Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.34 no.2 Texcoco 2016
https://doi.org/10.18781/R.MEX.FIT.1511-3
Phytopathological notes
Molecular identification of bacteria associated to ornamental plants obtained in vitro
1 Campo Experimental Zacatepec, INIFAP. km 0.5 Carretera Zacatepec-Galeana. C.P. 62780, Col Centro Zacatepec, Morelos, Tel. 734 3430230 ext. 108 y 139
2 Universidad Politécnica del Estado de Morelos (UPEMOR). Blvd. Cuauhnahuac #566, C.P. 62550, Col. Lomas del Texcal, Jiutepec, Morelos, México
3 Posgrado en Fitosanidad-Fitopatología. Colegio de Postgraduados, km 36.5 carretera México-Texcoco, Montecillo, Texcoco, CP 56230 México
In ornamental plants propagated in vitro at Tezoyuca Technology Development Center, belonging to FIRA, symptoms of necrosis on leaves and stems during the adaptation stage. The objective of this study was to isolate and to identify the agents related to these plants. Eleven bacterial isolates were obtained from necrotic leaves and stems, from which ADN was extracted using a commercial PCR kit. The PCR products were sequenced and analyzed using the Chromas Lite® program. We search for homology with BLAST after which the following bacteria were identified: Kosakonia oryzae, Pectobacterium cypripedii, Burkholderia tropica, Serratia marcescens, Pantoea dispersa, Erwinia cypripedii, Pantoea agglomerans y Erwinia rhapontici. The last three bacteria are plant disease in phase of acclimation in ornamental plants get in vitro.
Key words: Potted plant; Pathogens; Molecular identification; PCR
En plantas ornamentales reproducidas in vitro en viveros del Centro de Desarrollo Tecnológico Tezoyuca perteneciente al FIRA, se detectaron síntomas de necrosamiento en hojas y tallo durante la fase de adaptación. El objetivo de este trabajo fue aislar e identificar los agentes asociados a éstas plantas. De tejido vegetal con síntomas de necrosamiento, se obtuvieron once aislamientos bacterianos, a los que se les extrajo el ADN con el paquete correspondiente para su secuenciación. Todos los productos de PCR se secuenciaron y se analizaron con el programa Chromas Lite®. Utilizando la búsqueda de homología por BLAST se identificaron las siguientes bacterias: Kosakonia oryzae, Pectobacterium cypripedii, Burkholderia tropica, Serratia marcescens, Pantoea dispersa, Erwinia cypripedii, Pantoea agglomerans y Erwinia rhapontici: son de importancia fitosanitaria en su fase de aclimatación in vitro.
Palabras clave: Plantas en contenedor; patógenos; identificación molecular; PCR
Plants have the capability of reproducing sexually or asexually. In the first case, there is a greater variability in the progeny, whereas in the second case, descendants are obtained genetically equal to the mother (Corona-Nava-Esparza and Chimal-Hernández, 2006). The in vitro propagation technique is an asexual form of reproduction that takes advantage of the capability of peach plant cell to generate genetically similar individuals. The ornamental plant industry has used this technique to multiply plants with a high degree of uniformity and safety (Rout et al, 2006).
However, the plants obtained with this technique must undergo an acclimatization stage to come out to the definitive plantation. In this phase, plantlets obtained in vitro have their agar (artificial growth medium) removed and are placed in trays with a sterile substrate and a controlled climate, until they are ready to me transplanted to pots. This stage is considered critical, due to the tension plants undergo, because of the new condition, in which they are not given the nutrients present in the culture medium; the tension generated makes plants very vulnerable to some biotic diseases, mainly bacteria, fungi, and sporadically, viruses.
Bacteria are cosmopolitan microscopic organisms than can live as saprophytes, epiphytes, symbionts, parasites and pathogens of animals, plants, and humans. Some bacteria can behave as saprophytes, and under some conditions, may become opportunist pathogens (Madigan et al., 1997).
Potted flower-producing plants are subject to bacterial diseases frequently caused by Pectobacterium spp., Xanthomonas spp., and Pseudomonas spp. (Daughtrey et al., 2001). Each bacteria causes different symptoms in plants, which may include rotting, foliar spots, wilting, yellowing, canker, mainly (Agrios, 2005). To identify the pathogen it is necessary to carry out a pure isolation from mycelia in the case of fungi, and some exudates or bacterial discharge in the case of bacteria.
In the state of Morelos, the production of ornamental plants is calculated in 3 000 ha with an estimated production value of 1.2 million pesos per ha/year. Some of the most important species are Euphorbia pulcherrima, Bougainvillea glabra, Spathiphyllum uxpanapense, Rosa spp., Pteridium aquilinum, Cedrela odorata, Citrus limonum, Tulipa spp., Acanthocalycium spp., Thrinax radiata, Pelargonium zonale, Begonia x tuberhybrida. In the state, the municipal areas with the most important production are Cuautla, Jiutepec, Cuernavaca, Yautepec, Puente de Ixtla, Emiliano Zapata, Xochitepec, Jonacatepec, Temixco, and Tlaquiltenango.
The Tezoyuca Technology Development Center, which belongs to the regional Fideicomiso Instituido en Relación a la Agricultura (FIRA) office en Morelos, is responsible for reproducing Anthurium andreanum, Syngonium podophyllum, Spathiphyllum uxpanapense, Pteridium aquilinum with the in vitro technique, which are affected by diseases in this phase and in the phase of acclimatization.
To develop a disease control method, it is necessary to identify the causal agent of the symptom by a morphological, biochemical and/or molecular characterization, the latter being the most important due to the accuracy and speed with which is it carried out. The main objective of this investigation was to identify bacteria related to the necrosis of leaves and stems in ornamental plants produced in vitro during the phase of adaptation by molecular methods.
In Syngonium podophyllum, Philodendron spp., Orchidaceae spp., Pteridium aquilinum, and Spathiphyllum uxpanapense plants reproduced in vitro in FIRA in the state of Morelos, with symptoms of blackening during the phase of acclimatization, were transported in sterile plastic bags to the Plant Pathology laboratory of the Zacatepec experimental field, which belongs to the National Forestry, Agricultural and Livestock Research Center, and where they were processed to carry out the diagnosis of the etiologic agents of the disease.
Isolation of bacterial strains from diseased ornamental plants
From every diseased plant, a 0.1 g sample was taken from the base of the stem or leaves with some necrotic spot. They were washed with tap water and disinfected with a 2 % sodium hypochlorite solution for 2 min, removing the excess water with sterile absorbent paper. Each sample was placed in a Petri dish with potato dextrose agar (PDA), and the samples treated in this way were incubated at 25 °C for 48 h. The bacteria grown in this medium were re-isolated, taking bacterial mass with an inoculating loop and placed in Petri dishes with an NBY medium with nystatin at 28 °C for 48 h. Pure cultures were kept in an 80 % glycerol-distilled water solution at -80 °C.
Obtaining bacterial DNA and amplification with PCR
Of the bacterial colonies obtained from the ornamental plants developed and isolated in a solid NBY-N medium for 48 h at ambient temperature (25-28 °C), a sample of bacterial mass was taken using the inoculating loop and moved into a 2 mL test tube with NBY liquid medium. The tubes were incubated while shaking for 12 h at ambient temperature. At the end of the incubation period, 1 mL of the suspension obtained was taken and centrifuged in Eppendorf tubes at 13 000 rpm for 1 min. Later, the supernatant was poured out and the pellet was resuspended in sterile distilled water for DNA extraction. The extraction was carried out with a PCR (Wizard Genomic DNA Purification Kit, Promega®, Cat. A1120) package, following the factory protocol. The DNA extraction and integrity was verified in a 1 % agarose gel.
The initiators of the PCR reaction were rP2 (5' ACGGCTACCTTGTTACGACTT 3') y fD1 (5' AGAGTTTGATCCTGGCTCAG 3') for the amplification of a fragment of gene 16S rDNA (Weisburg et al., 1991); the genomic DNA was used as a template. The reaction took place in a thermocycler (Techne® PHC-3), in a final volume of 25 μL, which contained buffer 1 X, 200 μM of dNTP's, 1,5 μM de MgCl2, 0,4 μM of each oligonucleotide, 1 U of GoTaq enzyme (Promega, Cat. M8295) and 1 μg of genomic DNA. The PCR program consisted of an initial denaturation stage at 94 °C for 5 min, followed by 35 cycles of 94 °C for 15 s, 55 °C for 15 s, and 72 °C 15 s, as well as a final step of 72 °C for 5 min. The analysis of PCR was carried out by electrophoresis in agarose gel at 1.2 % a 80 V for 35 min, using 5 μL of each sample. The gel was stained with ethidium bromide (0.5 μg/mL) and observed in a transilluminator.
Sequencing the bacterial strains
Ll the PCR reactions for sequencing were treated with DNA Clean & Concentrator TM-25 Kit by Zymo (Cat. D4033). Afterwards, The DNA was quantified in a nanodrop (Epoch, BioTek). The sequencing reaction consisted of 10 ng of DNA for every 100 pb, 1 μL of oligo (10 pmol), in a final volume of 16 μL. Sequencing was carried out in the UNAM Biotechnology Institute, using Perkin equipment (Elmer/Applied Biosystems Modelo 3730), with the method Taq FS Dye Terminator Cycle Sequencing Fluorescence-Based.
Las secuencias obtenidas se analizaron con el programa Chromas Lite® y posteriormente, con BLAST (The Basic Local Alignment Search Tool: http://blast.ncbi.nlm.nih.gov/) para su alineamiento con la base de datos del GenBank del National Center for Biotechnology Information (NCBI). Of the quantitative values observed, only those with the greatest identity were considered (Table 1).
Table 1 Results of the molecular identification of the bacteria isolated in ornamental plants produced in vitro in the phase of acclimatization.
| Planta de la que se aisló | No. Accesión | Descripción | Máxima
identidad (%) |
| Singonio | KF479042.1 |
Kosakonia oryzae strain P-9 16S ribosomal RNA gene,
partial sequence |
99 |
| Orquídea | KF479042.1 |
Kosakonia oryzae, strain P-9 16S ribosomal RNA gene,
partial sequence |
99 |
| Cuna de moisés var.
Maunaloa |
FJ823047.1 |
Pectobacterium cypripedii strain gx-104 16S ribosomal RNA
gene, partial sequence |
99 |
| Filodendro | JQ659926.1 |
Burkholderia tropica strain R8-139 16S ribosomal RNA
gene, partial sequence |
100 |
| Cuna de moisés | KF528829.1 |
Serratia marcescens strain JASM1 16S ribosomal RNA
gene, partial sequence |
99 |
| Cuna de moisés var. Chopan | JX215555.1 |
Erwinia rhapontici strain ARB1 16S ribosomal RNA gene,
partial sequence |
100 |
| Cuna de moisés var. Chopan | JQ917111.1 |
Pantoea dispersa strain B-22 16S ribosomal RNA gene,
partial sequence |
100 |
| Helecho Boston | JF430157.1 |
Pectobacterium cypripedii strain B1 16S ribosomal RNA
gene, partial sequence |
100 |
| Helecho Boston | HM582877.1 |
Pantoea agglomerans strain AR PSBH2 16S ribosomal RNA
gene, partial sequence |
99 |
| Helecho Boston | JF430157.1 |
Pectobacterium cypripedii strain B1 16S ribosomal RNA
gene, partial sequence |
99 |
| Helecho Boston | JQ659926.1 |
Burkholderia tropica strain R8-139 16S ribosomal RNA
gene, partial sequence |
100 |
Out of the ornamental plants sampled and with symptoms of necrosis on stems and leaves, 12 bacterial isolations were obtained, most of which were characterized by having a white or creamy color, and only one showed a red pigment. After sequencing them (Figure 1), eight species were identified: Kosakonia oryzae, Pectobacterium cypripedii, Burkholderia tropica, Serratia marcescens, Pantoea dispersa, Erwinia cypripedii, Pantoea agglomerans, and Erwinia rhapontici (Table 1).
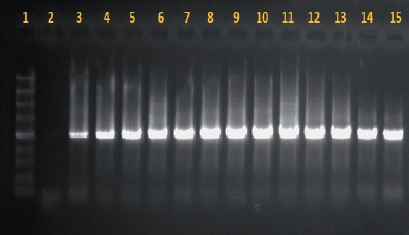
Figure 1 Amplifies fragment of 1,500 pb of the gene 16S with the oligos rP2 and with PCR. 1. Molecular weight marker #SM1373 (Fermentas); 2. Negative control (reaction of PCR with sterile distilled water); 3. Positive control (genomic DNA from Clavibacter sp.). 4. K. oryzae; 5. K. oryzae; 6. P. cypripedii; 7. B: tropica; 8. S. marcescens; 9. E. rhapontici/ Pantoea dispersa; 10. P. dispersa; 11. P. cypripedii; 12. P. agglomerans/P. cypripedii; 13. P. cypripedii; 14. B. tropica; 15. B. tropica.
The bacterial species identified were: Kosakonia oryzae, Pectobacterium cypripedii, Burkholderia tropica, Serratia marcescens, Erwinia rhapontici, Pantoea dispersa, Pantoea agglomerans, and Pectobacterium cypripedii. Most of the genuses identified, except for Burkholderia, belong to the Enterobacteriaceae family, which comprises several genuses of important pathogenic human, animal, and plant bacteria (Hauben et al., 1998; Young and Park, 2007). On the other hand, Burkholderia belongs to the Burkholderiaceae family. This genus is known for its adaptability to diverse habitats such as freshwater sediments and plant, animal, and human tissues. It is also used to promote plant growth, the biocontrol of plant pathogens, phytoremediation, and xenobiotic degradation (Paganin et al, 2011).
K. oryzae, synonymous with Enterobacter guangdongense, Enterobacter oryzae (UniProt Taxonomy, 2014), has also been reported as a bacteria that fixates nitrogen in roots of rice plants (Peng et al., 2009), and so far it has not been reported as phytopathogenic. In this same sense, the bacteria Burkholderia tropica is also considered nitrogen fixating, with important agro-biotechnological applications such as antifungal activity for the biocontrol of phytopathogenic fungi of agricultural interest (Tenorio-Salgado et al., 2013).
P. cypripedii, the previous name for which was Erwinia cypripedii (UniProt Taxonomy, 2014), produces the disease called orchid brown rot (Plantwise, 2014); it is a bacteria that affects plants such as the papaya tree (Carica papaya) and different types of orchids, such as the lady slipper or Venus's slipper (Paphiopedilum), the orchid var. Luna (Phalaenopsis amabilis), and others (Plantwise, 2014). It causes dark brown foliar spots, the yellowing of leaves, the bleaching of stems, and the death of the plant. In the case of orchids, the disease begins presenting itself as small moist, greasy-looking, brown, and slightly sunken lesions (Plantwise, 2014).
Two genuses of Pantoea were also identified: P. dispersa and P. agglomerans. The former has the potential to be used as a biological control against other bacteria such as Xanthomonas albilineans, which producesa foliar scalding in sugar cane (Zhang and Birch, 1997) or against some fungi such as Fusarium or Macrophomina, when the bacteria is placed under the proper treatments (Gohel et al., 2004); it also acts as a biostimulant of plant growth (Fernández et al., 2008). P. agglomerans, on the other hand, is better known for infecting several plant genuses such as Alocasia cucullata, in which it produces necrotic spots on leaves and their edges. The spots appear initially as irregular lesions that grow to form necrotic areas (Romeiro et al., 2006). In maize and sorghum, it causes smut and symptoms of vascular wilting (Morales-Valenzuela et al., 2007). In infected cotton capsules, fibers do not mature completely and the seed tissue shows a brown coloring (Ren et al., 2008). In rice, it causes leaf smut and stem rotting (Lee et al. 2010). In walnut trees (Carya or Juglans), it causes the premature falling of the fruit (Yang et al., 2011). However, the bacteria can behave as pathogens for humans (Cruz et al., 2007; Schmid et al., 2003; Holden et al., 2009; Cruz et al., 2007).
In this same context, Serratia marcescens, another bacteria identified in this study, presents a characteristically red color due to prodigiosin, a bright red pigment produced by certain strains of Serratia (Gerber, 1975). It used to be considered an opportunist pathogen that causes diseases in humans, such as cystitis (Liu et al., 2004), conjunctivitis (Hume and Willox, 2004), keratitis (Schaefer et al., 2001), meningitis (Zaidi et al., 1989), pneumonia (Carlon et al., 1977; Khan et al., 1997), and others. However, some reports show that this bacterium can cause yellow vine disease in cucurbits, in which it colonizes the phloem of plants, producing a yellow color and wilting of leaves (Wick et al., 2001; Bruton et al., 2003; Sikora et al., 2012).
Finally, although no differences were observed with 100 % reliability, according to the molecular identification between Erwinia rhapontici and Pantoea dispersa, further tests are considered necessary for its correct identification. Because P. dispersa is not considered a phytopathogen, E. rhapontici is, since it produces a pink color in the coating of some seeds (Schroeder et al., 2002; Huang et al., 2003a; Wise et al., 2008), as well as the rotting of the corona in other plants (Huang et al., 2003b).
Conclusions
Eight bacteria were found to be related to diseased ornamental plants in the phase of in vitro acclimatization: Kosakonia oryzae, Pectobacterium cypripedii, Burkholderia tropica, Serratia marcescens, Pantoea dispersa, Erwinia cypripedii, Pantoea agglomerans, and Erwinia rhapontici. The three latter represent a phytosanitary hazard.
Literatura citada
Agrios, G. N. 2005. Plant Pathology. Fifth ed. Elsevier Aca. Press. 948 p. [ Links ]
Bruton, B. D., F. Mitchell, J. Fletcher, S. D. Pair, A. Wayadande, U. Melcher, J. Brady, B. Bextine, and T. W. Popham. 2003. Serratia marcescens, a phloem-colonizing, squash bugtransmitted bacterium: Causal agent of cucurbit yellow vine disease. Plant Dis. 87:937-944. http://dx.doi.org/10.1094/pdis.2003.87.8.937 [ Links ]
Carlon, G. C., P. T. Dickinson P. L. Goldiner, A. D. Turnbull, and W. S Howland. 1977. Serratia marcescens pneumonia. Arch. Surgery 112:1220-1224. http://dx.doi.org/10.1001/archsurg.1977.01370100074015 [ Links ]
Corona Nava-Esparza, V. y A. Chimal-Hernández. 2006. Plantas mexicanas con potencial ornamental. Universidad Autónoma Metropolitana (UAM), división de ciencias biológicas y de la salud. No. 60. 626 p. [ Links ]
Cruz, A. T., A. C. Cazacu, and C. H Allen. 2007. Pantoea agglomerans, a plant pathogen causing human disease. J Clin. Microbiol. 45:1989-1992. http://dx.doi.org/10.1128/jcm.00632-07 [ Links ]
Daughtrey, M. L., R. L. Wick, and J. L Peterson. 2001. Plagas y enfermedades de las plantas en maceta con flores. The American Phytopathol. Soc. 90 p. [ Links ]
Fernández, A. I., M. Villaverde, J. A. Nicolás, A. García-Gómez, and J. Malo. 2008. Pantoea dispersa. Rhizobacteria promotora del crecimiento vegetal (PGPR). VII Congreso SEAE Bullas (Murcia). http://www.agroecologia.net/recursos/publicaciones/publicaciones-online/2009/eventos-seae/cds/congresos/actas-bullas/seae_bullas/verd/posters/9%20P.%20FER/16%20FER%20Pantoea.pdf [ Links ]
Gerber, N. N. 1975. Prodigiosin-like pigments. CRC critical reviews in microbiology, 3: 469-485. [ Links ]
Gohel, V., C. Megha, P. Vyas and H. S. Chatpar. 2004. Strain improvement of chitinolytic enzyme producing isolate Pantoea dispersa for enhancing its biocontrol potential against fungal plant pathogens. Ann Microbiol 54:503-515. [ Links ]
Hauben, L., E. R. Moore, L. Vauterin, M. Steenackers, J. Mergaert, L. Verdonck, and J. Swings. 1998. Phylogenetic position of phytopathogens within the Enterobacteriaceae. Syst. and Appli. Microbiol. 21:384-97. http://dx.doi.org/10.1016/s0723-2020(98)80048-9 [ Links ]
Holden, N., L. Pritchard, and I. Toth. 2009. Colonization outwith the colon: plants as an alternative environmental reservoir for human pathogenic enterobacteria. FEMS Microbial Revie 33: 689-703. http://dx.doi.org/10.1111/j.1574-6976.2008.00153.x [ Links ]
Huang, H. C., R. S. Erickson, L. J. Yanke, T. F. Hsieh, and R. A. A Morrall. 2003a. First report of pink seed of lentil and chickpea caused by Erwinia rhapontici in Canada. Plant Dis. 87:1398. http://dx.doi.org/10.1094/pdis.2003.87.11.1398a [ Links ]
Huang, H. C., T. F. Hsieh, and R. S. Erickson. 2003b. Biology and epidemiology of Erwinia rhapontici, causal agent of pink seed and crown rot of plants. Plant Pathol Bull. 12:6976. http://dx.doi.org/10.1094/pdis.2002.86.8.921c [ Links ]
Hume, E., and M. Willox. 2004. Aparición de Serratia marcescens como un patógeno de superficie ocular. Arch. Soci. Española de Oftalmol. [online]. 2004, vol.79, n.10 [citado 2014-03-24], pp. 475-481. http://dx.doi.org/10.4321/s0365-66912004001000002 [ Links ]
Khan, E. A., L. S. Wafelman, J. A. Garcia-Prats, and L. H. Taber. 1997. Serratia marcescens pneumonia, empyema and pneumatocele in a preterm neonate. The Pediatri Infec. Dis. J. 16:1003-1005. http://dx.doi.org/10.1097/00006454-199710000-00021 [ Links ]
Lee, H. B., J. P. Hong, and S. B. Kim. 2010. First report of leaf blight caused by Pantoea agglomerans on rice in Korea. Plant Dis. 94:1372. http://dx.doi.org/10.1094/pdis-05-10-0374 [ Links ]
Liu, J. W., Y. M. Hsu, and Y. F. Huang. 2004. Independent prognostic factors for fatality in patients with urinary tract infection caused by Serratia marcescens. Infect. Control and Hospital Epidemiol 25:80-82. http://dx.doi.org/10.1086/502297 [ Links ]
Madigan, M. T., J. M. Martinko y J. Parker. 1997. Biología de los Microorganismos. 8a ed. Prentice Hall. Madrid, España. 1064 p. http://dx.doi.org/10.1016/s0962-8924(97)83479-4 [ Links ]
Morales-Valenzuela, G., H.V. Silva-Rojas, D. Ochoa-Martínez, E. Valadez-Moctezuma, B. Alarcón-Zúñiga, L. X. Zelaya-Molina, L. Córdova-Téllez, L. Mendoza-Onofre, H. Vaquera-Huerta, A. Carballo-Carballo, A. Farfán-Gómez, and G. Ávila-Quezada. 2007. First report of Pantoea agglomerans causing leaf blight and vascular wilt in maize and sorghum in Mexico. Plant Dis. 91:1365. http://dx.doi.org/10.1094/pdis-91-10-1365a [ Links ]
Paganin, P., S. Tabacchioni, and L. Chiarini. 2011. Pathogenicity and biotechnological applications of the genus Burkholderia. Central Europ. J. of Biol. 6:997-1005. http://dx.doi.org/10.2478/s11535-011-0072-2 [ Links ]
Peng, G., W. Zhang, H. Luo, H. Xie, W. Lai, and Z. Tan. 2009. Enterobacter oryzae sp. nov., a nitrogen-fixing bacterium isolated from the wild rice species Oryza latifolia. Internati. J. of Syst. and Evol. Microbiol. 59:1650-1655. http://dx.doi.org/10.1099/00207713-59-10-2646-b [ Links ]
Plantwise, 2014. http://www.plantwise.org/KnowledgeBank/Datasheet.aspx?dsid=21918. [ Links ]
Ren, Y. Z., Y. Q. Liu, S. L. Ding, G. Y. Li, and H. Zhang. 2008. First report of boll rot of cotton caused by Pantoea agglomerans in China. Plant Dis. 92:1364. http://dx.doi.org/10.1094/pdis-92-9-1364b [ Links ]
Romeiro, R. S., D. Macagnan, H. L. Mendonça, and N. J. Rodrigues. 2006. Bact. spot of Chinese taro (Alocasia cucullata) in Brazil induced by Pantoea agglomeransNew Dis. Rep. 14:51. http://dx.doi.org/10.1111/j.1365-3059.2007.01631.x [ Links ]
Rout, G. R., A. Mohapatra, and S. M. Jain. 2006. Tissue culture of ornamental pot plant: A critical review on present scenario and future prospects. Biotechnol. Adv. 24:531-560. http://dx.doi.org/10.1016/j.biotechadv.2006.05.001 [ Links ]
Schaefer, F., O. Bruttin, L. Zografos, and Y. Guex-Crosier. 2001. Bacterial keratitis: a prospective clinical and microbiological study. Brit. J. Ophthalmol. 85:842-847. http://dx.doi.org/10.1136/bjo.85.7.842 [ Links ]
Schmid, H., S. Schubert, C. Weber, and J. R Bogner. 2003. Isolation of a Pantoea dispersa-like strain from a 71-year-old woman with acute myeloid leukemia and multiple myeloma Infection 31: 66-67. http://dx.doi.org/10.1007/s15010-002-3024-y [ Links ]
Schroeder, B. K., S. L. Lupien, and F. M. Dugan. 2002. First report of pink seed of pea caused by Erwinia rhapontici in the United States. Plant Dis. 86:188. http://dx.doi.org/10.1094/pdis.2002.86.2.188a [ Links ]
Sikora, E. J., B. D. Bruton, A. C. Wayadande, and J. Fletcher. 2012. First Report of the yellow vine disease caused by Serratia marcescens in watermelon and yellow squash in Alabama. Plant Dis. 96:761. http://dx.doi.org/10.1094/pdis-09-11-0739-pdn [ Links ]
Tenorio-Salgado, S., R. Tinoco, R. Vazquez-Duhalt, J. Caballero-Mellado, and E. Pérez-Rueda. 2013. Identification of volatile compounds produced by the bacterium Burkholderia tropica that inhibit the growth of fungal pathogens. Bioengineered 4:236-243. http://dx.doi.org/10.4161/bioe.23808 [ Links ]
UniProt Taxonomy. 2014.Disponible en linea: http://www.uniprot.org/taxonomy [ Links ]
Wick R. L., J. Lerner, S. D. Pair, J. Fletcher, F. Mitchell , and B.D. Bruton. 2001. Detection of cucurbit yellow vine disease in squash and pumpkin in Massachusetts. Plant Dis. 85:1031. http://dx.doi.org/10.1094/pdis.2001.85.9.1031c [ Links ]
Wise, K. A., Y. F. Zhao, C. A. and Bradley. 2008. First Report of Pink Seed of Pea Caused by Erwinia rhapontici in North Dakota. Plant Dis. 92:315. http://dx.doi.org/10.1094/pdis-92-2-0315a [ Links ]
Yang, K. Q., W. W. Qu, X. Liu, H. X. Liu, and L. Q. Hou. 2011. First report of Pantoea agglomerans causing brown apical necrosis of walnut in China. Plant Dis. 95:773. http://dx.doi.org/10.1094/pdis-01-11-0060 [ Links ]
Young, J. M., and D. C. Park. 2007. Relationships of plant pathogenic enterobacteria based on partial atpD, carA, and recA as individual and concatenated nucleotide and peptide sequences. Syst. and Appl. Microbiol. 30:343-354. http://dx.doi.org/10.1016/j.syapm.2007.03.002 [ Links ]
Zaidi, M., J. Sifuentes, M. Bobadilla, D. Moncada, and D. L. S. Ponce. 1989. Epidemic of Serratia marcescens bacteremia and meningitis in a neonatal unit in Mexico City. P. 14-20 : Infection control and hospital epidemiology: The official J. of the Soc. of Hosp. Epidemiol. of America. http://dx.doi.org/10.1086/645909 [ Links ]
Zhang, L., and R. G. Birch. 1997. Mechanisms of biocontrol by Pantoea dispersa of sugar cane leaf scald disease caused by Xanthomonas albilineans. J. of Appli. Microbiol. 82:448-54. http://dx.doi.org/10.1046/j.1365-2672.1997.00135.x [ Links ]
Received: November 23, 2016; Accepted: March 28, 2016











 texto en
texto en 


