Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.34 n.3 Texcoco Sep. 2016
https://doi.org/10.18781/r.mex.fit.1605-3
Scientific articles
Isolation of killer yeasts from ants of the genus Atta and their effect on the red tomato’s fungal pathogen Geotrichum candidum
1Universidad Autónoma de Nuevo León, UANL, Facultad de Ciencias Biológicas, Av. Universidad S/N Ciudad Universitaria, San Nicolás de los Garza, Nuevo León, México C.P. 66451.
2Facultad de Medicina, Universidad Autónoma de Nuevo León, Departamento de Microbiología, Madero y Dr. E. A. Pequeño s/n, Colonia Mitras Centro, Monterrey, Nuevo León, México C.P. 64460.
One of the diseases occurring in tomato is the sour rot caused by Geotrichum candidum. In order to evaluate the antagonism of killer yeasts, isolates were obtained from ants belonging to the genus Atta and those with killer activity were selected, resulting in 8 killer isolates (M1 - M8). Then, isolates of G. candidum were obtained from infected tomatoes and the most aggressive one was selected. Each killer yeast was inoculated in 1x1 cm2 cuts made to tomato fruits and after a 3 hour incubation, 50 μL of a G. candidum suspension of 1x104 cells/mL was inoculated. After incubation for 72 h in a moist chamber, the lesions were quantified using the Tukey test (p<0.05) resulting in yeast isolates M1 and M2 as the most protective ones. The auxanogram test API 20C AUX identified them as Candida (Pichia) guilliermondii.
Keywords: Killer yeasts; antagonism; Pichia guilliermondii; Geotrichum candidum
Una de las enfermedades que sufre el tomate es la pudrición ácida causada por Geotrichum candidum. Para lo cual se aislaron levaduras a partir de hormigas del género Atta y se seleccionaron aquellas que mostraron actividad killer frente, resultando en 8 cepas killer (M1 - M8). Posteriormente se aislaron cepas de G. candidum a partir de tomates infectados y se seleccionó la más agresiva. Cada levadura killer fue inoculada en cortes hechos a los tomates de 1x1 cm2 y después de 3 horas de incubación, la cepa seleccionada del fitopatógeno fue inoculada en un volumen de 50 µL con una concentración de 1x104 células/mL. Después de 72 h de incubación en cámara húmeda, los daños fueron cuantificados mediante la medición de las lesiones alrededor del sitio de inoculación. Los datos fueron analizados mediante la prueba de Tukey (p<0.05) resultando que las levaduras M1 y M2 presentaron las mayores actividades antagonistas. La identificación por API 20C AUX arrojó que las cepas corresponden a Candida (Pichia) guilliermondii.
Palabras clave: Levaduras killer; antagonismo; Pichia guilliermondii; Geotrichum candidum
Introduction
Tomato (Solanum lycopersicum L) is one of the most widely sold crops in the world, and one with the highest commercial value. Mexico is the tenth largest tomato producer worldwide, and the single most important exporter, providing the country with an average of one billion dollars. It has been estimated that 1 out of every 3 red tomatoes is produced in the state of Sinaloa, for a total of 867 thousand tons, out of the total of 2.8 millions produced in Mexico (SIAP, 2016). During postharvest, one of the diseases that appear in this fruit is sour rot, caused by the fungus G. candidum, a common alteration in practically any place in which this fruit is produced, and the temperature is preferably of around 30 °C (Ruiz-Martínez et al., 2012). The damage regularly begins through lesions caused by insects, or mechanically, when handling the product in the commercialization process. There are several alternatives to avoid the deterioration of tomatoes and to preserve their quality for longer time periods, including biological control using antagonistic microorganisms such as yeasts, the different action mechanisms include antibiosis, competition for space and nutrients, and direct interaction (Bautista-Baños, 2006; Khaled & Sivasithamparam, 2006). Among the antagonistic yeasts are what are called killer yeasts, capable of secreting metabolites of a protein nature and variable molecular weights, named killer toxins, capable of inhibiting other microorganisms by altering cell walls, membranes, or vulnerable cell nuclei (Buzzini et al., 2007). There are several reports on the effectiveness and the potential of killer yeasts for the control of fungi that cause plant deterioration. The fungus Botrytis cinerea, one of the most harmful pathogens in grape and strawberry crops, was inhibited using the partially purified of the strain Pichia membranifaciens CYC 1106 (Santos & Marquina, 2004). This same fungus was later inhibited using strains of Pichia anomala and Debaryomyces hansenii, increasing the biological arsenal with biocontrolling potential over this pathogen (Santos et al., 2004). Strains of Issatcehnkia orientalis, Candida guilliermondii, P. ohmeri and Torulaspora globosa, among others, have been reported to successfully inhibit important plant pathogenic fungi, such as Aspergillus cabonarius, A. niger, Penicillium expansum and Colletotrichum sublineolum in grape, pear, apple, and sorghum crops, respectively (Bleve et al., 2006; Coelho et al., 2009; Rosa et al., 2010). In Mexico, Hernández-Montiel et al., (2011) reported a significant reduction of Geotrichum citri-aurantii in limes during postharvest, using epiphytic D. hansenii yeasts.
Yeasts present an ecologically diverse association with insects; over 200 species of only beetles have been reported, along with 12 of termites, 17 for antes, and other insects such as wood-eaters, homoptera, dipterous, hymenoptera, neuroptera, etc. (Rosa & Gábor, 2006). Carreiro et al., (2002) reported that 77 out of 99 yeast strains isolated from ants of the genus Atta presented the killer phenotype, including some genuses in which this phenomenon had not been previously reported. To study the applicability of this knowledge in preventing the postharvest damage in tomatoes by G. candidum, the aim was set to evaluate the antagonistic capability of killer yeast strains taken from ants, against isolations of G. candidum obtained from tomato samples with characteristic symptoms of sour rot.
Materials and methods
Isolation of yeasts
Yeasts were isolated from red ants (Atta spp.) based on previous reports (Carreiro et al., 2002). The ants were gathered from different locations in plastic bags and transported to the lab in a low temperature. For the isolation, we carried out the procedure described by Torres-Mireles (2013); one by one, the antes were macerated in a sterile mortar adding 1 ml of sterile saline solution (0.85 % NaCl). From each homogenization obtained, we performed 4 dilutions in series, and 100 µl of each one were inoculated by extension in Petri dishes with potato dextrose agar (PDA) and incubated at 25 °C for up to 72 h. The yeasts obtained, recognized macroscopically by their creamy look and opaque color, and microscopically by their cell size, observable under an optic microscope at a 400x magnification, were preserved in vials with sterile distilled water at room temperature until used.
Determining the killer phenotype
To evaluate the presence of the phenotype killer in the isolated yeasts, we used the strains of Saccharomyces cerevisiae ATCC 26609 and ATCC 38527 as yeasts sensitive to the killer toxins (Robledo-Leal et al., 2012), using the medium YEPD-MB (0.3 % yeast extract, 0.3 % malt extract, 0.5% tryptone, 1 % glucose, 2 % agar, and 0.003 % methylene blue) adjusted to a pH of 4.5 with a citrate-phosphate regulator 0.1 M. Inóculos of 1x106 cells/ml were prepared from 24h cultures of the sensitive strains, out of which 400 µl were placed on plates with a YEPD-MB medium, using sterile cotton swabs, plating in 4 directions to generate an even “lawn”. Once the excess humidity was removed, the yeast strains to be evaluated were inoculated as a thick smear and the trays were incubated at 25 °C for 72h. The killer phenotype was considered positive if a clear area of inhibition appeared, surrounded by a blue edge.
Isolation and selection of G. candidum strains
Samples were taken of red tomato with symptoms compatible with sour rot (softening, exudate, white mycelium, production of gas). The samples were washed under the faucet, then pieces of the damaged area were cut (1 cm2 approx.) and submerged for 2 min in a 2 % hypochlorite solution. They were later moved to a Petri dish with sterile distilled water and washed; this process was carried out twice. Under conditions of sterility, the pieces of tomato were transferred to trays with PDA. The trays were incubated at 30 °C until the cultures appeared. The isolates were identified as G. candidum due to their macroscopic and microscopic characteristics, such as rapid growth, whitish color, dry culture aspect, hyaline, septed, branched hyphae, and the production by fragmentation of hyaline, unicellular arthroconidia, without conidiophores or blastoconidia (Watanabe, 2002). Once the pure isolations were obtained, they were kept in glycerol at 20 %, at a temperature of -20 °C, until they were used.
Under conditions of sterility, a tray with PDA was inoculated by puncturing for each strain obtained in the stage of isolation and incubated at 28ºC, allowing them to grow until the area of the tray was completely covered. Later, using a needle, a sample of the mycelium was taken and a healthy tomato was inoculated by puncturing after sanitizing with sodium hypochlorite at 2 %. Each tomato was punctured 10 times, then placed inside a damp chamber then incubated at 28 °C. Results were observed after 24 and 48 h. These experiments were carried out in two different moments, separated by replications per test. The most aggressive strain was chosen based on the amount of punctures infected (i.e. with mycelial growth), the diameter of the damages in the punctures and the degree of softening established visually.
Antagonism test on fruit
Two experiments were carried out independently with 3 replications each. Along with the strains of killer yeasts, we included a control treatment without added yeast, and another one with the strain of Candida albicans ATCC 90028 as a nonantagonistic yeast control. From young cultures of the strain of G. candidum chosen as the most aggressive for tomato, a suspension was prepared of 1 x 104 cells/ml. The yeasts of each treatment, including the C. albicans control, were adjusted to 1 x 108 cells/ml in a sterile salt solution (Ren et al., 2012).
Once the suspensions required for the tests were prepared, the tomatoes were sanitized using cotton soaked in 2 % sodium hypochlorite and using a scalpel, semicircle-shaped holes were carved around the pedicle, with incisions of 1 cm2 x 0.5 cm in depth in the mesocarp. In each hole 100 μl of the yeast suspension were inoculated, the tomato was placed inside a humidity chamber and incubated at 28 °C for 3 hours, to allow for the pre-establishment of the yeast in the fruit. After this time, 50 μl of the G. candidum suspension was inoculated in each hole; it was placed back into the humidity chamber and incubated at 28 °C. The effectiveness of each treatment was evaluated after 72h by measuring the lesions, which were quantified by adding their extension in millimeters towards both sides, and the bottom of each inoculated hole. The average values obtained for each treatment were analyzed with the Tukey test using the program SPSS version 20.0, at a level of significance of (p<0.05).
Results and Discussion
From the ants gathered we obtained 8 yeasts, the killer activity of which was observable by the formation of a halo of inhibition, surrounded by a darker blue edge, generated by the methylene precipitated as an indicator of cell death (Figure 1).
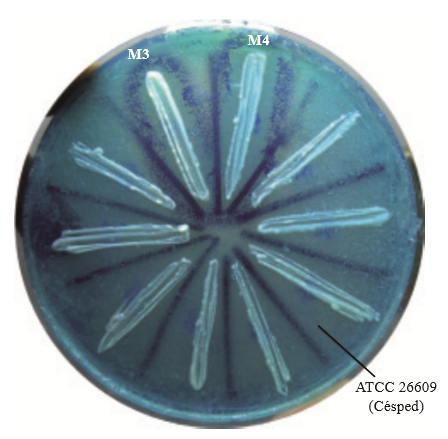
Figure 1. Determination of the killer phenotype of yeasts isolated from ants of the genus Atta. Blue edges indicate the cellular death of vulnerable yeast in the “lawn”.
From the tomato samples, the strain of G. candidum named MG2 produced the most damage in the tomatoes, and was therefore chosen as the most aggressive, and it was used in the antagonism test on the fruit (Table 1 and Figure 2). The comparison of averages using the Tukey test showed that all yeasts, except M5 and M6, helped reduce the significantly higher damage in comparison to the strain of C. albicans and the control without yeast. Strains M1 and M2 were the ones that allowed the least total damage (mm) on the fruits, although with no significant difference in comparison with M3, M4, M7, and M8 (Table 2). The identification of the 8 killer yeasts obtained using the API 20 C AUX test indicated that they all corresponded to Candida (Pichia) guilliermondii, a species in which killer activity has been known for over 20 years (Polonelli et al., 1987), yet it wasn’t until 1991 that its use as a biocontrol agent was first reported (Wisniewski et al., 1991) and its activity has been reported in several occasions (Coelho et al., 2009; de Lima et al., 2013; Papon et al., 2013). The difference between the damage in the presence of the killer yeasts and the treatments without yeast and with C. albicans suggest that the phenotype killer in yeasts M1 - M8 is related to its antagonism with G. candidum. The differences observed in the damage to the fruit allowed by each yeast could be explained by differences in the type of killer toxin secreted, which influences the affinity to the white area of the susceptible fungus and/or in differences in the expression of the toxin, since they are mostly codified in the nuclear genome (Buzzini et al., 2007). On the other hand, it is possible that these strains present, not only killer toxins, but also, in a variable way, additional previously reported action mechanisms in C. guilliermondii, such as the direct attack on hyphae and the competition for nutrients (Chanchaichaovivat et al., 2008; Nantawanit et al., 2010; Scherm et al., 2003). This diversity in mechanisms represents a diverse interval of antagonistic capabilities for the killer yeasts with plant pathogenic fungi, with the possibility that strains with little or no antagonistic activity towards a genus or species of fungi can show a high antagonistic activity towards others, which allows wide options of study. The use of insects as a nonconventional source of yeasts is attractive, given the size of the biodiversity related to these organisms (Rosa & Gábor, 2006) and the fact that, at least in some cases, associated yeasts represent a relation in which yeast protects the insect from pathogenic fungi (Rodrigues et al., 2009) and with this comes the possibility that this antagonistic mechanism acts against fungi which can be important as plant pathogens.
Table 1. Selection of the most aggressive G. candidum strain, out of a total of 4 repetitions. Results obtained by the inoculation of the pythopathogen by puncturing the fruits are enlisted.
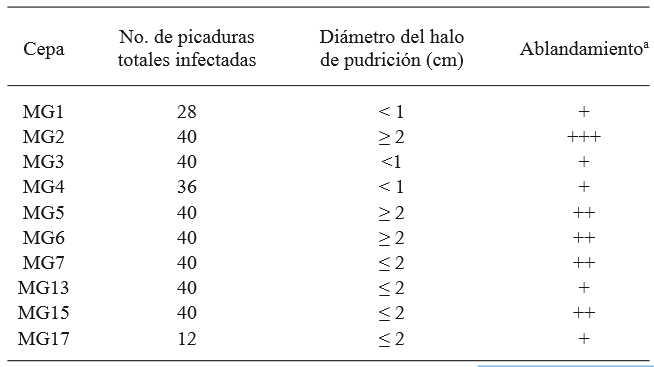
a + low, ++ medium, +++ high, determined visually.

Figure 2. Evaluation of the damages caused by different strains of G. candidum on red tomatoes. The presence of mycelia and softening of fruits can be observed.
Table 2. Average values of the extension of the damage caused by G. candidum in tomatoes in each treatment.
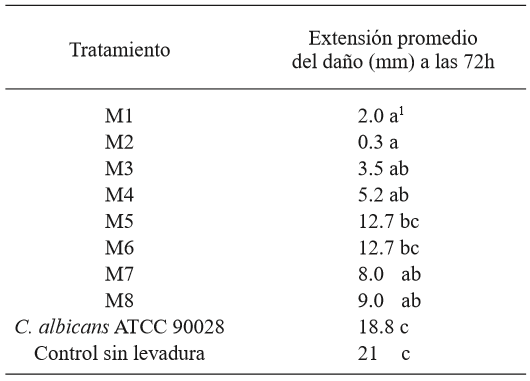
1 Each value represents the average of 6 tomatoes. Values in each row with different letters are statistically different (p<0.05).
Although it is clear that they do not represent a direct competition with the efficiency of chemical fungicides due to the speed at which they act, they are an alternative that would help reduce required doses without increasing the damage to the fruits, resulting in products with less concentrations of chemicals, and therefore a reduction in the risk of toxicity for humans.
Conclusions
Results show that it is possible to isolate yeasts with the killer phenotype from ants of the genus Atta and these yeasts display variable antagonism towards G. candidum. The mere presence of a non-killer yeast is not enough to protect the tomato from the damage caused by G. candidum, making it statistically equal to inoculating no yeast. Quantifying the damage to the fruit by accumulated measurement (mm) of the area affected to the sides and the bottom of the lesion help evaluate the protection conferred by the presence of antagonistic yeasts.
Literatura citada
Bautista-Baños S. 2006. El control biológico en la reducción de enfermedades de postcosecha en productos hortofrutícolas: uso de microorganismos antagónicos. Revista Iberoamericana de Tecnología Postcosecha 8(1):1-6. Disponible en línea: http://www.redalyc.org/articulo.oa?id=81380101 [ Links ]
Bleve G, Grieco F, Cozzi G, Logrieco A and Visconti A. 2006. Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. International Journal of Food Microbiology 108(2):204-209. http://dx.doi.org/10.1016/j.ijfoodmicro.2005.12.004 [ Links ]
Buzzini P, Turchetti B and Vaughan-martini AE. 2007. The use of killer sensitivity patterns for biotyping yeast strains: the state of the art, potentialities and limitations. FEMS Yeast Research 7(6):749-760. http://dx.doi.org/10.1111/j.15671364.2007.00238.x [ Links ]
Carreiro SC, Pagnocca FC, Bacci M, Bueno OC, Hebling MJ and Middelhoven WJ. 2002. Occurrence of killer yeasts in leaf-cutting ant nests. Folia Microbiol (Praha) 47(3):259-262. https://dx.doi.org/10.1007/BF02817648 [ Links ]
Chanchaichaovivat A, Panijpan B and Ruenwongsa P. 2008. Putative modes of action of Pichia guilliermondii strain R13 in controlling chilli anthracnose after harvest. Biological Control 47:207-215. http://dx.doi.org/10.1016/j.biocontrol.2008.07.018 [ Links ]
Coelho AR, Tachi M, Pagnocca FC, Andrade GM, Hoffman FL, Harada K and Hirooka EY. 2009. Purification of Candida guilliermondii and Pichia ohmeri killer toxin as an active agent against Penicillium expansum. Food Additives & Contaminants: Part A 26(1):73-81. http://dx.doi.org/10.1080/02652030802227227 [ Links ]
de Lima JR, Gonçalves LR, Brandão LR, Rosa CA and Viana FM. 2013. Isolation, identification, and activity in vitro of killer yeasts against Colletotrichum gloeosporioides isolated from tropical fruits. Journal of Basic Microbiology 53:590-599. http://dx.doi.org/10.1002/jobm.201200049 [ Links ]
Hernández-Montiel L, Holguín-Peña J, López-Aburto M y Troyo-Diéguez, E. 2011. Control poscosecha de Geotrichum citri-aurantii en limón mexicano (Citrus aurantifolia [Christm.] Swingle) mediante levaduras marinas y epífitas. Universidad Juárez Autónoma de Tabasco. Disponible en línea: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0186-29792011000200008&lng=es&nrm=iso [ Links ]
Khaled A and Sivasithamparam K. 2006. Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience. 47:25-35. http://dx.doi.org/10.1007/s10267-005-0268-2 [ Links ]
Nantawanit N, Chanchaichaovivat A, Panijpan B and Ruenwongsa P. 2010. Induction of defense response against Colletotrichum capsici in chili fruit by the yeast Pichia guilliermondii strain R13. Biological Control 52:145-152. http://dx.doi.org/10.1016/j.biocontrol.2009.10.011 [ Links ]
Papon N, Savini V, Lanoue A, Simkin AJ, Crèche J, Giglioli-Guivarc’h N, Clastre M, Courdavault V and Sibirny AA. 2013. Candida guilliermondii: biotechnological applications, perspectives for biological control, emerging clinical importance and recent advances in genetics. Current Genetics 59(3):73-90. http://dx.doi.org/10.1007/s00294-013-0391-0 [ Links ]
Polonelli LG. Dettori, C and Morace G. 1987. Biotyping of mycelial fungus cultures by the killer system. European Journal of Epidemiology 3:237-242. http://dx.doi.org/10.1007/bf00149730 [ Links ]
Ren X, Kong Q, Wang H, Yu t, Tang YJ, Zhou WW and Zheng X. 2012. Control of apple blue mold by Pichia pastoris recombinant strains expressing cecropin A. Bioprocess and Biosystems Engineering 35(5):761-767. http://dx.doi.org/10.1007/s00449-011-0656-2 [ Links ]
Robledo-Leal E, Villarreal-Treviño L and González GM. 2012. Occurrence of killer yeasts in isolates of clinical origin. Tropical Biomedicine 29(2):297-300. Disponible en línea: http://www.msptm.org/files/297_-_300_Robledo-Leal_E.pdf [ Links ]
Rodrigues A, Cable RN, Mueller UG, Bacci M and Pagnocca FC. 2009. Antagonistic interactions between garden yeasts and microfungal garden pathogens of leaf-cutting ants. Antonie Van Leeuwenhoek 96(3):331-342. https://dx.doi.org/10.1007/s10482-009-9350-7 [ Links ]
Rosa MM, Tauk-Tornisielo SM, Rampazzo PE and Ceccato-Antonini SR. 2010. Evaluation of the biological control by the yeast Torulaspora globosa against Colletotrichum sublineolum in sorghum. World Journal of Microbiology and Biotechnology 26(8):1491-1502. http://dx.doi.org/10.1007/s11274-010-0324-8 [ Links ]
Rosa, C and Gábor, P. 2006. Biodiversity and ecophysiology of yeasts. First edition. Springer. Berlin, Germany. 580p. [ Links ]
Ruiz-Martínez J, Vicente AA, Montánez-Saenz, JC, Rodríguez-Herrera R y Aguilar González, CN. 2012. Un tesoro perecedero en México: el tomate, tecnologías para prolongar su vida de anaquel. Investigación y Ciencia 54, 42-48. Disponible en línea: http://www.redalyc.org/articulo.oa?id=67424408008 [ Links ]
Santos A, Marquina D. 2004. Killer toxin of Pichia membranifaciens and its possible use as a biocontrol agent against grey mould disease of grapevine. Microbiology 150(Pt 8):2527-2534. http://dx.doi.org/10.1099/mic.0.27071-0 [ Links ]
Santos A, Sánchez A and Marquina D. 2004. Yeasts as biological agents to control Botrytis cinerea. Microbiological Research 159(4):331-338. http://dx.doi.org/10.1016/j.micres.2004.07.001 [ Links ]
Scherm B, Ortu G, Muzzu A, Budroni M, Arras G and Migheli Q. 2003. Biocontrol activity of antagonistic yeasts against Penicillium expansum on apple. Journal of Plant Pathology 85:205-213. Disponible en línea: http://www.jstor.org/stable/41998150 [ Links ]
SIAP, Servicio de Información Agroalimentaria y Pesquera. 2016. Cierre de la producción agrícola por estado. (consulta: 28 junio 2016). Disponible en línea: http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-estado/ [ Links ]
Torres-Mireles S. 2013. Actividad antagonista de levaduras frente a hongos en postcosecha. Tesis de licenciatura. Universidad Autónoma de Nuevo León. [ Links ]
Watanabe T. 2002. Pictorial atlas of soil and seed fungi: Morphologies of cultured fungi and key to species. Second edition. CRC Press. Boca Raton, Fla. 486p. [ Links ]
Wisniewski M, Biles C, Droby S, McLaughlin R, Wilson C and Chalutz E. 1991. Mode of action of the postharvest biocontrol yeast, Pichia guilliermondii. Characterization of attachment to Botrytis cinerea. Physiological and Molecular Plant Pathology 39:245-258. http://dx.doi.org/10.1016/0885-5765(91)90033-e [ Links ]
Received: May 11, 2016; Accepted: August 04, 2016











 text in
text in 


