, Waksman G, Nash C, Keon JPR and Bailey JA. 1993. Hemibiotrophic infection of Pisum sativum by Colletotrichum truncatum. Plant Pathology. 42:774-783. http://dx.doi.org/10.1111/j.1365-3059.1993.tb01564.x [ Links ]
O´Connell RJ, Perfect S, Hughes B, Carzaniga R, Bailey J and Green J. 2000. Dissecting the cell biology of Colletotrichum infection processes. 55-77p. Prusky D, Freeman S and Dickman MB. (eds.). In: Colletotrichum Host specificity, pathology, and host-pathogen interaction. APS press, Minnesota, USA. 399 p. [ Links ]
Pandey A, Pandey BK, Muthukumar M, Yadava LP and Chauhan UK. 2012. Histopathological study of infection process of Colletotrichum gloeosporioides Penz and Sacc. on Mangifera indica L. Plant Pathology Journal. 11:18-24. http://dx.doi.org/ppj.2012.18.24&linkid=pdf [ Links ]
Perfect SE, Hughes HB, O´Connell RJ and Green JR. 1999. Colletotrichum: A model genus for studies on pathology and fungal-plant interactions. Fungal Genetics and Biology. 27:186-198. http://dx.doi.org/10.1006/fgbi.1999.1143 [ Links ]
Pring RJ, Nash C, Zakaria M and Bailey JA. 1995. Infection process and host range of Colletotrichum capsici. Physiological and Molecular Plant Pathology. 46:137-152. http://dx.doi.org/10.1006/pmpp.1995.1011 [ Links ]
Ranathunge NP, Mongkolporn O, Ford R and Taylor PWJ. 2012. Colletotrichum truncatum pathosystem on Capsicum spp: infection, colonization and defense mechanisms. Australasian Plant Pathology. 41:463-473. http://dx.doi.org/10.1007/s13313-012-0156-0 [ Links ]
Rodríguez-López ES, Cárdenas-Soriano E, Hernández-Delgado S, Gutiérrez-Diez A y Mayek-Pérez N. 2013. Análisis de la infección de Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. de frutos de aguacatero. Revista Brasileira de Fruticultura. 35:898-905. Disponible en línea: http://www.scielo.org.mx/pdf/rmfi/v27n1/v27n1a7.pdf [ Links ]
Tapia-Tussell R, Quijano-Ramayo A, Cortes-Velázquez A, Lappe P, Larque-Saavedra A and Pérez-Brito D. 2008. PCR-based detection and characterization of the fungal pathogens Colletotrichum gloeosporioides and Colletotrichum capsici causing anthracnose in papaya (Carica papaya L.) in the Yucatán peninsula. Molecular Biotechnology. 40:293-298. http://dx.doi.org/10.1007/s12033-008-9093-0 [ Links ]
Torres-Calzada C, Tapia-Tussell R, Higuera-Ciapara I and Pérez-Brito D. 2012. Morphological, pathological and genetic diversity of Colletotrichum species responsible for anthracnose in papaya (Carica papaya L). European Journal of Plant Pathology. 135:67-79. http://dx.doi.org/10.1007/s10658-012-0065-7 [ Links ]
Received: April 14, 2016; Accepted: June 18, 2016











 texto em
texto em 


